Chapter 67 Care of Patients with Diabetes Mellitus
Safe and Effective Care Environment
1. Assess the person who has diabetes for specific current and ongoing factors that pose threats to safety.
2. Administer insulin and other antidiabetic agents in a safe and accurate manner.
3. Apply the principles of infection control in the care of patients with diabetes.
4. Individualize patient teaching methods for diabetes management.
5. Teach patients and families the safe use of insulin injection equipment and glucose monitoring equipment.
6. Teach patients with peripheral neuropathy how to avoid injury.
Health Promotion and Maintenance
7. Encourage everyone to prevent type 2 diabetes by achieving and maintaining ideal weight and participating in regular exercise.
8. Teach all patients with diabetes how to self-manage their disease.
9. Teach the patient and family about the manifestations and emergency treatment of hypoglycemia and hyperglycemia.
10. Allow the patient the opportunity to express concerns regarding the diagnosis of diabetes or the treatment regimen.
11. Explain all procedures, restrictions, drugs, and follow-up care to the patient and family.
12. Refer patients newly diagnosed with diabetes to local resources and support groups.
13. Compare the risk factors, age of onset, manifestations, and pathologic mechanisms of type 1 and type 2 diabetes mellitus.
14. Explain the effects of insulin on carbohydrate, protein, and fat metabolism.
15. Explain how to mix different kinds of insulin together.
16. Evaluate laboratory data to determine effectiveness of the prescribed dietary, drug, and exercise therapies for diabetes.
17. Explain how to perform foot assessment and foot care for the patient with diabetes.
18. Collaborate with members of the health care team to provide care for patients with diabetic ketoacidosis (DKA) or hyperglycemic-hyperosmolar state (HHS).
http://evolve.elsevier.com/Iggy/
Answer Keys for NCLEX Examination Challenges and Decision-Making Challenges
Concept Map: Diabetes Mellitus—Type 2
Review Questions for the NCLEX® Examination
Diabetes is a major public health problem, and its complications, especially hypertension and hyperlipidemia (high blood lipid levels), cause many serious health problems. In the United States, diabetes mellitus (DM) is a leading cause of blindness, end-stage kidney disease, and foot or leg amputations. Many people have undiagnosed diabetes and, among those who are diagnosed, many have continuous high blood glucose levels. The complications of DM can be greatly reduced with glycemic (blood glucose) control along with management of hypertension and hyperlipidemia. Thus nursing priorities focus on helping the patient with diabetes achieve and maintain lifestyle changes that prevent long-term complications by keeping blood glucose levels and cholesterol levels as close to normal as possible (Young, 2011).
Pathophysiology
Classification of Diabetes
For all types of diabetes mellitus (DM), the main feature is chronic hyperglycemia (high blood glucose level) resulting from problems with insulin secretion, insulin action, or both. The disease is classified by the underlying problem causing a lack of insulin and the severity of the insulin deficiency. Table 67-1 outlines the types of DM.
TABLE 67-1 CLASSIFICATION OF DIABETES MELLITUS
Data from American Diabetes Association (ADA). (2010a). Position statement: Diagnosis and classification of diabetes mellitus, Diabetes Care, 33(Suppl. 1), 62-69.
The Endocrine Pancreas
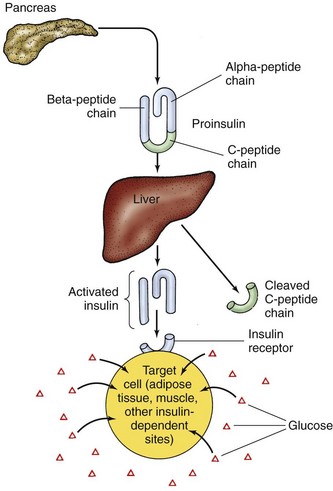
Active insulin is a protein made up of 51 amino acids. It is initially produced as inactive proinsulin, a prohormone that contains an additional amino acid chain (the C-peptide chain). Proinsulin is converted into active insulin by removal of the C-peptide (Fig. 67-1).
Absence of Insulin
Insulin is needed to move glucose into most body tissues. The lack of insulin in diabetes, from either a lack of production or a problem with insulin use at its cell receptor, prevents some cells from using glucose for energy. The body then breaks down fat and protein in an attempt to provide energy and also increases the levels of counterregulatory hormones in an attempt to make glucose from other sources. Table 67-2 outlines the body’s response to insufficient insulin.
TABLE 67-2 PHYSIOLOGIC RESPONSE TO INSUFFICIENT INSULIN
Insulin lack initially causes potassium depletion. With the increased fluid loss from hyperglycemia, excessive potassium is excreted in the urine, leading to low serum potassium levels. High serum potassium levels may occur in acidosis because of the shift of potassium from inside the cells to the blood. Serum potassium levels in DM, then, may be low (hypokalemia), high (hyperkalemia), or normal, depending on hydration, the severity of acidosis, and the patient’s response to treatment. Chapter 14 discusses acid-base balance and acidosis in more detail.
Health Promotion and Maintenance
A. “The extra glucose in the blood increases the blood sodium level, which increases your sense of thirst.”
B. “Without insulin, glucose is excreted rather than used in the cells. The loss of glucose directly triggers thirst, especially for sugared drinks.”
C. “The extra glucose in the blood makes the blood thicker, which then triggers thirst so that the water you drink will dilute the blood glucose level.”
D. “Without insulin, glucose combines with blood cholesterol, which damages the kidneys, making you feel thirsty even when no water has been lost.”
Acute Complications of Diabetes
Three glucose-related emergencies can occur in patients with diabetes:
• Diabetic ketoacidosis (DKA) caused by lack of insulin and ketosis
• Hyperglycemic-hyperosmolar state (HHS) caused by insulin deficiency and profound dehydration
Chronic Complications of Diabetes
• Chronic hyperglycemia thickens basement membranes, which causes organ damage.
• Glucose toxicity directly or indirectly affects functional cell integrity.
• Chronic ischemia in small blood vessels causes connective tissue hypoxia and microischemia.
Macrovascular Complications
Cardiovascular Disease
Cardiovascular complication rates can be reduced through aggressive management of hyperglycemia, hypertension, and hyperlipidemia. The American Diabetes Association (ADA) recommends that blood pressure be maintained below 130/80 mm Hg and that low-density lipoprotein (LDL) cholesterol remain below 100 mg/dL (2.60 mmol/L) for patients without manifestations of CVD and to less than 70 mg/dL (1.8 mmol/L) for patients with manifestations of CVD (ADA, 2010b). Diets high in saturated fat raise total cholesterol and LDL cholesterol levels, which increase the risk for coronary artery disease. Lifestyle modifications that focus on reducing saturated fat, trans fat, and cholesterol intake; increasing intake of omega-3 fatty acids, fiber, and plant sterols; weight loss (if indicated); and increasing physical activity are recommended to improve the lipid profile for patients with DM (ADA, 2010b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


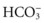 ] levels) and compensatory respiratory alkalosis (decreased partial pressure of arterial carbon dioxide [Pa
] levels) and compensatory respiratory alkalosis (decreased partial pressure of arterial carbon dioxide [Pa