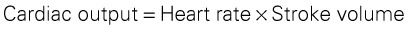Chapter 35 Assessment of the Cardiovascular System
Safe and Effective Care Environment
Health Promotion and Maintenance
2. Identify patients at risk for cardiovascular (CV) problems.
3. Differentiate modifiable and nonmodifiable risk factors for CV disease.
4. Teach patients about ways to decrease their risk for CV health problems.
5. Explain nursing implications related to CV changes associated with aging.
7. Review the anatomy and physiology of the CV system.
8. Describe the unique characteristics of heart disease in women.
9. Perform focused physical assessment for patients with CV problems.
10. Interpret laboratory test findings for patients with suspected or actual CV disease.
11. Differentiate invasive hemodynamic pressures and their significance.
http://evolve.elsevier.com/Iggy/
Animation: Auscultation of Heart Valves
Animation: Blood Flow: Circulatory System
Animation: Cardiac Cycle During Systole and Diastole
Animation: Heart Valves and Sounds
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Clip: S1 at Various Locations
Audio Clip: S2 at Various Locations
Audio Clip: Murmurs: High, Medium, and Low
Audio Clip: Murmurs: Blowing, Harsh or Rough, and Rumble
Audio Clip: Pericardial Friction Rub
Review Questions for the NCLEX® Examination
Video Clip: Auscultation with Diaphragm and Bell
Video Clip: Auscultatory Landmarks
Video Clip: Pulses, Lower Extremities
In almost every year since 1900, cardiovascular disease (CVD) has been the number-one cause of death in the United States. Nearly 2300 Americans die of CVD each day, an average of one death every 38 seconds (American Heart Association [AHA], 2010b). The disease kills more people than the next four causes of death combined, including cancer, chronic lower respiratory diseases, accidents, and diabetes. Of particular concern is that CVD is the leading cause of death for women. In addition, the American Heart Association (AHA) estimates that more than one in three adults is living with some form of the disease. About 20% of people who experience a myocardial infarction will die within 1 year from the initial cardiac event (AHA, 2010b).
Anatomy and Physiology Review
Heart
Structure
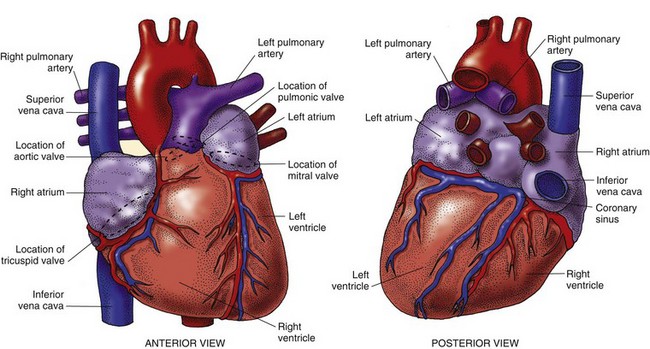
The human heart is a fist-sized, muscular organ located in the mediastinum between the lungs (Fig. 35-1). Each beat of the heart pumps about 60 mL of blood, or 5 L/min. During strenuous physical activity, it can double the amount of blood pumped to meet the body’s increased oxygenation needs. The heart is protected by a covering called the pericardium. A muscular wall (septum) separates the heart into two halves: right and left. Each half has an atrium and a ventricle (Fig. 35-2).
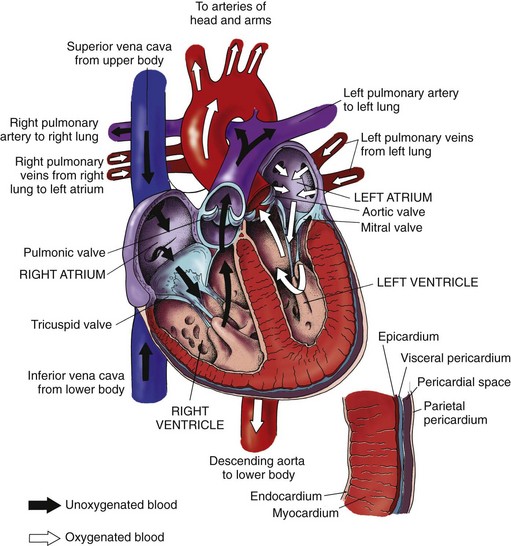
After blood is reoxygenated in the lungs, it flows freely from the four pulmonary veins into the left atrium. Blood then flows through an opened mitral valve into the left ventricle during ventricular diastole. When the left ventricle is almost full, the left atrium (LA) contracts, pumping the remaining blood volume into the left ventricle. With systolic contraction, the left ventricle (LV) generates enough pressure to close the mitral valve and open the aortic valve. Blood is propelled into the aorta and into the systemic arterial circulation. Blood flow through the heart is shown in Fig. 35-2.
The four cardiac valves are responsible for maintaining the forward flow of blood through the chambers of the heart (see Fig. 35-2). These valves open and close when pressure and volume change within the heart’s chambers. The cardiac valves are classified into two types: atrioventricular (AV) valves and semilunar valves.
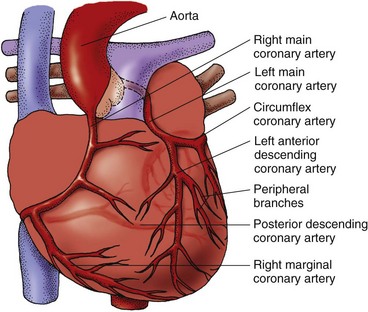
The heart muscle receives blood to meet its metabolic needs through the coronary arterial system (Fig. 35-3). The coronary arteries originate from an area on the aorta just beyond the aortic valve. All of the coronary arteries feeding the left heart originate from the left main coronary artery (LMCA). The right coronary artery (RCA) branches from the aorta to perfuse the right heart and inferior wall of the left heart.
Function
The electrophysiologic properties of heart muscle are responsible for regulating heart rate (HR) and rhythm. Cardiac muscle cells possess the characteristics of automaticity, excitability, conductivity, contractility, and refractoriness. Chapter 36 describes these properties and cardiac conduction in detail.
Sequence of Events During the Cardiac Cycle
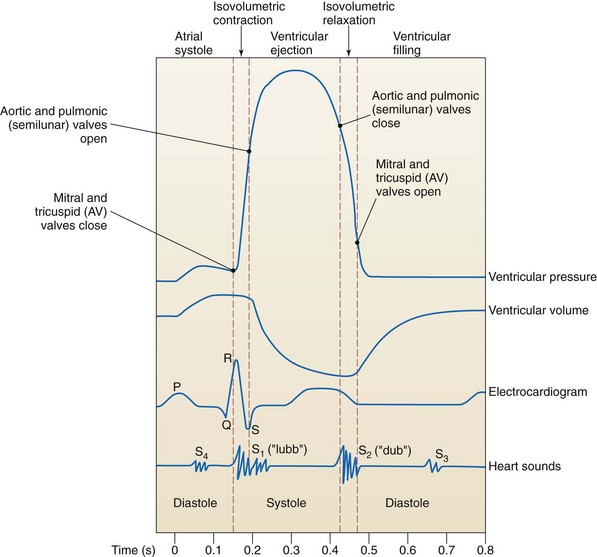
The phases of the cardiac cycle are generally described in relation to changes in pressure and volume in the left ventricle during filling (diastole) and ventricular contraction (systole) (Fig. 35-4). Diastole, normally about two thirds of the cardiac cycle, consists of relaxation and filling of the atria and ventricles. Systole consists of the contraction and emptying of the atria and ventricles.
Vascular System
The vascular system serves several purposes:
• Provides a route for blood to travel from the heart to nourish the various tissues of the body
• Carries cellular wastes to the excretory organs
• Allows lymphatic flow to drain tissue fluid back into the circulation
Arterial System
The primary function of the arterial system is to deliver oxygen and nutrients to various tissues in the body. Nutrients are carried through arteries to arterioles, then branch into smaller terminal arterioles and finally join with capillaries and venules to form a capillary network. Within this network, nutrients are exchanged across capillary membranes by three primary processes: osmosis, filtration, and diffusion. (See Chapter 13 for detailed discussions of these processes.)
• The autonomic nervous system (ANS), which excites or inhibits sympathetic nervous system activity in response to impulses from chemoreceptors and baroreceptors
• The kidneys, which sense a change in blood flow and activate the renin-angiotensin-aldosterone mechanism
• The endocrine system, which releases various hormones (e.g., catecholamine, kinins, serotonin, histamine) to stimulate the sympathetic nervous system at the tissue level
The kidneys also help regulate cardiovascular activity. When renal blood flow or pressure decreases, the kidneys retain sodium and water. BP tends to rise because of fluid retention and activation of the renin-angiotensin-aldosterone mechanism (see Fig. 13-6 in Chapter 13). This mechanism results in vasoconstriction and sodium retention (and thus fluid retention). Vascular volume is also regulated by the release of antidiuretic hormone (vasopressin) from the posterior pituitary gland (see Chapter 13).
Cardiovascular Changes Associated with Aging
A number of physiologic changes in the cardiovascular system occur with advancing age (Chart 35-1). Many of these changes result in a loss of cardiac reserve. Thus these changes are usually not evident when the older adult is resting. They become apparent only when the person is physically or emotionally stressed and the heart cannot meet the increased metabolic demands of the body.
Chart 35-1 Nursing Focus on the Older Adult
Changes in the Cardiovascular System Related to Aging
| CHANGE | NURSING INTERVENTIONS | RATIONALES |
|---|---|---|
| Cardiac Valves | ||
| Calcification and mucoid degeneration occur, especially in mitral and aortic valves. | Assess heart rate and rhythm and heart sounds for murmurs. Question patients about dyspnea. | Murmurs may be detected before other symptoms. Valvular abnormalities may result in rhythm changes. |
| Conduction System | ||
| Pacemaker cells decrease in number. Fibrous tissue and fat in the sinoatrial node increase. Few muscle fibers remain in the atrial myocardium and bundle of His. Conduction time increases. | Assess the electrocardiogram (ECG) and heart rhythm for dysrhythmias or a heart rate less than 60 beats/min. | The sinoatrial (SA) node may lose its inherent rhythm. Atrial dysrhythmias occur in many older adults; 80% of older adults experience premature ventricular contractions (PVCs). |
| Left Ventricle | ||
| The size of the left ventricle increases. The left ventricle becomes stiff and less distensible. Fibrotic changes in the left ventricle decrease the speed of early diastolic filling by about 50%. | Assess the ECG for a widening QRS complex and a longer QT interval. | Ventricular changes result in decreased stroke volume, ejection fraction, and cardiac output during exercise; the heart is less able to meet increased oxygen demands. |
| Assess the heart rate at rest and with activity. Assess for activity intolerance. | Maximum heart rate with exercise is decreased. The heart is less able to meet increased oxygen demands. | |
| Aorta and Other Large Arteries | ||
| The aorta and other large arteries thicken and become stiffer and less distensible. Systolic blood pressure increases to compensate for the stiff arteries. Systemic vascular resistance increases as a result of less distensible arteries; therefore the left ventricle pumps against greater resistance, contributing to left ventricular hypertrophy. | Assess blood pressure. Note increases in systolic, diastolic, and pulse pressures. Assess for activity intolerance and shortness of breath. Assess the peripheral pulses. | Hypertension may occur and must be treated to avoid target organ damage. |
| Baroreceptors | ||
| Baroreceptors become less sensitive. | Assess the patient’s blood pressure with the patient lying and then sitting or standing. Assess for dizziness when the patient changes from a lying to a sitting or standing position. Teach the patient to change positions slowly. | Orthostatic (postural) and postprandial changes occur because of ineffective baroreceptors. Changes may include blood pressure decreases of 10 mm Hg or more, dizziness, and fainting. |
Assessment Methods
Patient History
The focus of the patient history is on obtaining information about risk factors and symptoms of cardiovascular disease (Chart 35-2). Assess nonmodifiable (uncontrollable) risk factors including the patient’s age, gender, ethnic origin, and family history of cardiovascular disease. Ask about any chronic disease or illness that the patient may have. The incidence of conditions such as coronary artery disease (CAD) and valvular disease increases with age. The incidence of CAD also varies with the patient’s gender. Men have a higher risk for CAD than women of all ages except in the oldest age-group of 80 years and older (AHA, 2010b).
Cardiovascular Assessment
Using Gordon’s Functional Health Patterns
Health Perception–Health Management Pattern
Nutrition-Metabolic Pattern
• What is your usual daily diet? (Analyze the diet for saturated fat, cholesterol, total calorie, and sodium content.)
• How much fluid do you drink daily? Are you thirsty?
• What do you weigh? When did you last weigh yourself?
• How often do you weigh yourself?
• Do you know your cholesterol level? What is it?
• How often do you feel nauseated or not interested in eating?
Activity/Exercise Pattern
• What is the most strenuous exercise you did last week?
• How active are you compared with 6 months ago? 1 year ago?
• How often do you feel fatigued or tired?
• Can you climb a flight of stairs and walk a block without feeling short of breath or experiencing chest pain?
• Do you experience leg cramps when you walk or climb stairs?
Cognitive/Perceptual Pattern
• How is your memory? What does your family say about your memory?
• How often do you feel dizzy, disoriented, or faint?
• Do you ever have chest discomfort? How often? What precipitates it? What is it like? How do you relieve it? What is its level on a scale of 0 to 10?
• Do you ever have leg or buttock pain? What are its characteristics?
Based on Gordon, M. (2011). Manual of nursing diagnosis (12th ed.). Boston: Jones & Bartlett.
Postmenopausal women are two to three times more likely than premenopausal women to have CAD. The incidence for the disease in women is about 10 years later than in men and 20 years later for myocardial infarction (MI) and death to occur. After an acute MI, women tend to have a higher mortality rate and suffer more complications when compared with men (AHA, 2010b).
Heart disease is the leading cause of diabetes-related death for both men and women. Adults with diabetes have heart disease death rates 2 to 4 times higher than those without diabetes. The risk for stroke is also 2 to 4 times higher among people with diabetes. The number of premature deaths (younger than 65 years) from heart disease is greatest among American Indians and Alaska Natives and lowest among Asians (AHA, 2010b).
• How soon after you wake up in the morning do you smoke?
• Do you wake up in the middle of your sleep time to smoke?
• Do you find it difficult not to smoke in places where smoking is prohibited?
Three to four years after a patient has stopped smoking, his or her CVD risk appears to be similar to that of a person who has never smoked. Be sure to ask those who do not currently smoke whether they have ever smoked and when they quit. Passive smoke significantly reduces blood flow in healthy young adults’ coronary arteries, and the risk for dying increases among those who are exposed to secondhand smoke (AHA, 2010a).
A sedentary lifestyle is also a major risk factor for heart disease. Regular physical activity promotes cardiovascular fitness and produces beneficial changes in blood pressure and levels of blood lipids and clotting factors. Unfortunately, few people in the United States follow the recommended exercise guidelines: 30 minutes daily of light to moderate exercise, which is equivalent to a 30-minute brisk walk. According to the American Heart Association (AHA) (2010a), fewer than two thirds of people in the United States engage in this much exercise five times a week and fewer engage in more vigorous physical activity to promote cardiopulmonary fitness. Encourage increased physical exercise as part of a lifestyle change to reduce the risk for CAD. Ask patients about the type of exercise they perform, how long a period they have participated in the exercise, and the frequency and intensity of the exercise.
About two thirds of American adults are overweight when defined as a body mass index (BMI) of 25 to 30. Obesity, defined as a BMI greater than 30, is particularly a problem for African-American women, Mexican Americans, and native Hawaiians, but the exact cause of this cultural difference is unknown (AHA, 2010a). Obesity is also associated with hypertension, hyperlipidemia, and diabetes; all are known contributors to CVD.
The American Heart Association provides guidelines to combat obesity and improve cardiac health, including ingesting more nutrient-rich foods that have vitamins, minerals, fiber, and other nutrients but are low in calories. To get the necessary nutrients, teach patients to choose foods like vegetables, fruits, whole-grain products, and fat-free dairy products most often. Also teach patients to not eat more calories than they can burn every day (AHA, 2010a).
The social history includes information about the patient’s living situation, including having a domestic partner, other household members, environment, and occupation. Identification of support systems is especially important in exploring the possibility that the patient might have difficulty paying for medications or treatment. People who report an annual household income less than $30,000 have a greater risk for CVD than people who have an income over $50,000 (Metcalf et al., 2008; Wright et al., 2009).
Nutrition History
Health Promotion and Maintenance
A. “I can’t do anything about my disease risk because it is in my genes.”
B. “I will take my blood pressure medication only when I have symptoms.”
C. “I will start walking on the treadmill each day when I watch my favorite TV show.”
D. “I don’t need to stop smoking because damage to my blood vessels is irreversible.”
Family History and Genetic Risk
Review the family history, and obtain information about the age, health status, and cause of death of immediate family members. A positive family history for CAD in a first-degree relative (parent, sibling, or child) is a major risk factor. It is more important than other factors such as hypertension, obesity, diabetes, or sudden cardiac death (AHA, 2010b).
Genetic/Genomic Considerations
Cardiovascular disease has many contributory factors, including a genetic tendency. A significant association between familial cardiac history and cardiovascular disease is consistently demonstrated in the evidence of multiple large-scale prospective epidemiology studies (Lloyd-Jones et al., 2009). Although several genes have been reported to be associated with heart disease, stroke, and hypertension, the impact of each individual gene is not fully understood. Numerous genetic association studies are underway to determine more specific genetic variants that may underlie the family history (Lloyd-Jones et al., 2009). Additional discussions about genetic factors related to specific CV diseases are found in other chapters in this unit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree