Laura M. Dechant
Care of Patients with Cardiac Problems
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
8 Explain the pathophysiology of HF.
9 Compare and contrast left-sided and right-sided HF.
10 Identify priority problems for patients with HF.
11 Perform a comprehensive assessment of patients experiencing cardiac problems.
12 Explain how common drug therapies improve cardiac output and prevent worsening of HF.
13 Assess patients for adverse effects of drug therapy for cardiac problems.
14 Monitor the laboratory values for patients with cardiac problems.
15 Plan nursing interventions to improve the patient’s cardiovascular status when needed.
17 Identify the four Heart Failure Core Measures required by The Joint Commission.
18 Describe essential focused assessments used by the home care nurse for patients with heart failure.
19 Compare and contrast common valvular disorders.
20 Describe surgical management for patients with valvular disease.
21 Develop a teaching/learning plan for patients with valvular disease.
23 Provide postoperative care for patients having a heart transplant.
24 Identify clinical assessment findings for patients with cardiomyopathy.

http://evolve.elsevier.com/Iggy/
Animation: Congestive Heart Failure
Animation: Pericardial Tamponade
Answers to NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
This chapter focuses on heart failure and its common causes in the adult population; coronary artery disease is discussed in Chapter 40. Heart failure is the most common reason for hospital stays in patients older than 65 years in the United States. When the heart is diseased, it cannot effectively pump an adequate amount of arterial blood to the rest of the body. Arterial blood carries oxygen and nutrients to vital organs, such as the kidneys and brain, and peripheral tissues. When these organs and other body tissues are not adequately perfused, they may not function properly.
Heart Failure
Heart failure, sometimes referred to as pump failure, is a general term for the inability of the heart to work effectively as a pump. It results from a number of acute and chronic cardiovascular problems that are discussed later in this chapter and elsewhere in the cardiovascular unit.
Pathophysiology
Heart failure (HF) is a common chronic health problem, with acute episodes often causing hospitalization. Acute coronary disease and other structural or functional problems of the heart can lead to acute heart failure. Both acute and chronic HF can be life threatening if they are not adequately treated or if the patient does not respond to treatment.
Types of Heart Failure
The major types of heart failure are:
Because the two ventricles of the heart represent two separate pumping systems, it is possible for one to fail by itself for a short period. Most heart failure begins with failure of the left ventricle and progresses to failure of both ventricles. Typical causes of left-sided heart (ventricular) failure include hypertension, coronary artery disease, and valvular disease involving the mitral or aortic valve. Decreased tissue perfusion from poor cardiac output and pulmonary congestion from increased pressure in the pulmonary vessels indicate left ventricular failure (LVF).
Left-sided heart failure was formerly referred to as congestive heart failure (CHF); however, not all cases of LVF involve fluid accumulation. In the clinical setting, though, the term CHF is still commonly used. Left-sided failure may be acute or chronic and mild to severe. It can be further divided into two subtypes: systolic heart failure and diastolic heart failure.
Systolic heart failure (systolic ventricular dysfunction) results when the heart cannot contract forcefully enough during systole to eject adequate amounts of blood into the circulation. Preload increases with decreased contractility, and afterload increases as a result of increased peripheral resistance (e.g., hypertension) (McCance et al., 2010). The ejection fraction (the percentage of blood ejected from the heart during systole) drops from a normal of 50% to 70% to below 40% with ventricular dilation. As it decreases, tissue perfusion diminishes and blood accumulates in the pulmonary vessels. Manifestations of systolic dysfunction may include symptoms of inadequate tissue perfusion or pulmonary and systemic congestion. Systolic heart failure is often called “forward failure” because cardiac output is decreased and fluid backs up into the pulmonary system. Because these patients are at high risk for sudden cardiac death, patients with an ejection fraction of less than 30% are considered candidates for an implantable cardioverter/defibrillator (ICD; also known as an internal cardioverter/defibrillator) (see Chapter 36).
In contrast, diastolic heart failure (heart failure with preserved left ventricular function) occurs when the left ventricle cannot relax adequately during diastole. Inadequate relaxation or “stiffening” prevents the ventricle from filling with sufficient blood to ensure an adequate cardiac output. Although ejection fraction is more than 40%, the ventricle becomes less compliant over time because more pressure is needed to move the same amount of volume as compared with a healthy heart. Diastolic failure represents about 20% to 40% of all heart failure, primarily in older adults and in women who have chronic hypertension and undetected coronary artery disease. Clinical manifestations and management of diastolic failure are similar to those of systolic dysfunction (McCance et al., 2010).
Right-sided heart (ventricular) failure may be caused by left ventricular failure, right ventricular myocardial infarction (MI), or pulmonary hypertension. In this type of heart failure (HF), the right ventricle cannot empty completely. Increased volume and pressure develop in the venous system, and peripheral edema results.
High-output heart failure can occur when cardiac output remains normal or above normal, unlike left- and right-sided heart failure, which are typically low-output states. High-output failure is caused by increased metabolic needs or hyperkinetic conditions, such as septicemia, high fever, anemia, and hyperthyroidism. This type of heart failure is not as common as other types.
Classification and Staging of Heart Failure
The American College of Cardiology (ACC) and American Heart Association (AHA) have developed evidence-based guidelines for staging and managing heart failure as a chronic, progressive disease. These guidelines do not replace the New York Heart Association (NYHA) functional classification system, which is used to describe symptoms a patient may exhibit (see Table 35-2 in Chapter 35).
The ACC/AHA staging system when compared with the NYHA system categorizes patients as:
A Patients at high risk for developing heart failure (class I NYHA)
C Patients with current or prior symptoms of heart failure (class II or III NYHA)
D Patients with refractory end-stage heart failure (class IV NYHA)
Another method for staging HF is the Killip classification system, which is based on the heart’s hemodynamic ability. Table 40-3 in Chapter 40 outlines this system.
Compensatory Mechanisms
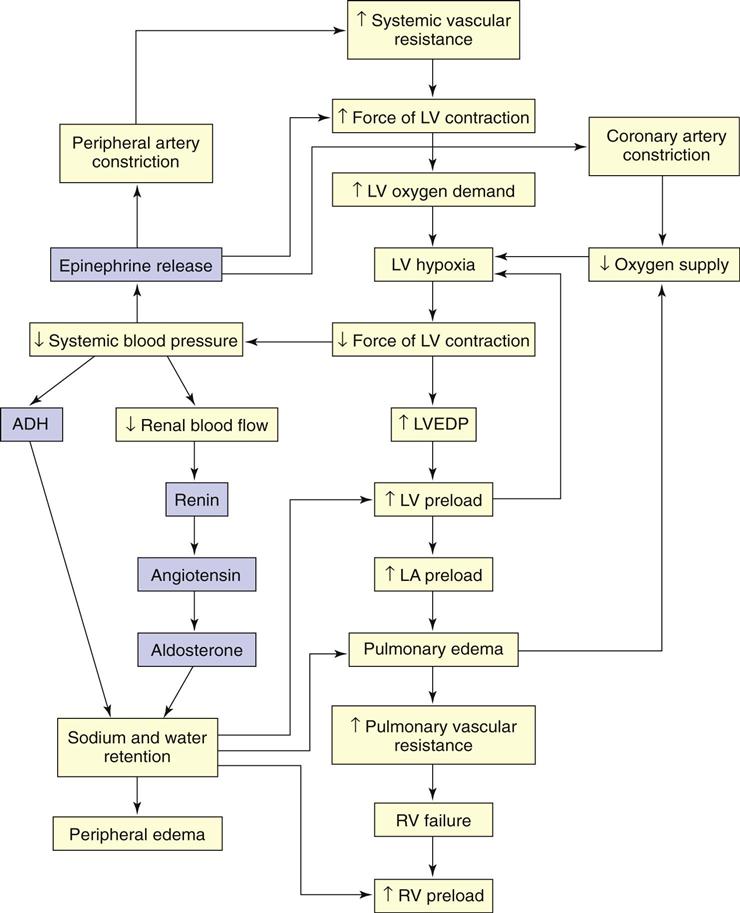
When cardiac output is insufficient to meet the demands of the body, compensatory mechanisms work to improve cardiac output (Fig. 37-1). Although these mechanisms may initially increase cardiac output, they eventually have a damaging effect on pump function. Major compensatory mechanisms include:
Stimulation of the Sympathetic Nervous System.
In heart failure (HF), stimulation of the sympathetic nervous system (i.e., increasing catecholamines) as a result of tissue hypoxia represents the most immediate compensatory mechanism. Stimulation of the adrenergic receptors causes an increase in heart rate (beta adrenergic) and blood pressure from vasoconstriction (alpha adrenergic).
Because cardiac output (CO) is the product of heart rate (HR) and stroke volume (SV), an increase in HR results in an immediate increase in cardiac output. The HR is limited, though, in its ability to compensate for decreased CO. If it becomes too rapid, diastolic filling time is limited and CO may start to decline. An increase in HR also significantly increases oxygen demand by the myocardium. If the heart is poorly perfused because of arteriosclerosis, HF may worsen.
Stroke volume (SV) is also improved by sympathetic stimulation. Sympathetic stimulation increases venous return to the heart, which further stretches the myocardial fibers causing dilation. According to Starling’s law, increased myocardial stretch results in more forceful contraction. More forceful contractions increase SV and CO. After a critical point is reached within the cardiac muscle, further volume and stretch reduce the force of contraction and cardiac output.
Sympathetic stimulation also results in arterial vasoconstriction. Vasoconstriction has the benefit of maintaining blood pressure and improving tissue perfusion in low-output states. However, constriction of the arteries increases afterload, the resistance against which the heart must pump. Afterload is the major determinant of myocardial oxygen requirements. As it increases, the left ventricle requires more energy to eject its contents and SV may decline.
Renin-Angiotensin System Activation.
Reduced blood flow to the kidneys, a common occurrence in low-output states, results in activation of the renin-angiotensin system (RAS). Vasoconstriction becomes more pronounced in response to angiotensin II, and aldosterone secretion causes sodium and water retention. Preload and afterload increase. Angiotensin II contributes to ventricular remodeling resulting in progressive myocyte (myocardial cell) contractile dysfunction over time (McCance et al., 2010).
Other Chemical Responses.
In addition to the sympathetic nervous system and RAS responses, other mechanisms are activated when a patient experiences heart failure (HF). Most of these actions contribute to worsening of the condition.
For example, in those who have had an MI, heart muscle cell injury causes an immune response. Pro-inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukins (IL-1 and IL-6), are released, especially with left-sided HF. These substances contribute to ventricular remodeling.
Natriuretic peptides are neurohormones that work to promote vasodilation and diuresis through sodium loss in the renal tubules. The B-type natriuretic peptide (BNP) is produced and released by the ventricles when the patient has fluid overload as a result of HF. It increases with age and has a greater concentration in women (Jessup et al., 2009). People who are obese have lower BNP levels compared with those who are not (Noveanu et al., 2009).
Low cardiac output (CO) causes decreased cerebral perfusion. As a result, the posterior pituitary gland secretes vasopressin (antidiuretic hormone [ADH]). The hormone causes vasoconstriction and fluid retention, which worsen HF.
Endothelin is secreted by endothelial cells when they are stretched. As the myocardial fibers are stretched in patients with HF, this potent vasoconstrictor is released, which increases peripheral resistance and hypertension. HF worsens as a result of these actions.
Myocardial Hypertrophy.
Myocardial hypertrophy (enlargement of the myocardium), with or without chamber dilation, is another compensatory mechanism. The walls of the heart thicken to provide more muscle mass, which results in more forceful contractions, further increasing cardiac output. Cardiac muscle, however, may hypertrophy more rapidly than collateral circulation can provide adequate blood supply to the muscle. Often a hypertrophied heart is slightly oxygen deprived.
All the compensatory mechanisms contribute to an increase in the consumption of myocardial oxygen. When the demand for oxygen increases and the myocardial reserve has been exhausted, clinical manifestations of HF develop.
Etiology
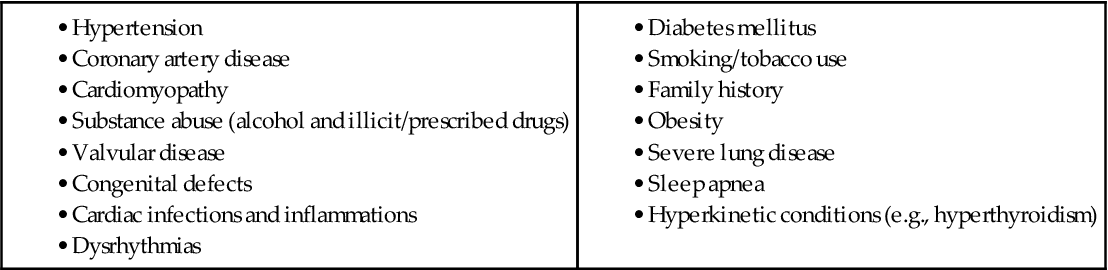
Heart failure (HF) is caused by systemic hypertension in most cases. About a third of patients experiencing myocardial infarction (MI, “heart attack”) also develop HF. The next most common cause is structural heart changes, such as valvular dysfunction, particularly pulmonic or aortic stenosis, which leads to pressure or volume overload on the heart. Common direct causes and risk factors for HF are listed in Table 37-1.
Right-sided HF in the absence of left-sided HF is usually the result of pulmonary problems such as chronic obstructive pulmonary disease (COPD) or pulmonary hypertension. Acute respiratory distress syndrome (ARDS) may also cause right-sided HF. These problems are discussed elsewhere in the cardiac unit.
Incidence/Prevalence
Over five million people in the United States have HF, causing about 875,000 hospitalizations each year. HF is the most common reason for hospital admission for people over 65 years of age. African Americans are affected more often than Euro-Americans, probably because they have more risk factors that can lead to HF. The disease is a major cause of disability and death after MI, often due to nonadherence to the treatment plan and recommended lifestyle changes.
Patient-Centered Collaborative Care
Assessment
History
When obtaining a history, keep in mind the many conditions that can lead to HF. Carefully question the patient about his or her medical history, including hypertension, angina (cardiac pain), MI, rheumatic heart disease, valvular disorders, endocarditis, and pericarditis. Ask about the patient’s perception of his or her activity tolerance, breathing pattern, sleeping pattern, urinary pattern, and fluid volume status, as well as his or her knowledge about HF.
Left-Sided Heart Failure.
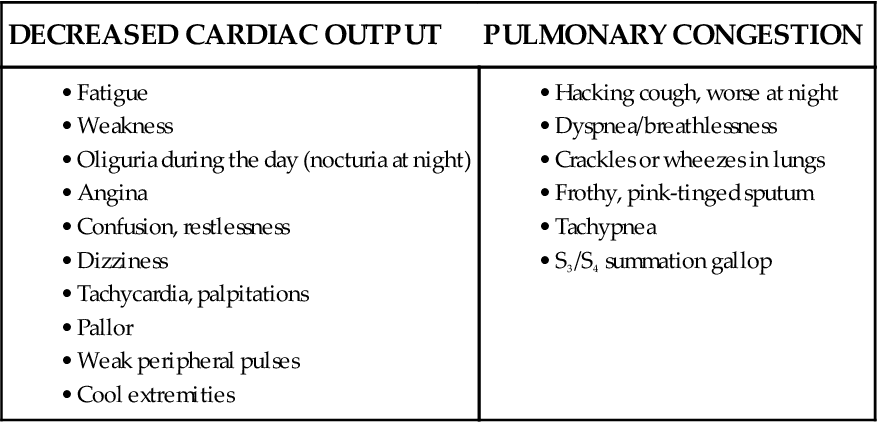
With left ventricular systolic dysfunction, cardiac output (CO) is diminished, leading to impaired tissue perfusion, anaerobic metabolism, and unusual fatigue. Assess activity tolerance by asking whether the patient can perform normal ADLs or climb flights of stairs without fatigue or dyspnea. Many patients with heart failure (HF) experience weakness or fatigue with activity or have a feeling of heaviness in their arms or legs. Ask about their ability to perform simultaneous arm and leg work (e.g., walking while carrying a bag of groceries). Such activity may place an unacceptable demand on the failing heart. Ask the patient to identify his or her most strenuous activity in the past week. Many people unconsciously limit their activities in response to fatigue or dyspnea and may not realize how limited they have become.
Perfusion to the myocardium is often impaired as a result of left ventricular failure, especially with cardiac hypertrophy. The patient may report chest discomfort or may describe palpitations, skipped beats, or a fast heartbeat.
As the amount of blood ejected from the left ventricle diminishes, hydrostatic pressure builds in the pulmonary venous system and results in fluid-filled alveoli and pulmonary congestion, which results in a cough. The patient in early HF describes the cough as irritating, nocturnal (at night), and usually nonproductive. As HF becomes very severe, he or she may begin expectorating frothy, pink-tinged sputum—a sign of life-threatening pulmonary edema.
Dyspnea also results from increasing pulmonary venous pressure and pulmonary congestion. Carefully question about the presence of dyspnea and when and how it developed. The patient may refer to dyspnea as “trouble in catching my breath,” “breathlessness,” or “difficulty in breathing.”
As exertional dyspnea develops (also called dyspnea upon or on exertion [DUE/DOE]), the patient often stops previously tolerated levels of activity because of shortness of breath. Dyspnea at rest in the recumbent (lying flat) position is known as orthopnea. Ask how many pillows are used to sleep or whether the patient sleeps in an upright position in a bed, recliner, or other type of chair.
Patients who describe sudden awakening with a feeling of breathlessness 2 to 5 hours after falling asleep have paroxysmal nocturnal dyspnea (PND). Sitting upright, dangling the feet, or walking usually relieves this condition.
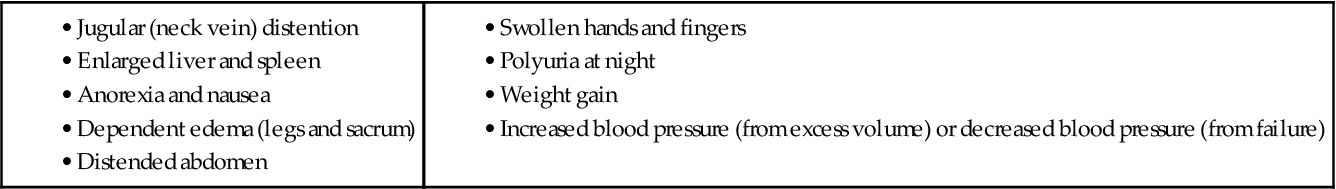
Right-Sided Heart Failure.
Signs of systemic congestion occur as the right ventricle fails, fluid is retained, and pressure builds in the venous system. Edema develops in the lower legs and may progress to the thighs and abdominal wall. Patients may notice that their shoes fit more tightly, or their shoes or socks may leave indentations on their swollen feet. They may have removed their rings because of swelling in their fingers and hands. Ask about weight gain. An adult may retain 4 to 7 liters of fluid (10 to 15 lb [4.5 to 6.8 kg]) before pitting edema occurs.
Reports of nausea and anorexia may be a direct consequence of liver engorgement (congestion) resulting from fluid retention. In advanced heart failure (HF), ascites and an increased abdominal girth may develop from severe liver congestion. Another common finding related to fluid retention is diuresis at rest. At rest, fluid in the peripheral tissue is mobilized and excreted and the patient describes frequent awakening at night to urinate.
Obtain a careful nutritional history, questioning about the use of salt and the types of food consumed. Ask about daily fluid intake. Patients with HF may experience increased thirst and drink excessive fluid (4000 to 5000 mL/day) because of sodium retention.
Physical Assessment/Clinical Manifestations
Manifestations of HF depend on the type of failure, the ventricle involved, and the underlying cause. Impaired tissue perfusion, pulmonary congestion, and edema are associated with left ventricular failure (Chart 37-1). Conversely, systemic venous congestion and peripheral edema are associated with right ventricular failure (Chart 37-2).
Left-Sided Heart Failure.
Left ventricular failure is associated with decreased cardiac output and elevated pulmonary venous pressure. It may appear clinically as:
Decreased blood flow to the major body organs can cause dysfunction, especially renal failure. Nocturia may occur when the patient is at rest.
The pulse may be tachycardic, or it may alternate in strength (pulsus alternans). Take the apical pulse for a full minute, noting any irregularity in heart rhythm. An irregular heart rhythm resulting from premature atrial contractions (PACs), premature ventricular contractions (PVCs), or atrial fibrillation (AF) is common in HF (see Chapter 36). The sudden development of an irregular rhythm may further compromise CO. Carefully monitor the patient’s respiratory rate, rhythm, and character, as well as oxygen saturation. The respiratory rate typically exceeds 20 breaths/min.
Assess whether the patient is oriented to person, place, and time. A short mental status examination may be used if there are concerns about orientation. Objective data are important because in daily conversation many people are skillful at covering up memory losses. Older adults are frequently disoriented or confused when the heart fails due to brain hypoxia (decreased oxygen).
Increased heart size is common with a displacement of the apical impulse to the left. A third heart sound, S3 gallop, is an early diastolic filling sound indicating an increase in left ventricular pressure. This sound is often the first sign of HF. A fourth heart sound (S4) also can occur; it is not a sign of failure but is a reflection of decreased ventricular compliance.
Auscultate for crackles and wheezes of the lungs. Late inspiratory crackles and fine profuse crackles that repeat themselves from breath to breath and do not diminish with coughing indicate HF. Crackles are produced by intra-alveolar fluid and are often noted first in the bases of the lungs and spread upward as the condition worsens. Wheezes indicate a narrowing of the bronchial lumen caused by engorged pulmonary vessels. Identify the precise location of crackles and wheezes and whether the wheezes are heard on inspiration, expiration, or both.
Right-Sided Heart Failure.
Right ventricular failure is associated with increased systemic venous pressures and congestion. On inspection, assess the neck veins for distention and measure abdominal girth. Hepatomegaly (liver engorgement), hepatojugular reflux, and ascites may also be assessed. Abdominal fluid can reach volumes of more than 10 liters.
Assess for dependent edema. In ambulatory patients, edema commonly presents in the ankles and legs. When patients are restricted to bedrest, the sacrum is dependent and fluid accumulates there.
Psychosocial Assessment
Chronic heart failure (HF) is typically a slow, debilitating disease. Anxiety and frustration are common. Symptoms such as dyspnea increase the patient’s anxiety level.
Patients with HF, especially those with advanced disease, are at high risk for depression. It is not certain whether the functional impairments contribute to the depression or depression affects functional ability. Older hospitalized patients may be depressed, particularly those who have been readmitted for an acute episode of HF. Lifestyle changes and quality-of-life issues can also cause depression many months after the initial diagnosis of HF (Thomas et al., 2008).
Assess patients and their families for anxiety and depression. Ask them about their usual methods of coping, as well as any history of depression. If anxiety or depression is present, notify the health care provider for further assessment. Social workers, certified clinical chaplains, or psychologists may administer specific assessment tools to determine the extent of the problem. Some patients need drug therapy and nonpharmacologic modalities, such as cognitive behavior therapy, biofeedback, or relaxation training.
Hope is a major indicator of well-being for patients with HF. Those who are hopeful tend to feel better and are more socially involved. Ask patients about their daily activities and how often they interact with the significant people in their life to help determine patient and family coping strategies.
Laboratory Assessment
Electrolyte imbalance may occur from complications of HF or as side effects of drug therapy, especially diuretic therapy. Regular evaluations of a patient’s serum electrolytes, including sodium, potassium, magnesium, calcium, and chloride, are essential. Any impairment of renal function resulting from inadequate perfusion causes elevated blood urea nitrogen and serum creatinine and decreased creatinine clearance levels. Hemoglobin and hematocrit tests should be performed to identify HF resulting from anemia. If the patient has fluid volume excess, the hematocrit levels may be low as a result of hemodilution.
B-type natriuretic peptide (BNP) is used for diagnosing HF (in particular, diastolic HF) in patients with acute dyspnea. As discussed earlier, it is part of the body’s response to decreased cardiac output from either left or right ventricular dysfunction. An increase in BNP, in conjunction with history and physical, best differentiates between the dyspnea of HF and that associated with lung dysfunction (Wexler et al., 2009). However, patients with renal disease may also have elevated BNP levels (Chen et al., 2010).
Urinalysis may reveal proteinuria and high specific gravity. Microalbuminuria is an early indicator of decreased compliance of the heart and occurs before the BNP rises. It serves as an “early warning detector” that lets the health care provider know that the heart is experiencing early signs of decreased compliance, long before symptoms occur.
Arterial blood gas (ABG) values often reveal hypoxemia (low blood oxygen level) because oxygen does not diffuse easily through fluid-filled alveoli. Respiratory alkalosis may occur because of hyperventilation; respiratory acidosis may occur because of carbon dioxide retention. Metabolic acidosis may indicate an accumulation of lactic acid.
Imaging Assessment
Chest x-rays can be helpful in diagnosing left ventricular failure. Typically the heart is enlarged (cardiomegaly), representing hypertrophy or dilation. Pleural effusions develop less often and generally reflect biventricular failure. Echocardiography is considered the best tool in diagnosing heart failure. Cardiac valvular changes, pericardial effusion, chamber enlargement, and ventricular hypertrophy can be diagnosed using this noninvasive technique. The test can also be used to determine ejection fraction.
Radionuclide studies (thallium imaging or technetium pyrophosphate scanning) can also indicate the presence and cause of HF. Multigated angiographic (MUGA) scans provide information about left ventricular ejection fraction and velocity, which are typically low in patients with HF. These tests are discussed in Chapter 35.
Other Diagnostic Assessment
An electrocardiogram (ECG) is also performed. It may show ventricular hypertrophy, dysrhythmias, and any degree of myocardial ischemia, injury, or infarction. It is not helpful in determining the presence or extent of HF.
Invasive hemodynamic monitoring allows the direct assessment of cardiac function and volume status in acutely ill patients. These measurements can confirm the diagnosis and guide the management of HF. Right atrial pressure may be normal or elevated in left ventricular failure and is elevated in right ventricular failure. Pulmonary artery pressure (PAP) and pulmonary artery wedge pressure (PAWP) are elevated in left-sided HF because volumes and pressures are increased in the left ventricle. (See Chapter 35 for a more detailed description of hemodynamic monitoring.)
Analysis
The priority problems for patients with heart failure (HF) include:
Planning and Implementation
The patient-centered collaborative care that patients with HF need depends on their disease stage and severity of signs and symptoms.
Improving Gas Exchange
Planning: Expected Outcomes.
The expected outcome is that the patient will have an optimal spontaneous breathing pattern that increases oxygenation and maintains a serum carbon dioxide level that is within normal limits.
Interventions.
The purpose of collaborative care is to help promote oxygenation and gas exchange. Ventilation assistance may be needed because the oxygen content of the blood is often decreased in patients who have pulmonary congestion. Monitor or have assistive personnel monitor the patient’s respiratory rate, rhythm, and quality every 1 to 4 hours. Auscultate breath sounds every 4 to 8 hours.
Improving Cardiac Output
Planning: Expected Outcomes.
The expected outcome is that the patient will have increased cardiac output by improving stroke volume (SV) (determined by preload, afterload, and contractility) and heart rate (HR).
Interventions.
Collaborative care begins with nonsurgical interventions, but the patient may need surgery if these are not successful in meeting optimal outcomes.
Nonsurgical Management.
Nonsurgical management relies primarily on a variety of drugs (Table 37-3). If drug therapy is ineffective, other nonsurgical options are available.
TABLE 37-3
COMMONLY USED DRUG CLASSIFICATIONS FOR PATIENTS WITH SYSTOLIC HEART FAILURE
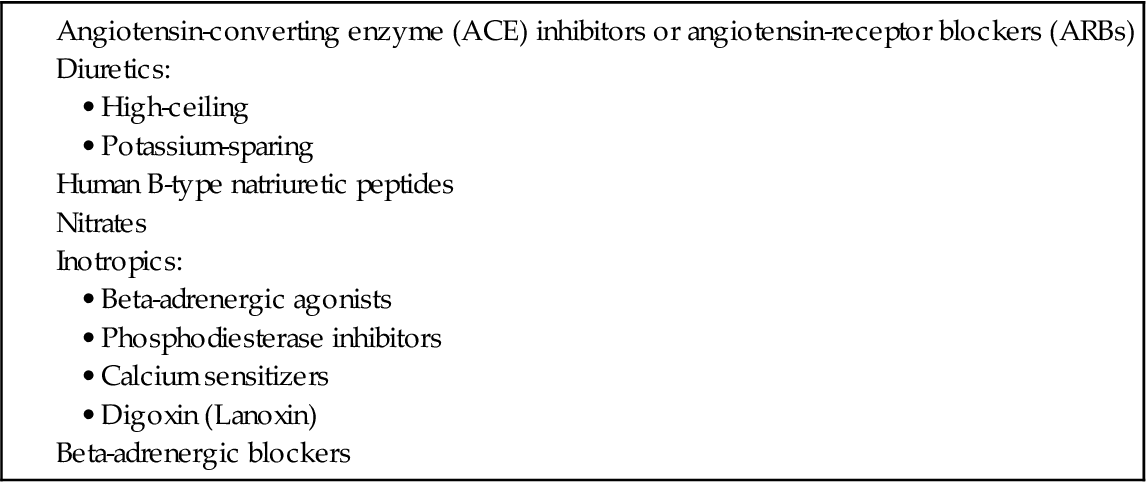
Drugs to improve stroke volume include those that reduce afterload, reduce preload, and improve cardiac muscle contractility. A major role of the nurse is to give medications as prescribed, monitor for their therapeutic and adverse effects, and teach the patient and family about drug therapy. A variety of classes of drugs that reduce afterload and preload are used to manage heart failure (see Table 37-3).
Drugs That Reduce Afterload.
By relaxing the arterioles, arterial vasodilators can reduce the resistance to left ventricular ejection (afterload) and improve CO. These drugs do not cause excessive vasodilation but reverse some of the inappropriate or excessive vasoconstriction common in HF.
Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin-Receptor Blockers (ARBs).
Patients with even mild heart failure (HF) resulting from left ventricular dysfunction are given a trial of ACE inhibitors or ARBs. Both ACE inhibitors (e.g., enalapril [Vasotec] and fosinopril [Monopril]) and ARBs (e.g., valsartan [Diovan], irbesartan [Avapro], and losartan [Cozaar]) improve function and quality of life for patients with HF. ACE inhibitors are the first-line drug of choice, but some health care providers prefer to start the patient on an ARB because ACE inhibitors can cause a nagging, dry cough. For patients with acute HF, the health care provider may prescribe an IV-push ACE inhibitor such as Vasotec IV.
The ACE inhibitors and ARBs suppress the renin-angiotensin system (RAS), which is activated in response to decreased renal blood flow. ACE inhibitors prevent conversion of angiotensin I to angiotensin II, resulting in arterial dilation and increased stroke volume. ARBs block the effect of angiotensin II receptors and thus decrease arterial resistance and arterial dilation. In addition, these drugs block aldosterone, which prevents sodium and water retention, thus decreasing fluid overload. Both ACEIs and ARBs work more effectively for Euro-Americans than for African-American populations. Volume-depleted patients should receive a low starting dose, or the fluid volume should be restored before beginning the prescribed drug. Monitor for hyperkalemia, a potential adverse drug effect in patients who have renal dysfunction.
Assess for orthostatic hypotension, acute confusion, poor peripheral perfusion, and reduced urine output in patients with low systolic blood pressure. Monitor serum potassium and creatinine levels to determine renal dysfunction. Additional nursing implications for selected ACE inhibitor/ARB drugs are described in Chapter 38 on p. 783 in the Drug Therapy section.
Human B-type Natriuretic Peptides.
Human B-type natriuretic peptides (hBNPs) such as nesiritide (Natrecor) are often used to treat acute HF. Endogenous BNP is released in response to decreased CO and causes natriuresis, or loss of sodium in the renal tubules, as well as vasodilation. Natrecor lowers pulmonary capillary wedge pressure (PCWP) and improves renal glomerular filtration. It is given as an IV bolus over 60 seconds followed with a continuous infusion for up to 48 hours.
Interventions That Reduce Preload.
Ventricular fibers contract less forcefully when they are overstretched, such as in a failing heart. Interventions aimed at reducing preload attempt to decrease volume and pressure in the left ventricle, increasing ventricular muscle stretch and contraction. Preload reduction is appropriate for HF accompanied by congestion with total body sodium and water overload.
Nutrition Therapy.
In HF, nutrition therapy is aimed at reducing sodium and water retention to decrease the workload of the heart. The health care provider may restrict sodium intake in an attempt to decrease fluid retention. Many patients need to omit table salt (no added salt) from their diet, thus reducing sodium intake to about 3 g daily.
If salt intake must be reduced further, the patient may need to eliminate all salt in cooking and high-sodium foods (e.g., ham, bacon, pickles), thus reducing sodium intake to 2 g daily. If needed, collaborate with the dietitian to help the patient select foods that meet such a restricted therapeutic diet.
Few patients are placed on severe fluid restrictions. However, patients with excessive aldosterone secretion may experience thirst and drink 3 to 5 liters of fluid each day. As a result, their fluid intake may be limited to a more normal 2 liters daily. Supervise unlicensed assistive personnel (UAP) to ensure that they limit the prescribed intake and accurately record intake and output.
Weigh the patient daily, or delegate this activity to UAP and supervise that it is done. Keep in mind that 1 kg of weight gain or loss equals 1 liter of retained or lost fluid. The same scale should be used every morning before breakfast for the most accurate assessment of weight. Monitor for an expected decrease in weight because excess fluid is excreted from the body.
Drug Therapy.
Common drugs prescribed to reduce preload are diuretics and venous vasodilators. Morphine sulfate is also given for patients in acute heart failure (HF) to reduce anxiety, decrease preload and afterload, slow respirations, and reduce the pain associated with a myocardial infarction (MI).
The health care provider adds diuretics to the regimen when diet and fluid restrictions have not been effective in managing the symptoms of HF. Diuretics are the first-line drug of choice in older adults with HF and fluid overload (Joseph et al., 2009). These drugs enhance the renal excretion of sodium and water by reducing circulating blood volume, decreasing preload, and reducing systemic and pulmonary congestion.
The type and dosage of diuretic prescribed depend on the severity of HF and renal function. High-ceiling (loop) diuretics, such as furosemide (Lasix, Furoside ![]() , Novosemide
, Novosemide ![]() ), torsemide (Demadex), and bumetanide (Bumex), are most effective for treating fluid volume overload.
), torsemide (Demadex), and bumetanide (Bumex), are most effective for treating fluid volume overload.
For those patients with acute HF, Lasix or Bumex can be administered by IV push (IVP). Lasix can be given in doses of 20 to 40 mg IV and increased by 20 mg every 2 hours until the desired diuresis is obtained. The usual IV initial dose for Bumex is 1 to 2 mg once or twice daily, but it is more often given in a continuous infusion of 10 mg over 24 hours.
The practitioner may initially use a thiazide diuretic, such as hydrochlorothiazide (HCTZ) (HydroDIURIL, Urozide ![]() ) and metolazone (Zaroxolyn), for older adults with mild volume overload. Zaroxolyn is a long-acting agent and is therefore often given every second, third, or fourth day, depending on patient need and tolerance.
) and metolazone (Zaroxolyn), for older adults with mild volume overload. Zaroxolyn is a long-acting agent and is therefore often given every second, third, or fourth day, depending on patient need and tolerance.
Unlike loop diuretics, the action of thiazides is self-limiting (i.e., diuresis decreases after edema fluid is lost). Therefore the dehydration that may occur with loop diuretics is not common with these drugs. Patients also prefer thiazides because of the gradual onset of diuresis.
As HF progresses, many patients develop diuretic resistance with refractory edema. The health care provider may choose to manage this problem by prescribing both types of diuretics.
Monitor for and prevent potassium deficiency (hypokalemia) from diuretic therapy. The primary signs of hypokalemia are nonspecific neurologic and muscular symptoms, such as generalized weakness, depressed reflexes, and irregular heart rate. A potassium supplement may be prescribed for some patients. Other practitioners prescribe a potassium-sparing diuretic, such as spironolactone (Aldactone), for patients at risk for dysrhythmias from hypokalemia. Although not as effective as other diuretics, Aldactone helps retain potassium and thus decrease the risk of ventricular dysrhythmias.
Patients being managed with ACE inhibitors or ARBs and diuretics at the same time may not experience hypokalemia. However, if their kidneys are not functioning well, they may develop hyperkalemia (elevated serum potassium level). Review the patient’s serum creatinine level. If the creatinine is greater than 1.8 mg/dL, notify the health care provider before administering supplemental potassium.
The health care provider may prescribe venous vasodilators (e.g., nitrates) for the patient with HF who has persistent dyspnea. Significant constriction of venous and arterial blood vessels occurs to compensate for reduced CO. Constriction reduces the volume of fluid that the vascular bed can hold and increases preload. Venous vasodilators may benefit by:
• Returning venous vasculature to a more normal capacity
• Decreasing the volume of blood returning to the heart
Nitrates may be administered IV, orally, or topically. IV nitrates are used most often for acute HF. These drugs cause primarily venous vasodilation but also a significant amount of arteriolar vasodilation. Monitor the patient’s blood pressure when starting nitrate therapy or increasing the dosage. Patients may initially report headache, but assure them that they will develop a tolerance to this effect and that the headache will cease or diminish. Acetaminophen (Tylenol, Exdol ![]() ) can be given to help relieve discomfort.
) can be given to help relieve discomfort.
Unfortunately, tolerance to the vasodilating effects develops when nitrates are given around-the-clock. To prevent this tolerance, the health care provider may prescribe at least one 12-hour nitrate-free period out of every 24 hours (usually overnight). Nitrates such as isosorbide (Imdur, ISMO) are prescribed to provide nitrate-free periods and reduce the problem of tolerance. Chapter 40 discusses nitrates in more detail.
Drugs That Enhance Contractility.
Contractility of the heart can also be enhanced with drug therapy. Positive inotropic drugs are most commonly used, but vasodilators and beta-adrenergic blockers may also be administered. For chronic HF, low-dose beta blockers are most commonly used. Digoxin (Lanoxin) may be prescribed to improve symptoms, thereby decreasing dyspnea and improving functional activity. This older and long-used drug is not expensive. In some settings, nesiritide (Natrecor) may be administered for end-stage HF, although this drug is very expensive (see discussion of Natrecor for acute HF on p. 751).
Digoxin.
Although not as commonly used today, digoxin (Lanoxin, Novodigoxin ![]() ), a cardiac glycoside, has been demonstrated to provide symptomatic benefits for patients in chronic heart failure (HF) with sinus rhythm and atrial fibrillation. Digoxin (sometimes called “dig”) therapy reduces exacerbations of HF and hospitalizations when added to a regimen of ACE inhibitors or ARBs, beta blockers, and diuretics. However, it may increase mortality due to drug toxicity, especially in older adults.
), a cardiac glycoside, has been demonstrated to provide symptomatic benefits for patients in chronic heart failure (HF) with sinus rhythm and atrial fibrillation. Digoxin (sometimes called “dig”) therapy reduces exacerbations of HF and hospitalizations when added to a regimen of ACE inhibitors or ARBs, beta blockers, and diuretics. However, it may increase mortality due to drug toxicity, especially in older adults.
The potential benefits of digoxin include:
• Slowing of conduction through the atrioventricular node
• Inhibition of sympathetic activity while enhancing parasympathetic activity
Digoxin is erratically absorbed from the GI tract. Many drugs, especially antacids, interfere with its absorption. It is eliminated primarily by renal excretion. Older patients should be maintained on lower doses of the drug than younger patients.
Other Inotropic Drugs.
Patients experiencing acute heart failure (HF) are candidates for IV drugs that increase contractility. For example, beta-adrenergic agonists, such as dobutamine (Dobutrex), are used for short-term treatment of acute episodes of HF. Dobutamine improves cardiac contractility and thus cardiac output and myocardial-systemic perfusion.
A more potent drug used for acute HF, milrinone (Primacor), functions as a vasodilator/inotropic medication with phosphodiesterase activity. Also known as a phosphodiesterase inhibitor, this drug increases cyclic adenosine monophosphate (cAMP), which enhances the entry of calcium into myocardial cells to increase contractile function. Like the beta-adrenergic agonists, Primacor is given IV.
Levosimendan (Simdax) is a calcium-sensitizing medication and a positive inotropic drug. It appears to bind to troponin C in the heart muscle and therefore increases the contraction of the heart. Simdax is used most often in patients who have had or are at high risk for myocardial infarction. Chapter 40 discusses inotropic drugs in more detail.