Chapter 24 Care of Patients with Cancer
Safe and Effective Care Environment
1. Modify the environment to protect patients who have neutropenia or thrombocytopenia.
2. Use personal protective equipment (PPE) when administering IV and oral chemotherapy and when handling excreta of a patient within 48 hours of receiving chemotherapy.
3. Coordinate with the health care team to adjust the environment for protection of the patient having photodynamic therapy.
4. Maintain radiation precautions when caring for a patient receiving radiation therapy using implanted sources.
Health Promotion and Maintenance
5. Teach patients and family members how to avoid injury and infection when blood counts are low.
6. Teach patients and family members skin protection measures for radiation therapy and photodynamic therapy.
7. Teach patients and family members the proper scheduling of antiemetic therapy to ensure prevention or relief of chemotherapy-induced nausea and vomiting.
8. Teach patients and family members about what to expect during therapeutic procedures.
9. Encourage patients and family members to express their concerns about the diagnosis of cancer, its prognosis, and its treatment.
10. Assess the patient’s and family’s use of coping strategies related to cancer, cancer treatment, possible role changes, and possible outcomes of the disease and its treatment.
11. Teach patients strategies to improve body image changes occurring with cancer treatment.
12. Interpret laboratory data and clinical manifestations to determine the presence of cancer treatment side effects and oncologic emergencies.
13. Prioritize nursing interventions for the patient with neutropenia or thrombocytopenia.
14. Prioritize nursing care for the patient receiving radiation therapy.
15. Explain the rationale for hormonal manipulation therapy.
16. Discuss the uses of biological response modifiers and growth factors as supportive therapy in the treatment of cancer.
17. Explain the basis and effects of targeted therapy for cancer.
18. Identify patients at risk for oncologic emergencies.
19. Teach patients and families the manifestations of oncologic emergencies and when to notify the health care provider.
http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Review Questions for the NCLEX® Examination
Cancer is a common problem in North America and other affluent societies. Most people fear cancer and consider a cancer diagnosis to involve suffering and death. In affluent countries, more than 50% of people diagnosed with cancer are cured and thousands of others live 5 years or longer (American Cancer Society, 2011; Canadian Cancer Society, 2010). Regardless of treatment type, cancer always affects a person’s physical and psychological functioning.
Providing care to patients and families experiencing cancer is complex and challenging. This chapter describes the general interventions for cancer and the problems associated with cancer treatment. For specific treatment regimens and patient problems that occur with specific cancer types, consult the chapters in which the cancer is described. Table 24-1 lists common cancer types and the specific locations within this text where the interventions are presented.
TABLE 24-1 TEXT LOCATION OF SPECIFIC CANCER CONTENT
| CANCER TYPE | CHAPTER | pages |
|---|---|---|
| Bladder (urolithial) | 69 | 1512-1515 |
| Brain | 47 | 1031-1036 |
| Breast | 73 | 1591-1608 |
| Cervical | 74 | 1624-1626 |
| Colorectal | 59 | 1245-1254 |
| Esophageal | 57 | 1211-1217 |
| Head and Neck | 31 | 590-600 |
| Leukemia | 42 | 880-889 |
| Lung | 32 | 632-640 |
| Lymphoma | 42 | 892-894 |
| Ovarian | 74 | 1626-1627 |
| Prostate | 75 | 1637-1642 |
| Renal cell carcinoma | 70 | 1532-1533 |
| Skin | 27 | 502-505 |
| Stomach (gastric) | 58 | 1234-1238 |
General Disease-Related Consequences of Cancer
Cancer can develop in any organ or tissue and destroys normal tissue, which decreases function of that tissue or organ. Even when cancers occur in nonvital tissues or organs, they can cause death by metastasizing (spreading) into vital organs and disrupting critical physiologic processes (see Chapter 23). Cancers that are left untreated often cause:
Reduced Immunity and Blood-Producing Functions
When cancer invades the bone marrow, it also causes anemia by decreasing the number of red blood cells (RBCs) and causes thrombocytopenia by decreasing the number of platelets (Fitch et al., 2009). These changes may be caused by the cancer itself or by cancer treatment, especially chemotherapy. The patient feels weak and fatigued and is at risk for bleeding.
Altered Gi Structure and Function
Many patients with cancer have anorexia that often interferes with their ability to meet energy needs. Cachexia (extreme body wasting and malnutrition) develops from an imbalance between food intake and energy use (increased catabolism). This problem may occur even when nutritional intake appears adequate (Granda-Cameron et al., 2010). Changes in taste can result from the cancer or the treatment and reduce appetite.
Motor and Sensory Deficits
The patient with cancer may also have pain, especially chronic pain. Pain does not always accompany cancer, but it can be a major problem for those with terminal cancer. Chapter 5 provides an in-depth discussion of the causes and management of cancer pain. The Concept Map on p. 42 presents nursing care issues related to chronic cancer pain.
Reduced Oxygenation
Health Promotion and Maintenance
A. “With lung cancer, you are more likely to develop pneumonia and could pass this on to other people who are already ill.”
B. “When lung cancer is in the bones, it becomes a bone marrow malignancy, which stops producing immune system cells.”
C. “The large amount of mucus produced by the cancer cells is a good breeding ground for bacteria and other microorganisms.”
D. “When lung cancer is in the bones, it can prevent production of immune system cells, making you less resistant to infection.”
Cancer Management
Surgery
Patient-Centered Collaborative Care
The physical care needs of the patient having surgery for cancer are similar to those related to surgery for other reasons (see Chapters 16, 17, and 18). For cancer surgery, two additional priority care needs are psychosocial support and assisting the patient to achieve or maintain maximum function.
Coordinate with the health care team to support the patient. Encourage the patient and family to express their concerns. Help the patient accept changes in appearance or function by encouraging him or her to look at the surgical site, touch it, and participate in dressing changes. Provide information about support groups such as those sponsored by the American Cancer Society (www.cancer.org) or Canadian Cancer Society (www.cancer.ca). Some cancer organizations have support groups for patients and separate support groups for patients’ spouses and children. Discuss having a person who has coped with the same issues come for a visit. Such visits can be valuable in showing the patient that many aspects of life can be the same after cancer treatment. If the patient is open to this type of support, arrange for a visit. For patients who have persistent sadness or depression as a result of appearance changes from cancer surgery, a referral to a mental health counselor and drug therapy may be needed (Weaver, 2009).
Radiation Therapy
Overview
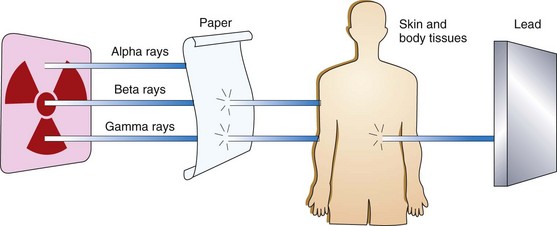
Three different types of energy, or rays, are produced by radioactive elements: gamma rays, alpha particles, and beta particles. These energies vary in their ability to penetrate tissues and damage cells (Fig. 24-1). Gamma rays are used most commonly for radiation therapy because of their ability to deeply penetrate tissues (Camporeale, 2008). Beta particles are weaker and must be placed within or very close to the cancer cells for cancer therapy (see discussion of brachytherapy on pp. 412-413). Alpha particles are not used as cancer therapy.
The amount of radiation delivered to a tissue is called the exposure; the amount of radiation absorbed by the tissue is called the radiation dose. The dose is always less than the exposure because some energy is lost as scatter on the way to the tissue. The three factors determining the absorbed dose are the intensity of exposure, the duration of exposure, and the closeness of the radiation source to the cells (Camporeale, 2008).
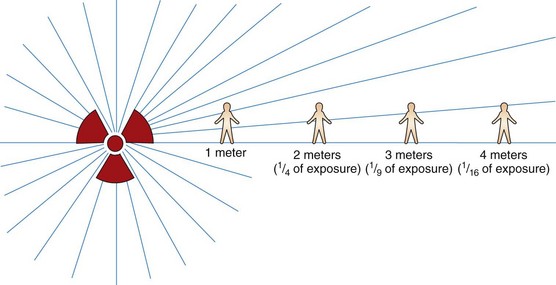
The intensity of the radiation decreases with the distance from the radiation source (Fig. 24-2). This factor is known as the inverse square law. For example, the radiation dose received at a distance of 2 meters from the radiation source is only one fourth of the dose received at a distance of 1 meter from the radiation source; the dose of radiation received at 3 meters is only one ninth of the dose received at 1 meter.
Radiation Delivery Methods and Devices
Another newer method of teletherapy is stereotactic body radiotherapy (SBRT). This method uses three-dimensional tumor imaging to identify the exact tumor location, which allows more precise delivery of higher radiation doses and spares more of the surrounding tissue. Usually, the total dosage is delivered in one to five separate treatment sessions. Currently, this approach is most commonly used for small, localized lung cancers (Smink & Schneider, 2008).
Regardless of the teletherapy delivery method, the exact location of the tumor is determined for therapy accuracy. Once the pattern of radiation delivery is determined, the patient must always be in exactly the same position for all treatments (Camporeale, 2008). Ensure that the patient can get into and maintain this position. Position-fixing devices and markings, either on the patient’s body or on the devices, ensure the proper position each day of treatment. The markings may be small permanent “tattoos,” ink outlines on the skin, or a marked covering laid over the skin during positioning. Position-fixing devices include customized external vacuum-type body molds, foam-based body molds, and fiberglass splints (Smink & Schneider, 2008).
Brachytherapy uses radioactive isotopes either in solid form or within body fluids. Isotopes can be delivered to the tumor tissues in several ways. With all types of brachytherapy, the radiation source is within the patient. Therefore the patient emits radiation for a period of time and is a hazard to others (Waring & Gosselin, 2010). When the isotopes used are unsealed and suspended in a fluid, they are given by the oral or IV routes or instilled within body cavities. An example of brachytherapy with soluble isotopes is the ingestion or injection of the radionuclide iodine-131 (131I) (an iodine base with a half-life of 8.05 days) to treat some thyroid cancers. The iodine concentrates in the thyroid gland and destroys the thyroid cancer cells. When the isotopes are unsealed, they enter body fluids and eventually are eliminated in waste products, which are radioactive and should not be directly touched by other people. After the isotope is completely eliminated from the body, neither the patient nor the body wastes are radioactive.
Traditional implants deliver “low-dose rates” (LDRs) of radiation continuously and patients are hospitalized for several days. “High-dose rate” (HDR) implant radiation is another delivery type. The patient comes into the radiation therapy department several times a week, and a stronger radiation implant is placed for only an hour or so each time. The patient goes home between treatments and is radioactive only when the implant is in place (Waring & Gosselin, 2010). Chart 24-1 lists the best practices for care of the patient with sealed implant radiation sources and for the safety of the personnel providing the care.
Chart 24-1 Best Practice for Patient Safety & Quality Care
Care of the Patient with Sealed Implants of Radioactive Sources
• Assign the patient to a private room with a private bath.
• Place a “Caution: Radioactive Material” sign on the door of the patient’s room.
• If portable lead shields are used, place them between the patient and the door.
• Keep the door to the patient’s room closed as much as possible.
• Wear a dosimeter film badge at all times while caring for patients with radioactive implants. The badge offers no protection but measures a person’s exposure to radiation. Each person caring for the patient should have a separate dosimeter to calculate his or her specific radiation exposure.
• Wear a lead apron while providing care. Always keep the front of the apron facing the source of radiation (do not turn your back toward the patient).
• Pregnant nurses should not care for these patients; do not allow pregnant women or children younger than 16 years to visit.
• Limit each visitor to one-half hour per day. Be sure visitors stay at least 6 feet from the source.
• Never touch the radioactive source with bare hands. In the rare instance that it is dislodged, use a long-handled forceps to retrieve it. Deposit the radioactive source in the lead container kept in the patient’s room.
• Save all dressings and bed linens in the patient’s room until after the radioactive source is removed. After the source is removed, dispose of dressings and linens in the usual manner. Other equipment can be removed from the room at any time without special precautions and does not pose a hazard to other people.
Side Effects of Radiation Therapy
The immediate and long-term side effects of all types of radiation are limited to the tissues exposed to the radiation. Therefore the side effects vary according to the site (Ruppert, 2011). Skin changes and hair loss are local but are often permanent depending on the total absorbed dose.
Altered taste sensations and fatigue are two common systemic side effects noted by patients receiving teletherapy, regardless of the radiation site. Taste changes are thought to be caused by metabolites released from dead and dying cells. Many patients develop an aversion to the taste of red meats. Fatigue may be related to the increased energy demands needed to repair damaged cells. Regardless of the cause, radiation-induced fatigue can be debilitating and may last for months (Kuchinski et al., 2009).
Patient-Centered Collaborative Care
Skin in the path of radiation becomes very dry and may break down. Teaching patients about skin care needs during radiation therapy is a priority nursing intervention. Chart 24-2 lists skin care and other precautions needed with external radiation therapy. Instruct the patient to not remove any temporary ink markings when cleaning the skin until the entire course of radiation therapy is completed. At one time, patients were told to avoid using lotions or other skin care products within 4 hours of the radiation therapy; however, this practice is not evidence-based and is now controversial (Bieck, Phillips, & Steele-Moses, 2010). Teach patients to follow the radiation-oncology department’s policy regarding the use and timing of skin care products. Use of skin care products designed to manage or protect the skin from radiation damage does reduce the degree of skin problems that develop during a full course of radiation therapy (Gosselin et al., 2010) (see the Evidence-Based Practice box on p. 414).
Chart 24-2 Patient and Family Education
Preparing for Self-Management: Skin Protection During Radiation Therapy
• Wash the irradiated area gently each day with either water or a mild soap and water as prescribed by your radiologist.
• Use your hand rather than a washcloth to be gentler.
• Rinse soap thoroughly from your skin.
• If ink or dye markings are present to identify exactly where the beam of radiation is to be focused, take care not to remove them.
• Dry the irradiated area with patting motions rather than rubbing motions; use a clean, soft towel or cloth.
• Use only powders, ointments, lotions, or creams on your skin at the radiation site that are prescribed by the radiation oncology department.
• Wear soft clothing over the skin at the radiation site.
• Avoid wearing belts, buckles, straps, or any type of clothing that binds or rubs the skin at the radiation site.
• Avoid exposure of the irradiated area to the sun.
Do Skin Care Products Reduce Radiation Skin Effects?
Action Alert
The normal tissues most sensitive to external radiation are bone marrow cells, skin, mucous membranes, hair follicles, and germ cells (ova and sperm). When possible, these tissues are shielded from radiation during therapy. At times, they are in the radiation path and cannot be protected from exposure. Some changes caused by radiation are permanent. The long-term problems vary with the location and dose of radiation received. For example, radiation to the throat and upper chest can cause difficulty in swallowing. Combined with taste alteration, this problem can lead to reduced nutrition. A registered dietitian is part of the radiation oncology team (Gosselin et al., 2008). Head and neck radiation may damage the salivary glands and cause dry mouth (xerostomia), which increases the patient’s lifelong risk for tooth decay. Bone exposed to radiation therapy is less dense and breaks more easily. Teach about the symptoms that might be expected from the location and dose of radiation (see Table 24-1 for the location of this information for different cancer types).
Health Promotion and Maintenance
Chemotherapy
Overview
As described in Chapter 23, cancer cells can separate from the original tumor, spread to new areas, and establish new cancers at distant sites (metastasize). Patients with metastatic cancer will die unless treatment eliminates the metastatic cancer cells along with the original cancer cells. Chemotherapy is useful in treating cancer because its effects are systemic, providing the opportunity to kill metastatic cancer cells that may have escaped local treatment. Chemotherapy used along with surgery or radiation is termed adjuvant therapy.
Drugs used for chemotherapy usually are given systemically and exert their cell-damaging (cytotoxic) effects against healthy cells as well as cancer cells. The normal cells most affected by chemotherapy are those that divide rapidly, including skin, hair, intestinal tissues, spermatocytes, and blood-forming cells. These drugs are classified by the specific types of action they exert in the cancer cell. Table 24-2 lists categories, drugs, and their potential to induce nausea and vomiting (emetogenic) or to damage surrounding tissue.
TABLE 24-2 CATEGORIES OF CHEMOTHERAPEUTIC DRUGS
| DRUG | EMETOGENIC POTENTIAL | TISSUE DAMAGE POTENTIAL |
|---|---|---|
| Antimetabolites | ||
| Azacitidine (Vidaza) | High | Irritant |
| Capecitabine (Xeloda) | Low | N/A (oral drug) |
| Cladribine (Leustatin) | Moderate | Bruising |
| Clofarabine (Clofar) | Low—Moderate | Irritant |
| Cytarabine (Cytosar, ara-C) | Moderate | Irritant |
| Decitabine (Dacogen) | Moderate | Irritant |
| Floxuridine (FUDR) | High | Bruising |
| 5-Fluorouracil (Adrucil, Efudex, Fluoroplex) | Moderate | Irritant |
| Fludarabine (Fludara, FLAMP) | Low | Bruising |
| Gemcitabine (Gemzar) | Moderate | Bruising |
| 6-Mercaptopurine (Purinethol) | Low | N/A (oral drug) |
| Methotrexate (MTX, Mexate) | Low—Moderate | Bruising |
| Nelarabine (Arranon) | Moderate | Irritant |
| Pemetrexed (Alimta) | Moderate—High | Bruising |
| Pentostatin (Nipent) | Low—Moderate | Irritant |
| 6-Thioguanine (Tabloid) | Moderate—High | N/A (oral drug) |
| Antitumor Antibiotics | ||
| Bleomycin (Blenoxane) | Moderate | Irritant |
| Dactinomycin (Cosmegen) | High | Vesicant |
| Daunorubicin (Cerubidine, Daunomycin) | High | Vesicant |
| Doxorubicin (Adriamycin, Rubex) | High | Vesicant |
| Doxorubicin liposomal (Doxil) | High | Vesicant |
| Epirubicin (Ellence) | High | Vesicant |
| Idarubicin (Idamycin) | Moderate—High | Vesicant |
| Mitomycin C (Mutamycin) | Moderate | Vesicant |
| Mitoxantrone (Novantrone) | Low | Irritant |
| Valrubicin (Valstar) | N/A | N/A (intravesicular drug) |
| Antimitotics | ||
| Cabazitaxel (Jevtana) | Moderate | Irritant |
| Docetaxel (Taxotere) | Moderate | Bruising |
| Etoposide (VP-16, VePesid) | Low | Irritant |
| Eribulin mesylate (Halaven) | Low—Moderate | Irritant |
| Paclitaxel (Taxol) | Moderate | Irritant & potential vesicant |
| Teniposide (Vumon) | Moderate | Vesicant |
| Vinblastine (Velban, Velbe, Velsar) | Low—Moderate | Vesicant |
| Vincristine (Oncovin) | Low | Vesicant |
| Vinorelbine (Navelbine) | Moderate | Vesicant |
| Alkylating Agents | ||
| Altretamine (Hexalen) | Moderate | N/A (oral drug) |
| Bendamustine (Treanda) | Moderate | Vesicant |
| Busulfan (Busulfex) | High | Bruising |
| Carboplatin (Paraplatin) | High | Irritant |
| Carmustine (BiCNU; Gliadel) | High | Irritant |
| Chlorambucil (Leukeran) | High | N/A (oral drug) |
| Cisplatin (Platinol) | High | Irritant |
| Cyclophosphamide (Cytoxan, Procytox) | High | Bruising |
| Dacarbazine (DTIC) | Moderate—High | Irritant |
| Estramustine (Emcyt, Estracyte) | Moderate | Vesicant |
| Ifosfamide (Ifex) | High | Bruising |
| Lomustine (CCNU, CeeNU) | High | N/A (oral drug) |
| Mechlorethamine (Mustargen) | High | Vesicant |
| Melphalan (Alkeran) (available in oral or IV form) | High | Vesicant (IV drug) |
| Oxaliplatin (Eloxatin) | Moderate—High | Vesicant |
| Streptozocin (Zanosar) | High | Bruising |
| Temozolomide (Temodar) | Moderate—High | N/A (oral drug) |
| Thiotepa (Thioplex) | Low | Bruising |
| Topoisomerase Inhibitors | ||
| Irinotecan (Camptosar) | Moderate | Irritant |
| Topotecan (Hycamtin) | Moderate | Irritant |
| Miscellaneous Agents | ||
| Arsenic trioxide (Trisenox) | Moderate | Not known |
| Asparaginase (Elspar) | Low | Vesicant |
| Hydroxyurea (Droxia, Hydrea) | Low | N/A (oral drug) |
| Ixabepilone (Ixempra) | Low—Moderate | Irritant |
| Pegaspargase (Oncaspar) | Low | Bruising |
| Procarbazine (Matulane, Natulan | High | N/A (oral drug) |
| Vorinostat (Zolinza) | Moderate | N/A (oral drug) |
Data from www.fda.gov/cder/index.html; and www.mdconsult.com/php/82925233-2/homepage.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree