Linda LaCharity
Care of Patients with Acute Kidney Injury and Chronic Kidney Disease
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
13 Discuss interventions to prevent AKI.
15 Coordinate nursing care for the patient with severe CKD or end-stage kidney disease (ESKD).
16 Plan prevention strategies for the complications of PD.
17 Coordinate nursing care for the patient during the first 24 hours after kidney transplantation.

http://evolve.elsevier.com/Iggy/
Animation: Renal and Urinary Disorders
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Concept Map: End-Stage Kidney Disease
Key Points
Review Questions for the NCLEX® Examination
Severe kidney disease leading to renal failure is common in North America. Acute kidney injury (AKI) is most common in the acute care setting, and chronic kidney disease (CKD), which may take years to develop, is more common in the community. Both types of kidney dysfunction cause problems by interfering with urinary elimination and disrupting homeostasis of fluid volume, blood pressure, electrolytes, wastes, and acid-base balance (see Fig. 70-1 in Chapter 70). These problems can reduce general function, shorten life, and decrease quality of life. Diabetes, hypertension, and cardiovascular disease are much more common in people with CKD, and their incidence increases as the stage of CKD worsens (U.S. Renal Data Systems [USRDS], 2010). Between 2003 and 2006, the number of patients with stage 3 CKD rose by 1.8% (USRDS, 2010). Thus overall, the incidence has increased only slightly and is most likely related to the use of kidney protective therapy with antihypertensive drugs such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blocker (ARB) drugs, as well as the benefits associated with use of beta-blocker drugs in the treatment of heart failure. The incidence is expected to continue to rise as a result of the aging population and the huge increase in the incidence of type 2 diabetes. In the United States, about half a million people with end-stage-kidney disease (ESKD) are treated yearly with dialysis or kidney transplantation (Okusa et al., 2009). Kidney dysfunction has many causes. CKD is most commonly caused by hypertension (HTN) and diabetes.
As described in Chapter 68, kidney functions include excretion of waste, water and salt balance, acid-base balance, and hormone secretion. When kidney function declines gradually, as occurs most often with CKD (also known as chronic renal failure [CRF]), 90% to 95% of the nephrons must be destroyed before kidney dysfunction is obvious. The patient may have many years of reduced kidney function (renal insufficiency) before the uremia of ESKD develops. During this time of decreased kidney function, the patient is at increased risk for acute kidney injury because of the stress on remaining nephrons.
When kidney decline is sudden, the functioning nephrons are overworked and kidney dysfunction may develop with the loss of only 50% of functioning nephrons. Acute kidney injury and chronic kidney disease are compared in Table 71-1. Acute kidney injury affects many body systems. Chronic kidney disease affects every body system. The problems that occur with loss of kidney function are related to fluid overload, electrolyte and acid-base abnormalities, buildup of nitrogen-based wastes, and loss of kidney hormone function.
TABLE 71-1
CHARACTERISTICS OF ACUTE KIDNEY INJURY AND CHRONIC KIDNEY DISEASE
| CHARACTERISTIC | ACUTE KIDNEY INJURY | CHRONIC KIDNEY DISEASE |
| Onset | Sudden (hours to days) | Gradual (months to years) |
| % of nephron involvement | ≈50% | 90%-95% |
| Duration | 2-4 wks; less than 3 months | Permanent |
| Prognosis | Good for return of kidney function with supportive care; high mortality in some situations | Fatal without a renal replacement therapy such as dialysis or transplantation |
When kidney function declines to the point that the kidneys can no longer maintain homeostasis by urine elimination, renal replacement therapy is needed to prevent death.
Acute Kidney Injury
Pathophysiology
Acute kidney injury (AKI), which used to be known as acute renal failure (ARF) is a rapid decrease in kidney function, leading to the collection of metabolic wastes in the body. AKI can result from conditions that reduce blood flow to the kidneys (prerenal acute kidney injury); damage to the glomeruli, interstitial tissue, or tubules (intrarenal/intrinsic acute kidney injury); or obstruction of urine flow (postrenal acute kidney injury). When AKI occurs in patients who already have reduced kidney function, it may lead to end-stage kidney disease (ESKD) or it may resolve to nearly the pre-AKI level of kidney function. Many factors contribute to kidney insults in AKI, but the acute syndrome may be reversible, especially with prompt intervention.
The pathologic process of AKI is related to the cause of the sudden decrease in kidney function and to the affected kidney site(s). Reduced blood flow (poor perfusion), toxins, tubular ischemia, infections, and obstruction have different effects on the kidney and its function. Any of these processes can reduce glomerular filtration rate (GFR), damage nephron cells, and obstruct urine flow in the kidney tubules.
With shock or other problems causing an acute reduction in blood flow to the kidney (hypoperfusion), the kidney compensates by constricting renal blood vessels, activating the renin-angiotensin-aldosterone pathway, and releasing antidiuretic hormone (ADH). These responses increase blood volume and improve kidney perfusion. However, these same responses reduce urine volume, resulting in oliguria (urine output less than 400 mL/day) and azotemia (the retention and buildup of nitrogenous wastes in the blood). Nephron cell injury is more likely to occur from the lack of oxygen (ischemia) related to reduced blood flow (McCance et al., 2010). Toxins can cause blood vessel constriction in the kidney, leading to reduced kidney blood flow and kidney ischemia.
Kidney tissue inflammation caused by infection, drugs, or cancer results in immune-mediated changes in kidney tissue. With extensive tubular damage, tubular cells slough and combine with other formed elements (e.g., red blood cell [RBC] casts), which then obstruct tubular lumens and prevent urine outflow. Obstruction anywhere within the urinary tract may result in reduced urine formation and full or partial obstruction to urine outflow.
When pressure in the kidney tubules (intrarenal pressure) exceeds glomerular pressure, glomerular filtration stops. This problem allows nitrogen-based wastes to collect in the blood, increasing the blood urea nitrogen (BUN) and serum creatinine levels. When the BUN rises faster than the serum creatinine level, the cause is usually related to protein breakdown or dehydration. When both the BUN and the creatinine levels rise and the ratio between the two remains constant, this indicates kidney dysfunction.
Classification of Acute Kidney Injury Risk
The recent change in terminology from acute renal failure to acute kidney injury resulted in the development of standardized criteria to recognize the changes associated with the problem earlier, when interventions are more likely to reverse the process and prevent permanent kidney damage (Dirkes, 2011; Martin, 2010). This change was aimed at preventing kidney damage both in people who had healthy kidneys before a specific precipitating event and in those who already had some degree of chronic kidney disease (CKD). (An acute kidney injury occurring in a person with CKD can greatly increase the rate of progression to end-stage kidney disease.)
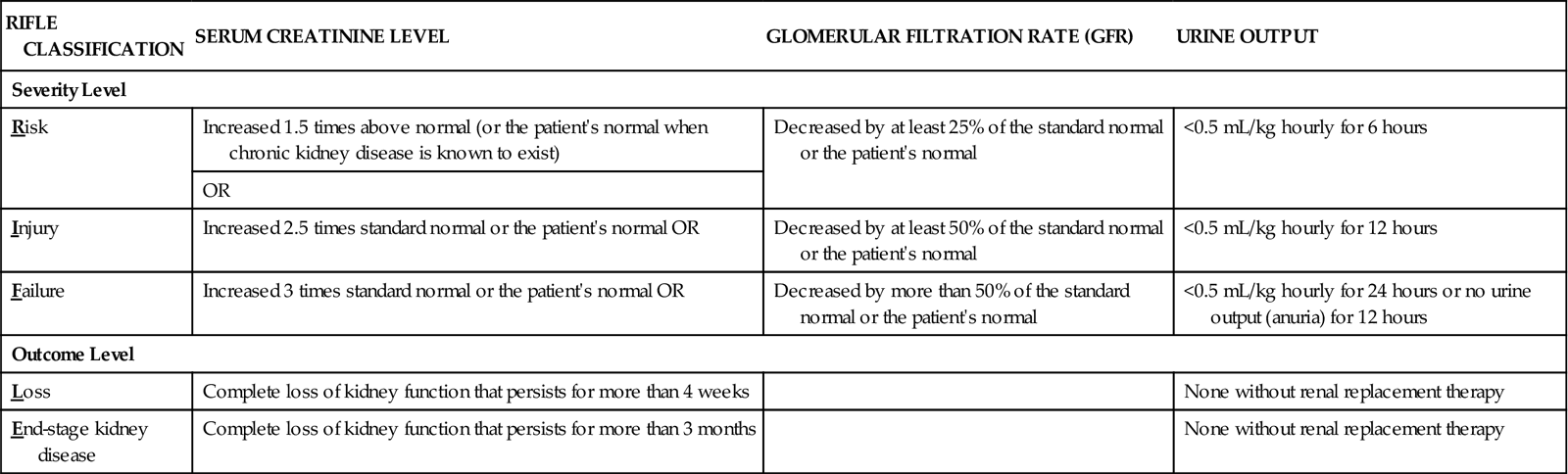
Table 71-2 lists the criteria for classification of acute kidney injury based on the five RIFLE categories of changes in glomerular filtration, serum creatinine levels, and reduction of hourly urine output. (RIFLE stands for Risk, Injury, Failure, Loss, and End-stage kidney disease.) These criteria apply to patients with no known kidney problems and to patients with CKD. The first three criteria (R,I,F), are injury severity levels. Identification of AKI at these levels and proper management can prevent progression to more serious injury that may not be reversible. The last two criteria (L,E) indicate serious injury that requires renal replacement therapy at least on a temporary basis. Even at level L, some people have return of kidney function. It is the responsibility of all health care professionals to be alert to the possibility of AKI, implement prevention strategies, and identify the need to implement appropriate interventions to prevent permanent impairment of kidney function.
TABLE 71-2
THE RIFLE CLASSIFICATION SYSTEM FOR DIAGNOSIS AND SEVERITY OF ACUTE KIDNEY INJURY
| RIFLE CLASSIFICATION | SERUM CREATININE LEVEL | GLOMERULAR FILTRATION RATE (GFR) | URINE OUTPUT |
| Severity Level | |||
| Risk | Increased 1.5 times above normal (or the patient’s normal when chronic kidney disease is known to exist) | Decreased by at least 25% of the standard normal or the patient’s normal | <0.5 mL/kg hourly for 6 hours |
| OR | |||
| Injury | Increased 2.5 times standard normal or the patient’s normal OR | Decreased by at least 50% of the standard normal or the patient’s normal | <0.5 mL/kg hourly for 12 hours |
| Failure | Increased 3 times standard normal or the patient’s normal OR | Decreased by more than 50% of the standard normal or the patient’s normal | <0.5 mL/kg hourly for 24 hours or no urine output (anuria) for 12 hours |
| Outcome Level | |||
| Loss | Complete loss of kidney function that persists for more than 4 weeks | None without renal replacement therapy | |
| End-stage kidney disease | Complete loss of kidney function that persists for more than 3 months | None without renal replacement therapy | |
Types of Acute Kidney Injury
The types of AKI are described by their causes. These include prerenal azotemia, intrarenal (intrinsic) AKI, and postrenal azotemia. Table 71-3 lists causes of AKI.
TABLE 71-3
CAUSES OF THE THREE TYPES OF ACUTE KIDNEY INJURY

Prerenal azotemia is kidney injury caused by poor blood flow to the kidneys. The most common problems leading to AKI are hypovolemic shock and heart failure (Ali & Gray-Vickrey, 2011). Early AKI (levels R and I) often can be reversed by correcting blood volume, increasing blood pressure, and improving cardiac output. When the reduced blood flow is prolonged, the kidneys are severely damaged and intrarenal kidney injury results.
The term intrarenal AKI is often referred to as oliguric AKI in the clinical setting. Other terms include acute tubular necrosis (ATN) and lower nephron nephrosis. Infections (bacteria, viral, fungal), drugs (especially aminoglycoside antibiotics and NSAIDs), and invading tumors (e.g., lymphomas or leukemias) can cause acute interstitial nephritis. Other causes of intrarenal AKI include inflammation of the glomeruli (glomerulonephritis) or of the small vessels of the kidneys (vasculitis) or an obstruction of blood flow to the kidneys.
Postrenal azotemia develops from obstruction to the outflow of formed urine anywhere within the kidney or urinary tract.
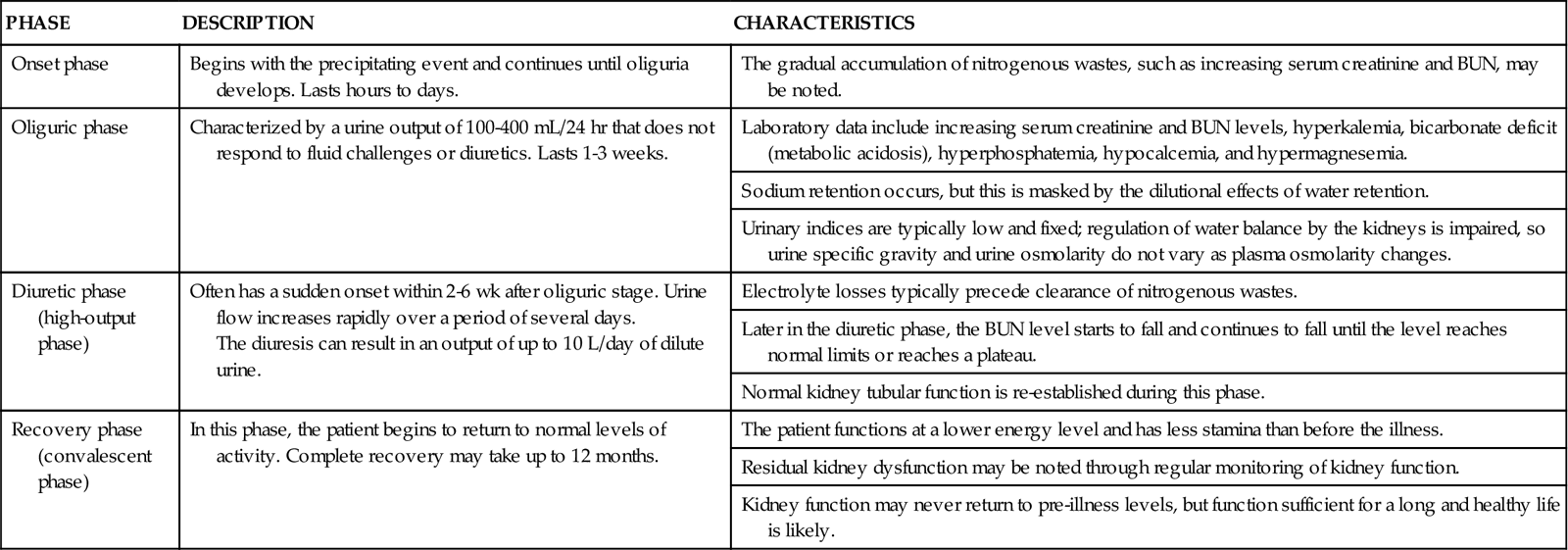
When kidney function declines, the oliguric phases of AKI begin (Table 71-4). Some patients have a nonoliguric form of AKI in which urine output remains near normal but serum creatinine levels rise. Ideally, interventions to restore circulating volume, improve cardiac output, and increase blood pressure prevent progression to a more severe level of kidney injury.
TABLE 71-4
THE PHASES OF OLIGURIC ACUTE KIDNEY INJURY
| PHASE | DESCRIPTION | CHARACTERISTICS |
| Onset phase | Begins with the precipitating event and continues until oliguria develops. Lasts hours to days. | The gradual accumulation of nitrogenous wastes, such as increasing serum creatinine and BUN, may be noted. |
| Oliguric phase | Characterized by a urine output of 100-400 mL/24 hr that does not respond to fluid challenges or diuretics. Lasts 1-3 weeks. | Laboratory data include increasing serum creatinine and BUN levels, hyperkalemia, bicarbonate deficit (metabolic acidosis), hyperphosphatemia, hypocalcemia, and hypermagnesemia. |
| Sodium retention occurs, but this is masked by the dilutional effects of water retention. | ||
| Urinary indices are typically low and fixed; regulation of water balance by the kidneys is impaired, so urine specific gravity and urine osmolarity do not vary as plasma osmolarity changes. | ||
| Diuretic phase (high-output phase) | Often has a sudden onset within 2-6 wk after oliguric stage. Urine flow increases rapidly over a period of several days. The diuresis can result in an output of up to 10 L/day of dilute urine. | Electrolyte losses typically precede clearance of nitrogenous wastes. |
| Later in the diuretic phase, the BUN level starts to fall and continues to fall until the level reaches normal limits or reaches a plateau. | ||
| Normal kidney tubular function is re-established during this phase. | ||
| Recovery phase (convalescent phase) | In this phase, the patient begins to return to normal levels of activity. Complete recovery may take up to 12 months. | The patient functions at a lower energy level and has less stamina than before the illness. |
| Residual kidney dysfunction may be noted through regular monitoring of kidney function. | ||
| Kidney function may never return to pre-illness levels, but function sufficient for a long and healthy life is likely. |
Etiology
Many types of problems can reduce kidney function. Severe hypotension from shock or dehydration reduces kidney blood flow and can lead to prerenal AKI. Cardiac disease or heart failure also can reduce kidney blood flow. The patient may be oliguric or even anuric (less than 100 mL/24 hr) if kidney blood flow reduction is severe. Conditions causing AKI are listed in Table 71-3.
Incidence/Prevalence
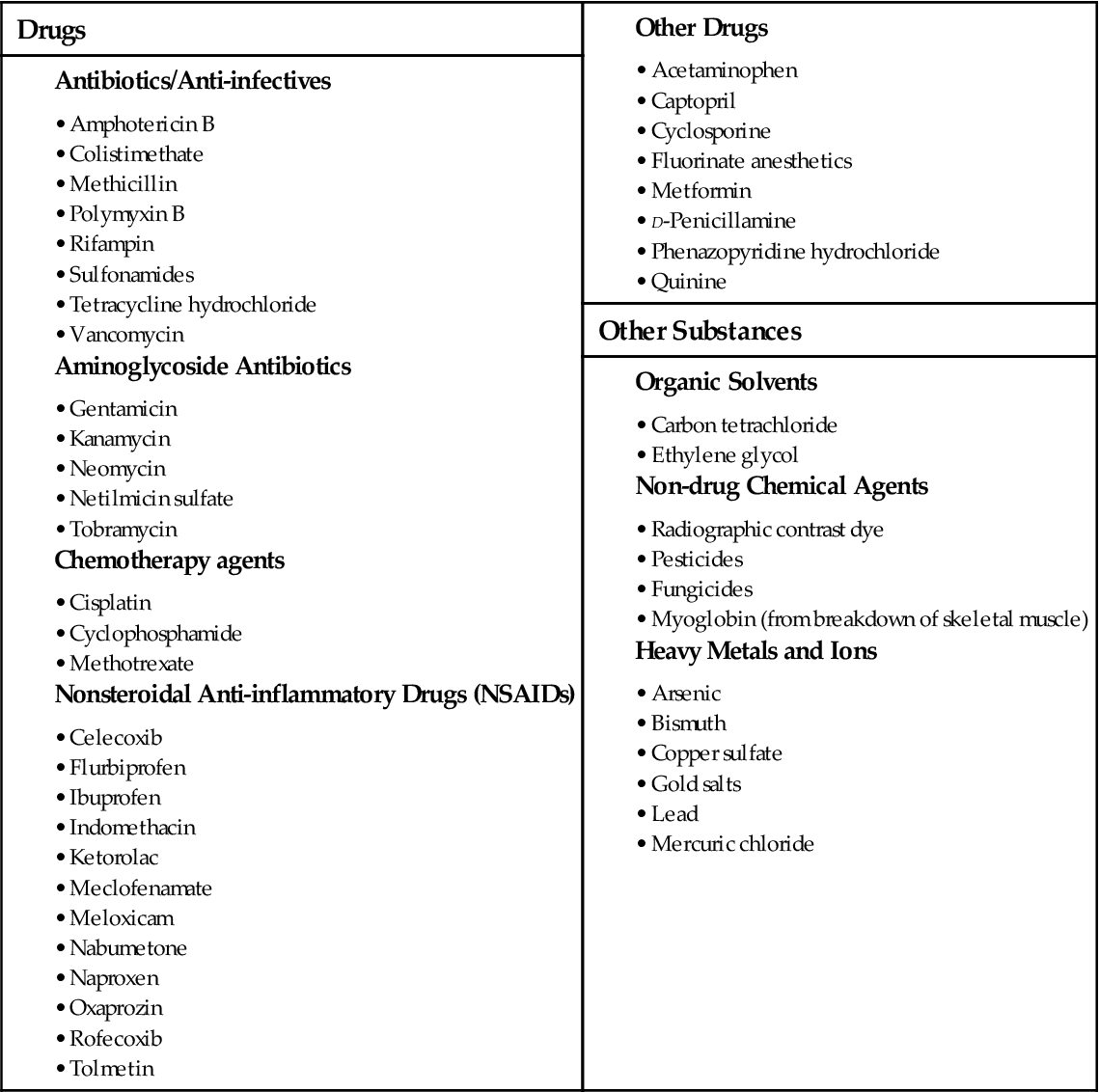
Health care–acquired AKI occurs in as many as 4% of hospital admissions and 20% of critical care admissions (Peacock & Sinert, 2010). Most AKI episodes are due to events that lead to hypotension with poor kidney perfusion and worsening of chronic kidney problems. For patients who survive the precipitating event, the chance for return of kidney function is good. However, complications during the course of AKI can greatly increase the risk for death. Bloodstream infections from IV line contamination are frequent complications that lead to death. However, the highest death rate occurs with trauma (70%) and surgery. AKI caused by nephrotoxic (kidney damaging) substances (Table 71-5) has the lowest rates of recovery. The prognosis for AKI caused by obstruction or glomerulonephritis is much better.
Health Promotion and Maintenance
Keep in mind that severe blood volume depletion can lead to kidney injury even in people who have no known kidney problems. Urge all people to avoid dehydration by drinking at least 2 to 3 L of fluids daily. This is especially important for athletes or any person who performs strenuous exercise or work and sweats heavily.
Nurses have an essential role in the prevention of acute kidney injury in hospitalized patients. Always be on the lookout for signs of reduced kidney function through careful physical assessment (especially of urine output and weight), close monitoring of laboratory values, and evaluation of fluid status. Early recognition and correction of problems causing reduced kidney blood flow usually restore function before tissue damage can occur. Evaluate the patient’s fluid status. Accurately measure intake and output and check body weight to identify changes in fluid balance.
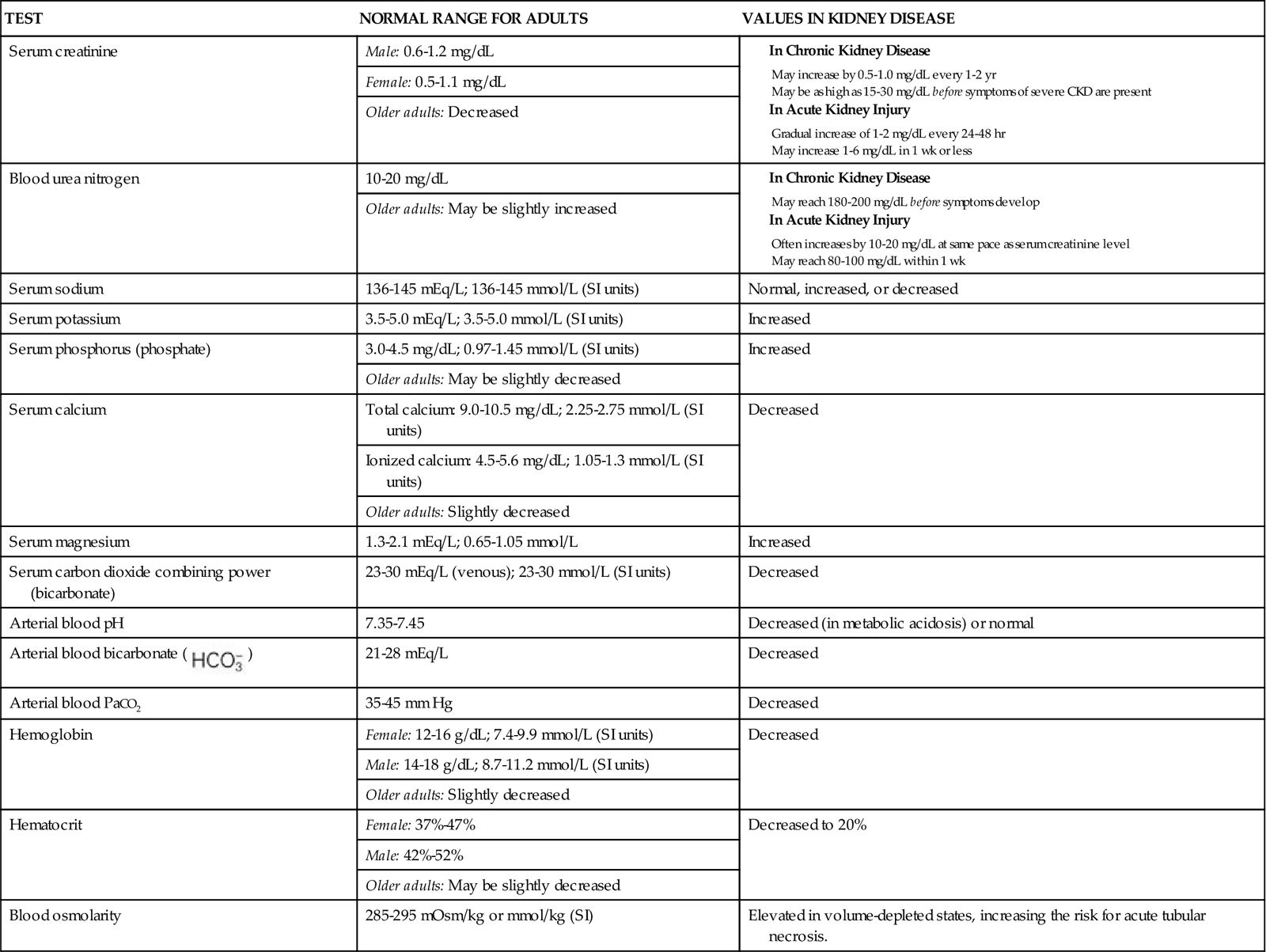
Also monitor laboratory values for any changes that reflect poor kidney function. Decreased urine specific gravity indicates a loss of urine-concentrating ability and is the earliest sign of kidney tubular damage. Other laboratory values that are helpful in monitoring kidney function include serum creatinine, urine and serum electrolytes, and blood urea nitrogen (BUN).
Be aware of nephrotoxic substances that the patient may ingest or be exposed to (see Table 71-5). Question any prescription for potentially nephrotoxic drugs, and validate the dose before the patient receives the drug. Antibiotics are common drugs that have nephrotoxic side effects. NSAIDs can cause or increase the risk for AKI. Combining two or more nephrotoxic drugs dramatically increases the risk for AKI. If a patient must receive a known nephrotoxic drug, closely monitor laboratory values, including BUN, creatinine, and drug peak and trough levels, for indications of reduced kidney function.
Patient-Centered Collaborative Care
Assessment
History
The accurate diagnosis of AKI, including its type and its cause, depends on a detailed history of potential causes of AKI. Ask the patient about exposure to nephrotoxins, recent surgery or trauma, transfusions, or other factors that might lead to reduced kidney blood flow. Obtain a drug history, especially treatment with antibiotics, ACE inhibitors, and NSAIDs. Ask about recent imaging procedures requiring injection of a contrast dye. These dyes can cause AKI, especially in older patients with reduced kidney function. AKI must be differentiated from chronic kidney disease (CKD). Ask the patient about diseases that impair kidney function, such as diabetes mellitus, long-term hypertension, systemic lupus erythematosus, and other connective tissue diseases.
To identify possible acute glomerulonephritis, ask about acute illnesses such as influenza, colds, gastroenteritis, and sore throats. Ask whether urine color is darker or appears smoky.
Reversible prerenal azotemia may occur after any episode of acute hypotension, hemorrhage or shock, burns, heart failure, or any problem in which the blood volume is depleted. Extensive bowel preparations, being NPO before surgery without fluid replacement, and fluid loss during surgery can cause prerenal AKI in some patients.
Postrenal kidney injury is identified by focusing on urinary obstructive problems. Ask the patient about any difficulty in starting the urine stream, changes in the amount or appearance of the urine, narrowing of the urine stream, nocturia, urgency, or symptoms of kidney stones. Also ask about any cancer history that may cause urinary obstruction.
Physical Assessment/Clinical Manifestations
The manifestations of AKI are related to the buildup of nitrogenous wastes (azotemia), as well as to as the underlying cause (Chart 71-1). Manifestations of prerenal azotemia are hypotension, tachycardia, decreased urine output, decreased cardiac output, decreased central venous pressure (CVP), and lethargy. The appearance of a patient with prerenal azotemia is similar to that of a patient with heart failure or dehydration, depending on the cause of the poor kidney blood flow.
Intrarenal (intrinsic) AKI usually occurs with damage to the glomeruli, interstitial tissue, or tubules. Manifestations are related to the retention of fluid and nitrogenous wastes. These include oliguria (decreased urine output) or anuria (absence of urine), edema, hypertension, tachycardia, shortness of breath, distended neck veins, elevated CVP, weight gain, respiratory crackles, anorexia, nausea, vomiting, and lethargy or changes in levels of consciousness. Manifestations of electrolyte imbalances (particularly elevated potassium levels and low calcium levels), such as electrocardiographic (ECG) changes, may also be present.
In patients with postrenal AKI, monitor for oliguria or intermittent anuria, symptoms of uremia, and lethargy. Report changes in the urine stream, difficulty starting urination, and the presence of blood or particles in the urine.
Laboratory Assessment
The many changes in laboratory values in the patient with AKI are similar to those occurring in chronic kidney disease (CKD) (Chart 71-2; see also p. 1552 in the discussion of Laboratory Assessment in the Chronic Kidney Disease section). Expect to see rising BUN and serum creatinine levels and abnormal blood electrolyte values. Patients with AKI, however, usually do not have the anemia associated with CKD unless there is hemorrhagic blood loss or unless blood urea levels are high enough to break (lyse) red blood cells.
In early AKI, urine tests provide important information. Urine sodium levels are often less than 10 to 20 mEq/L in patients with prerenal azotemia. The urine is concentrated, with a specific gravity greater than 1.030. The presence of urine sediment (e.g., red blood cells [RBCs], RBC casts, and tubular cells), myoglobin, or hemoglobin; a urine sodium level lower than 40 mEq/L; and a specific gravity of less than 1.010 indicate intrarenal kidney injury. In postrenal AKI, urine sodium levels may be normal (about 40 mEq/L), with a specific gravity of 1.000 to 1.010.
Imaging Assessment
X-rays help determine the cause of AKI. An abdominal x-ray is used to check the size of the kidneys. Enlarged kidneys, possibly due to obstruction, may result from hydronephrosis. X-rays may show stones obstructing the renal pelvis, ureters, or bladder.
Renal ultrasonography is a noninvasive procedure using high-energy sound waves. It is useful in the diagnosis of urinary tract obstruction. Dilation of the renal calyces and collecting ducts, as well as stones, can be detected. Ultrasonography can show kidney size and patency of the ureters.
CT scans without contrast dye can identify obstruction or tumors. Contrast dyes are usually avoided to prevent further kidney damage. A nuclear medicine study called MAG3 may be used to determine the nature of the renal failure, GFR, or tubular function and its severity. A renal scan can determine whether blood flow to the kidneys is sufficient. Cystoscopy or retrograde pyelography may be needed to identify obstructions of the lower urinary tract.
Other Diagnostic Assessment
Kidney biopsy is performed if the cause of AKI is uncertain, an immunologic disease is suspected, or the reversibility of the kidney dysfunction needs to be determined after AKI has persisted for an extended period. Prepare the patient before the test, and provide follow-up care. Be aware of all test results and understand how they might affect the treatment regimen. (See Chapter 68 for a detailed discussion of renal diagnostic tests.)
Interventions
The patient with AKI may move from the oliguric phase (in which fluid and electrolytes are retained) to the diuretic phase. In the oliguric phase, the plan of care focuses on close monitoring for life-threatening electrolyte changes and nitrogen retention that may require intervention. During the diuretic phase, hypovolemia and electrolyte loss are the main problems. The patient in the diuretic phase of AKI needs a plan of care that focuses on fluid and electrolyte replacement and monitoring.
These examples of output variation reflect the continually changing nature of AKI and the need for the plan of care to be constantly updated to reflect the stages of the disease process. Drug therapy, nutrition therapy, and renal replacement therapy (peritoneal dialysis [PD], hemodialysis [HD], or hemofiltration) are commonly used to manage AKI.
Drug Therapy
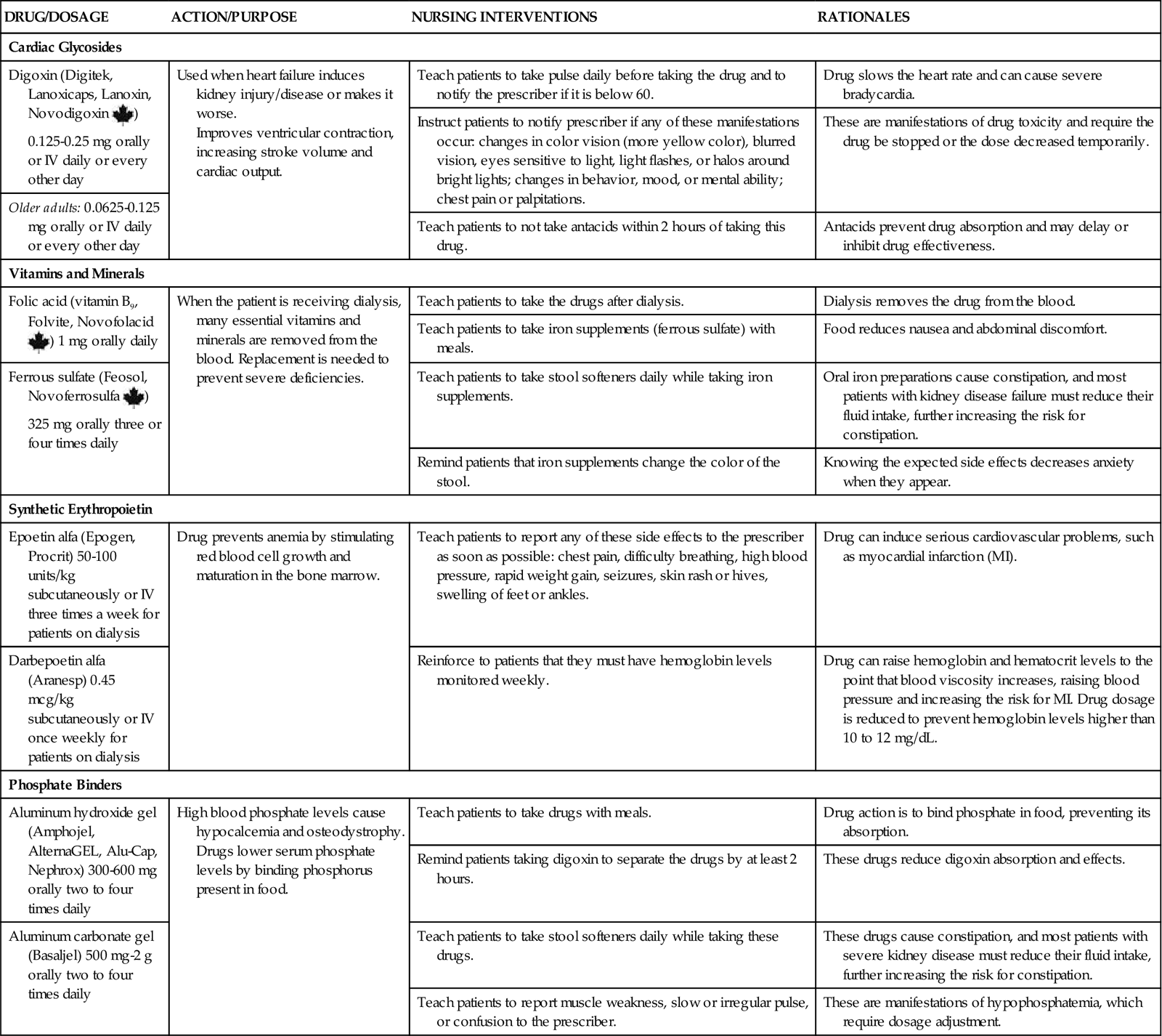
Patients with AKI receive many drugs. As kidney function changes, drug dosages are changed. It is important to be knowledgeable about the site of drug metabolism and especially careful when giving drugs. Constantly monitor for possible side effects and interactions of the drugs that the patient with AKI is receiving (Chart 71-3; see also the discussion of drug therapy on pp. 1553, 1556, and 1557 in the Chronic Kidney Disease section). Diuretics may be used to increase urine output.
In patients with prerenal azotemia, fluid challenges and diuretics are often used to promote kidney blood flow. In the patient without fluid overload, 500 to 1000 mL of normal saline may be infused over 1 hour. In prerenal azotemia, the patient responds to the fluid challenge by producing urine soon after the initial bolus. Diuretics such as furosemide (Lasix) also may be prescribed along with a fluid bolus. If oliguric kidney injury is diagnosed, the fluid challenges and diuretics are discontinued. Patients often require central venous pressure (CVP) monitoring or measurement of pulmonary arterial pressure by means of a pulmonary artery catheter for accurate evaluation of their hemodynamic status. They also require constant nursing supervision for assessment of the response to fluid and drug therapy. Carefully monitor for signs of possible fluid overload.
Calcium channel blockers may be used to treat AKI resulting from nephrotoxic acute tubular necrosis (ATN). These drugs prevent the movement of calcium into the kidney cells, maintain kidney cell integrity, and improve the glomerular filtration rate (GFR) by improving kidney blood flow.
Nutrition Therapy
Patients who have AKI often have a high rate of protein breakdown. The exact cause for this state is not known. Increases in metabolism and protein breakdown may be related to the stress of a critical illness, causing an increase in blood levels of catecholamines, cortisol, and glucagon. The rate of protein breakdown correlates with the severity of uremia and azotemia. This state causes the breakdown of muscle for protein, which leads to an increase in azotemia and an even more elevated blood urea nitrogen (BUN) level.
If the patient with AKI has a good dietary intake (see discussion of Enhancing Nutrition on pp. 1555-1556 in the Chronic Kidney Disease section), nutritional support may not be needed. A dietary consult with a dietitian, who will calculate the patient’s caloric needs, may be prescribed. Work with the dietitian to provide a diet with specified amounts of protein, sodium, and fluids. For the patient who does not require dialysis, 0.6 g/kg of body weight or 40 g/day of protein is usually prescribed. For patients who do require dialysis, the protein level needed will range from 1 to 1.5 g/kg. The amount of dietary sodium ranges from 60 to 90 mEq. If high blood potassium levels are present, dietary potassium is restricted to 60 to 70 mEq. The amount of fluid permitted is generally calculated to be equal to the urine volume plus 500 mL. Assess food intake every shift to ensure that caloric intake is adequate.
Many patients with AKI are too ill or their appetite is too poor to eat enough food. For these patients, some form of nutritional support (e.g., total parenteral nutrition [TPN] or hyperalimentation) is needed. The purposes of nutritional support in AKI are to provide sufficient nutrients to maintain or improve nutritional status, to preserve lean body mass, to restore or maintain fluid balance, and to preserve kidney function.
If TPN is used, the solutions are mixed to meet the patient’s specific needs. Because kidney function is unstable in AKI, constantly monitor the serum electrolyte levels to indicate when the TPN solution needs to be changed. IV fat emulsion (Intralipid) infusions can provide a nonprotein source of calories. In uremic patients, fat emulsions are used in place of glucose to avoid the problems of excessive sugars.
Dialysis Therapies
Hemodialysis (HD) and peritoneal dialysis (PD) are used for patients with L and E levels of AKI. Indications for dialysis use include the presence of uremia, persistent high potassium levels, metabolic acidosis, continued fluid overload, uremic pericarditis, and encephalopathy.
Immediate vascular access for HD in patients with AKI is made by placement of a dual- or triple-lumen catheter specific for HD. When HD is expected to be used for several weeks, the catheter is usually placed in the subclavian or internal jugular vein. If only one or two HD treatments are needed, as for removal of drugs or toxins, a femoral site may be selected. Longer use of the femoral site is avoided because the patient’s mobility is restricted and complications, such as hematomas and infection, are common. Repeatedly accessing the femoral site increases the risk for hematoma formation and makes repeated use of the vein impossible.
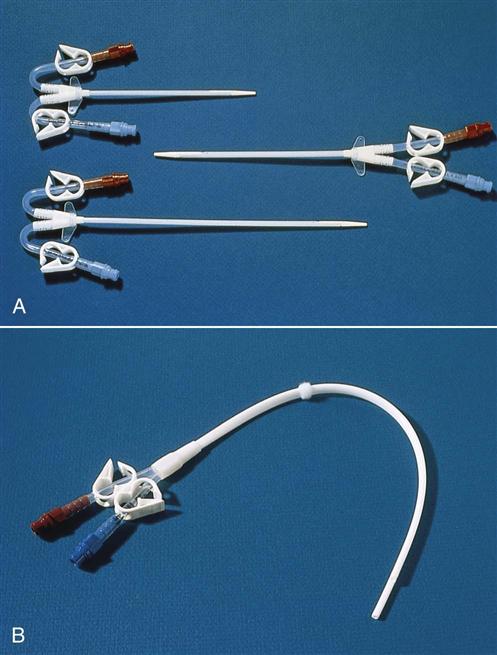
The subclavian vein is used, when possible, instead of the femoral site because the catheter can be left in place between dialysis treatments. However, the longer the catheter is left in this place, the greater the chance for infection. The subclavian dialysis catheter (Fig. 71-1) is inserted at the bedside. A physician or nurse practitioner performs the sterile procedure, and then the catheter is covered with a sterile dressing. Monitor for manifestations of procedure complications such as pneumothorax (reduced breath sounds, tracheal deviation away from midline, prominence and poor movement of one side of the chest) or subcutaneous emphysema (crackling and swelling of tissue around the site). Catheter placement is checked by chest x-ray before its use.
If hemodialysis is needed for more than a few days, a long-term dialysis catheter may be used. Most of these catheters are placed in the radiology department using a tunneling technique. The patient receives moderate sedation. Under sonographic or fluoroscopic guidance, the physician makes a small incision where the internal jugular vein passes behind the clavicle. A 6- to 8-cm tunnel is created out from the side of the incision. A long-term hemodialysis catheter is inserted through the tunnel and into the jugular vein. Keeping a segment of the catheter within the subcutaneous tissues before entering the jugular vein reduces the risk for infection.
Hemodialysis catheters have two lumens, one for outflow and one for inflow. This allows the outflow of blood for dialysis to be separated from the dialyzed blood returned through the inflow lumen. A triple-lumen catheter for HD is available. The third lumen is an access for drawing venous blood or giving drugs and fluid without interrupting dialysis.
Peritoneal dialysis (PD) may also be used in the treatment of AKI, although some patients, such as those being mechanically ventilated, may not be able to tolerate the abdominal distention that occurs with PD. PD uses the peritoneum as the dialyzing membrane. The dialysate is infused through a catheter implanted in the peritoneum. A complete discussion of PD is provided in the Chronic Kidney Disease section, pp. 1564-1567.
Continuous Renal Replacement Therapy
Continuous renal replacement therapies (CRRTs) are the standard treatment for AKI levels L and E. Renal replacement therapies in the form of hemofiltration are often better tolerated than HD for critically ill patients because this method avoids rapid shifts of fluids and electrolytes.
Continuous venovenous hemofiltration (CVVH), continuous venovenous hemodiafiltration (CVVHD), continuous arteriovenous hemofiltration (CAVH), and continuous arteriovenous hemofiltration with dialysis (CAVHD) are the renal replacement therapies most commonly used for patients with AKI. The venovenous methods are being used more often than the arteriovenous methods. These procedures are similar to HD but their use is temporary.
Continuous venovenous hemofiltration (CVVH) is often used with critically ill patients. CVVH uses only a double-lumen venous catheter for access and is powered by a pump, making the rate of filtration more reliable than methods using mean arterial pressure. The pump increases the risk for an air embolus, but most pumps have alarms that detect air. These systems also require the use of anticoagulants but at lower doses than needed for AV systems. These procedures are used in critical care units, and patients require continuous nursing care.
The technique of CVVHD is a very common method of renal replacement therapy. It uses an infusion pump, hemodialysis membrane, and dialysate solution, as well as the same type of blood access as the CVVH technique. Similar to the CAVHD system, CVVHD adds the dialysis membrane and the dialysate solution instead of just filtering the blood and, as a result, increases the efficiency of removing waste products from the blood. CVVHD is somewhat less effective than CAVHD because the lower pressure in the venous system compared with the arterial system does not filter as much blood in the same amount of time. Thus CVVHD needs to be performed for longer periods to achieve the same effects as CAVHD. However, because the removal is slower, fewer adverse changes in blood volume and blood pressure are likely to occur.
CAVH is used for patients who have fluid volume overload, are resistant to diuretics, and have unstable blood pressures and cardiac output. The use of CAVH requires placement of both arterial and venous catheters and that the patient have a mean arterial pressure (MAP) of at least 60 mm Hg. (Mean arterial pressure is a person’s average arterial pressure and is based on cardiac output and systemic vascular resistance. The normal adult MAP is 100 mm Hg.) CAVH continuously removes large amounts of plasma water, wastes, and electrolytes. Electrolytes are replaced through prescribed amounts of IV electrolyte solutions. A major disadvantage of arteriovenous (AV) filtration is the risk for bleeding caused by anticoagulants used to prevent membrane clotting.
A double-lumen dialysis catheter is inserted into a large vein (subclavian, jugular) for CAVHD. A dialysate (a solution composed of water, glucose, sodium chloride, potassium, magnesium, calcium, and bicarbonate) delivery system is used to remove waste products in addition to plasma water in patients with limited cardiac output, those with severe hypotension, or those who do not respond to diuretic therapy. These patients cannot tolerate HD, and PD could not remove the large amount of excess fluid.
Posthospital Care
The care for a patient with AKI after discharge from the hospital varies, depending on the status of the disorder when the patient is discharged. The course of AKI varies, with recovery lasting up to several months. If the kidney injury is resolving, follow-up care may be provided by a nephrologist or by the family physician in consultation with the nephrologist. However, AKI may result in permanent kidney damage and CKD with the need for chronic dialysis or even transplantation. In these cases, follow-up care is similar to that needed for patients with chronic kidney disease (see Community-Based Care, pp. 1571-1572).
If the AKI is beginning to resolve, the follow-up care may involve many services. Frequent medical visits are necessary, as are scheduled laboratory blood and urine tests to monitor kidney function. A dietitian is needed to modify the patient’s diet according to the degree of kidney function and ongoing nutritional needs. Teach patients continuing dialysis after discharge to limit foods high in potassium, sodium, and phosphorus and to observe protein restrictions. Also teach about any needed fluid intake limitation. Instruct patients to weigh themselves daily, keep a daily log, and report any daily weight gain of 2 pounds or more immediately to the health care provider.
Some patients may need temporary dialysis until their kidneys can eliminate fluid and waste products. The dialysis started while the patient was an inpatient can be continued at an outpatient dialysis center. Teaching about the type of dialysis, how to care for vascular access sites, dietary restrictions, fluid restrictions, and prevention of complications is ongoing throughout the recovery phase. Depending on their level of independence and family support, some patients may also need home care nursing or social work assistance.
Chronic Kidney Disease
Pathophysiology
Unlike acute kidney injury (AKI), chronic kidney disease (CKD) is a progressive, irreversible disorder and kidney function does not recover. When kidney function is too poor to sustain life, CKD becomes end-stage kidney disease (ESKD). Terms used with kidney dysfunction include azotemia (buildup of nitrogen-based wastes in the blood), uremia (azotemia with clinical symptoms [Chart 71-4]), and uremic syndrome. Table 71-1 compares AKI and CKD.
Stages of Chronic Kidney Disease
The kidneys lose function (fail) in an organized fashion involving five stages based on estimated glomerular filtration rate (GFR). Progression toward ESKD usually starts with a gradual decrease in GFR (Table 71-6). In this first stage, the person may have a normal GFR (greater than 90 mL/min) with normal kidney function and no obvious kidney disease. However, there may be a reduced renal reserve in which reduced kidney function occurs without buildup of wastes in the blood because the unaffected nephrons overwork to compensate for the diseased nephrons. Although no manifestations of kidney dysfunction are usually present at this stage, if the patient is stressed with infection, fluid overload, pregnancy, or dehydration, kidney function at this stage can appear reduced.
TABLE 71-6
PROGRESSION OF CHRONIC KIDNEY DISEASE
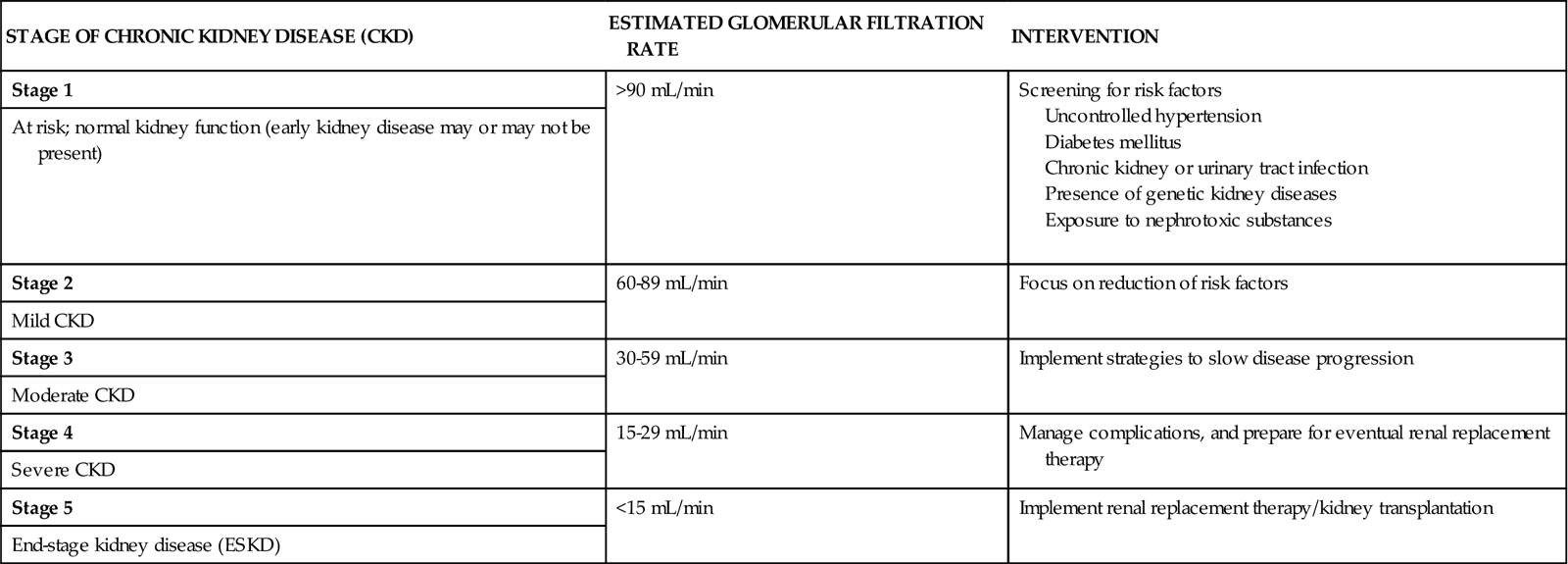
In the next stage, mild CKD, GFR is reduced, ranging between 60 and 89 mL/min. Kidney nephron damage has occurred, and there may be slight elevations of metabolic wastes in the blood because not enough healthy nephrons remain to compensate completely for the damaged nephrons. Levels of blood urea nitrogen (BUN), serum creatinine, uric acid, and phosphorus are not sensitive enough to define this stage, however, and reduced GFR is the best measure of CKD. Increased output of dilute urine may occur at this stage of CKD and, if the problem is untreated at this stage, can cause severe dehydration.
In moderate CKD, GFR reduction continues and ranges between 30 and 59 mL/min. Nephron damage has continued, and the remaining nephrons cannot manage metabolic wastes, fluid balance, and electrolyte balance. Restriction of fluids, proteins, and electrolytes is needed.
Over time, patients progress to severe CKD (the fourth stage) and end-stage kidney disease (ESKD) (the fifth stage). Excessive amounts of urea and creatinine build up in the blood, and the kidneys cannot maintain homeostasis. Severe fluid, electrolyte, and acid-base imbalances occur (Pradeep & Verrelli, 2010). Without renal replacement therapy, fatal complications occur.
Kidney Changes
Kidney dysfunction with greatly reduced GFR causes many problems, including abnormal urine production, poor water excretion, electrolyte imbalances, and metabolic abnormalities. Because the healthy nephrons become larger and work harder, the GFR is effective until about three fourths of kidney function is lost. Homeostasis of electrolytes, acid-base, and nitrogenous wastes is maintained until late in the course of kidney disease.
As the disease progresses, the ability to produce dilute urine is reduced, resulting in urine with a fixed osmolarity (isosthenuria). As kidney function continues to decline, the BUN increases and urine output decreases. At this point, the patient is at risk for fluid overload.
Metabolic Changes
Urea and creatinine excretion are disrupted by kidney dysfunction. Creatinine comes from proteins present in skeletal muscle. The rate of creatinine excretion depends on muscle mass, physical activity, and diet. Without major changes in diet or physical activity, the serum creatinine level is constant. Creatinine is partially excreted by the kidney tubules, and a decrease in kidney function leads to a buildup of serum creatinine. Urea is made from protein metabolism and is excreted by the kidneys. The BUN level normally varies directly with protein intake and hydration status.
The method for assessing the GFR is the use of a formula that considers the serum creatinine level, age, gender, race, and body size. The most common formula is the Cockcroft-Gault equation.
Sodium excretion changes are common. Early in CKD, the patient is at risk for hyponatremia (sodium depletion) because there are fewer healthy nephrons to reabsorb sodium. Thus sodium is lost in the urine. The polyuria of early kidney dysfunction also causes sodium loss.
In the later stages of CKD, kidney excretion of sodium is reduced as urine production decreases. Then sodium retention and high serum sodium levels (hypernatremia) can occur with only modest increases in dietary sodium intake. This problem leads to severe fluid and electrolyte imbalances (see Chapter 13). Sodium retention causes hypertension and edema.
Even with sodium retention, the serum sodium level may appear normal because plasma water is retained at the same time. If fluid retention occurs at a greater rate than sodium retention, the serum sodium level is falsely low because of dilution (see Chart 71-2).
Potassium excretion occurs mainly through the kidney. Any increase in potassium load during the later stages of CKD can lead to hyperkalemia (high serum potassium levels). Normal serum potassium levels of 3.5 to 5 mEq/L are maintained until the 24-hour urine output falls below 500 mL. High potassium levels then develop quickly, reaching 7 to 8 mEq/L or greater. Severe ECG changes result from this elevation, and fatal dysrhythmias can occur. Other factors contribute to high potassium levels in CKD, including the ingestion of potassium in drugs, failure to restrict dietary potassium, tissue breakdown, blood transfusions, and bleeding or hemorrhage. (See Chapter 13 for discussion of potassium problems.)
Acid-base balance is affected by CKD. In the early stages, blood pH changes little because the remaining healthy nephrons increase their rate of acid excretion. As more nephrons are lost, acid excretion is reduced and metabolic acidosis results (see Chapter 14).
Many factors lead to acidosis in CKD. First, the kidneys cannot excrete excessive hydrogen ions (acids). Normally, tubular cells move hydrogen ions into the urine for excretion, but ammonium and bicarbonate are needed for this movement to occur. In patients with CKD, ammonium production is decreased and reabsorption of bicarbonate does not occur. This process leads to a buildup of hydrogen ions and reduced levels of bicarbonate (base deficit). High potassium levels further reduce kidney ammonium production and excretion.
As CKD worsens and acid retention increases, increased respiratory action is needed to keep blood pH normal. The respiratory system adjusts or compensates for the increased blood hydrogen ion levels (decreased pH) by increasing the rate and depth of breathing to excrete carbon dioxide through the lungs. This breathing pattern, called Kussmaul respiration, increases with worsening kidney disease. Although hydrogen ions (acids) can leave the body this way, when too much carbon dioxide is “blown off,” respiratory alkalosis results. Serum bicarbonate measures the extent of metabolic acidosis (bicarbonate deficit). Patients with CKD usually need alkali replacement to counteract acidosis.
 )
)