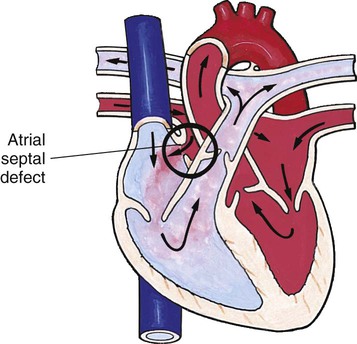
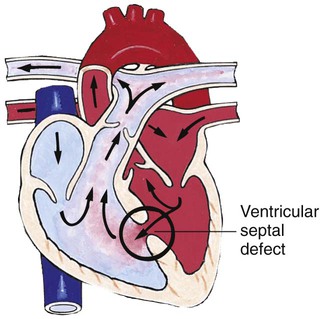
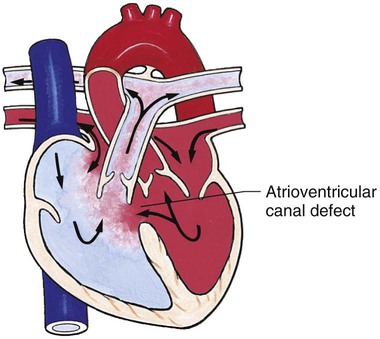
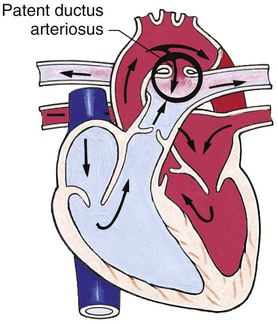
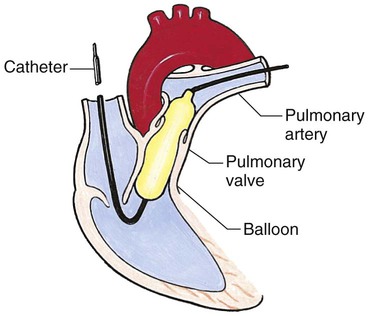
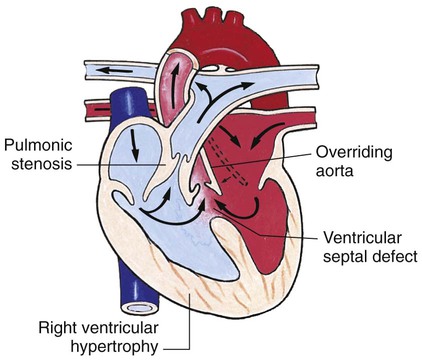
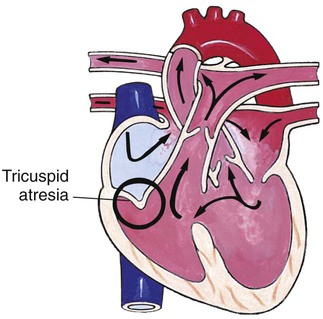
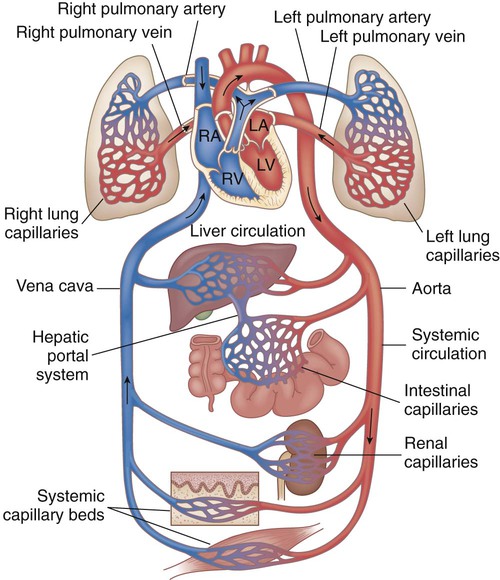
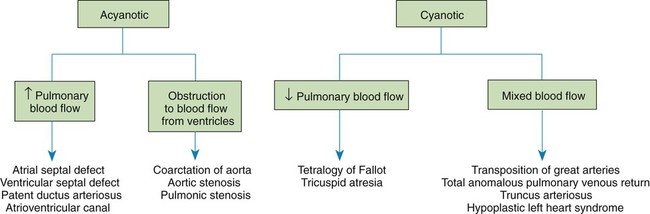
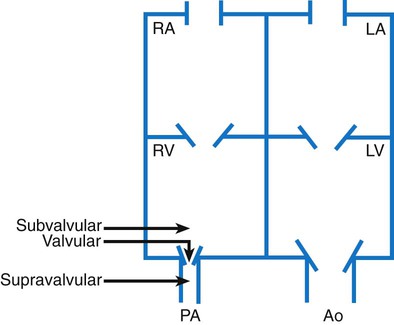
Chapter 42 On completion of this chapter, the reader will be able to: • Design a plan for assisting children during cardiac diagnostic procedures. • Demonstrate an understanding of the hemodynamics, distinctive manifestations, and therapeutic management of congenital heart disease. • Outline a care plan for an infant or child with heart failure. • Describe the care for a child who has hypoxia. • Describe the care for an infant or a child with a congenital heart defect and its surgical repair. • Discuss the nurse’s role in helping the child and family cope with congenital heart disease. • Differentiate between rheumatic fever and rheumatic heart disease. • List the criteria for selected cholesterol screening of children. • Discuss the assessment and management of hypertension in children and adolescents. • Outline a care plan for a child with Kawasaki disease. • Describe the emergency treatment for shock, including anaphylaxis. Cardiovascular disorders in children are divided into two major groups—congenital heart disease and acquired heart disorders. Congenital heart disease (CHD) includes primarily anatomic abnormalities present at birth that result in abnormal cardiac function. The clinical consequences of congenital heart defects fall into two broad categories—heart failure (HF) and hypoxemia. Acquired cardiac disorders are disease processes or abnormalities that occur after birth and can be seen in the normal heart or in the presence of congenital heart defects. They result from various factors, including infection, autoimmune responses, environmental factors, and familial tendencies. The pathophysiology review found in Fig. 42-1 describes the flow of blood through the heart. The physical assessment of suspected cardiac disease begins with observation of general appearance and then proceeds with more specific observations. The following are supplementary to the general assessment techniques described for physical examination of the chest and heart in Chapter 29: Nutritional state—Failure to thrive or poor weight gain is associated with heart disease. Color—Cyanosis is a common feature of CHD, and pallor is associated with poor perfusion. Chest deformities—An enlarged heart sometimes distorts the chest configuration. Unusual pulsations—Visible pulsations of the neck veins are seen in some patients. Respiratory excursion—This refers to the ease or difficulty of respiration (e.g., tachypnea, dyspnea, expiratory grunt). Diagnostic catheterizations—These studies are used to diagnose congenital cardiac defects, particularly in symptomatic infants and before surgical repair. They are divided into (1) right-sided catheterizations, in which the catheter is introduced through a vein (usually the femoral vein) and threaded to the right atrium (most common); and (2) left-sided catheterizations, in which the catheter is threaded through an artery into the aorta and into the heart. Interventional catheterizations (therapeutic catheterizations)—A balloon catheter or other device is used to alter the cardiac anatomy. Examples include dilating stenotic valves or vessels or closing abnormal connections. Electrophysiology studies—Catheters with tiny electrodes that record the impulses of the heart directly from the conduction system are used to evaluate dysrhythmias and sometimes destroy accessory pathways that cause some tachydysrhythmias. Cardiac catheterization has become a routine diagnostic procedure and may be done on an outpatient basis. However, it is not without risks, especially in neonates and seriously ill infants and children. Possible complications include acute hemorrhage from the entry site (more likely with interventional procedures because larger catheters are used), low-grade fever, nausea, vomiting, loss of pulse in the catheterized extremity (usually transient, resulting from a clot, hematoma, or intimal tear), and transient dysrhythmias (generally catheter induced) (Uzark, 2001). Rare risks include stroke, seizures, tamponade, and death. Preparing the child and family for the procedure is the joint responsibility of the patient care team. School-age children and adolescents benefit from a description of the catheterization laboratory and a chronologic explanation of the procedure, emphasizing what they will see, feel, and hear. Older children and adolescents may bring earphones and favorite music so they can listen during the catheterization procedure. Preparation materials such as picture books, videotapes, or tours of the catheterization laboratory may be helpful. Preparation should be geared to the child’s developmental level. The child’s caregivers often benefit from the same explanations. Additional information, such as the expected length of the catheterization, description of the child’s appearance after catheterization, and usual postprocedure care, should be outlined. (See also Prepare the Child and Family for Invasive Procedures, p. 1341.) • Pulses, especially below the catheterization site, for equality and symmetry (Pulse distal to the site may be weaker for the first few hours after catheterization but should gradually increase in strength.) • Temperature and color of the affected extremity, because coolness or blanching may indicate arterial obstruction • Vital signs, which are taken as frequently as every 15 minutes, with special emphasis on heart rate, which is counted for 1 full minute for evidence of dysrhythmias or bradycardia • Blood pressure (BP), especially for hypotension, which may indicate hemorrhage from cardiac perforation or bleeding at the site of initial catheterization • Dressing, for evidence of bleeding or hematoma formation in the femoral or antecubital area • Fluid intake, both IV and oral, to ensure adequate hydration (Blood loss in the catheterization laboratory, the child’s NPO status, and diuretic actions of dyes used during the procedure put children at risk for hypovolemia and dehydration.) • Blood glucose levels, for hypoglycemia, especially in infants, who should receive dextrose-containing IV fluids Depending on hospital policy, the child may be kept in bed with the affected extremity maintained straight for 4 to 6 hours after venous catheterization and 6 to 8 hours after arterial catheterization to facilitate healing of the cannulated vessel. If younger children have difficulty complying, they can be held in the parent’s lap with the leg maintained in the correct position. The child’s usual diet can be resumed as soon as tolerated, beginning with sips of clear liquids and advancing as the condition allows. The child is encouraged to void to clear the contrast material from the blood. Generally, there is only slight discomfort at the percutaneous site. To prevent infection, the catheterization area is protected from possible contamination. If the child wears diapers, the dressing can be kept dry by covering it with a piece of plastic film and sealing the edges of the film to the skin with tape. However, the nurse must be careful to continue observing the site for any evidence of bleeding (see Family-Centered Care box and Critical Thinking Case Study). The incidence of CHD in children is approximately 5 to 8 per 1000 live births (Park, 2008). About 2 or 3 in 1000 infants will be symptomatic during the first year of life with significant heart disease that requires treatment (Hoffman and Kaplan, 2002). CHD is the major cause of death (other than prematurity) in the first year of life. Although there are more than 35 well-recognized cardiac defects, the most common heart anomaly is ventricular septal defect (VSD). There are typically two classification systems used to categorize congenital heart defects. Traditionally, cyanosis, a physical characteristic, has been used as the distinguishing feature, dividing anomalies into acyanotic defects and cyanotic defects (Fig. 42-2). In clinical practice, this system is problematic because children with acyanotic defects may develop cyanosis. Also, more often, those with cyanotic defects may appear pink and have more clinical signs of HF. A more useful classification system is based on hemodynamic characteristics (blood flow patterns within the heart). These blood flow patterns are (1) increased pulmonary blood flow; (2) decreased pulmonary blood flow; (3) obstruction to blood flow out of the heart; and (4) mixed blood flow, in which saturated and desaturated blood mix within the heart or great arteries. As a comparison, Fig. 42-3 outlines both classification systems. With the hemodynamic classification system, the clinical manifestations of each group are more uniform and predictable. Defects that allow blood flow from the higher-pressure left side of the heart to the lower-pressure right side (left-to-right shunt) result in increased pulmonary blood flow and cause heart failure (HF). Obstructive defects impede blood flow out of the ventricles; whereas obstruction on the left side of the heart results in HF, severe obstruction on the right side causes cyanosis. Defects that cause decreased pulmonary blood flow result in cyanosis. Mixed lesions present a variable clinical picture based on the degree of mixing and amount of pulmonary blood flow; hypoxemia (with or without cyanosis) and HF usually occur together. Using this classification system, the clinical presentation and management of the most common defects are outlined in the following sections and Box 42-1. In this group of cardiac defects, intracardiac communications along the septum or an abnormal connection between the great arteries allows blood to flow from the higher pressure left side of the heart to the lower pressure right side of the heart. Increased blood volume on the right side of the heart increases pulmonary blood flow at the expense of systemic blood flow. Clinically, patients demonstrate signs and symptoms of HF. ASD, VSD, and patent ductus arteriosus are typical anomalies in this group (see Box 42-1). Obstructive defects are those in which blood exiting the heart meets an area of anatomic narrowing (stenosis), causing obstruction to blood flow. The pressure in the ventricle and in the great artery before the obstruction is increased, and the pressure in the area beyond the obstruction is decreased. The location of the narrowing is usually near the valve (Fig. 42-4), as follows: • Valvular—At the site of the valve itself • Subvalvular—Narrowing in the ventricle below the valve (also referred to as the ventricular outflow tract) • Supravalvular—Narrowing in the great artery above the valve Coarctation of the aorta (narrowing of the aortic arch), aortic stenosis, and pulmonic stenosis are typical defects in this group (Box 42-2). Hemodynamically, there is a pressure load on the ventricle and decreased cardiac output. Clinically, infants and children exhibit signs of HF. Children with mild obstruction may be asymptomatic. Rarely, as in severe pulmonic stenosis, hypoxemia may be seen. In this group of defects, there is obstruction of pulmonary blood flow and an anatomic defect (ASD or VSD) between the right and left sides of the heart (Fig. 42-5). Because blood has difficulty exiting the right side of the heart via the pulmonary artery, pressure on the right side increases, exceeding left-sided pressure. This allows desaturated blood to shunt right to left, causing desaturation in the left side of the heart and in the systemic circulation. Clinically, these patients have hypoxemia and usually appear cyanotic. Tetralogy of Fallot and tricuspid atresia are the most common defects in this group (Box 42-3).
Cardiovascular Dysfunction
Cardiovascular Dysfunction
History and Physical Examination
Inspection
Diagnostic Evaluation
Cardiac Catheterization
Care Management
Preprocedural Care
Postprocedural Care
Congenital Heart Disease
Classification of Defects
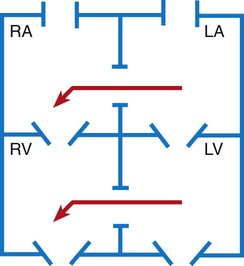
Defects with Increased Pulmonary Blood Flow
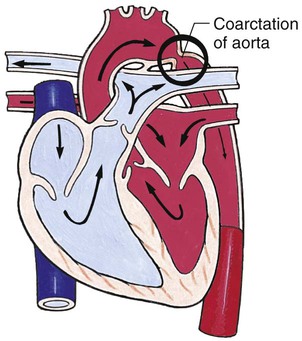
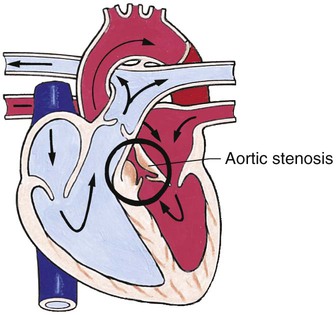
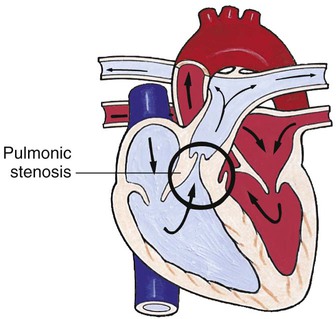
Obstructive Defects
Defects with Decreased Pulmonary Blood Flow

Cardiovascular Dysfunction
Get Clinical Tree app for offline access