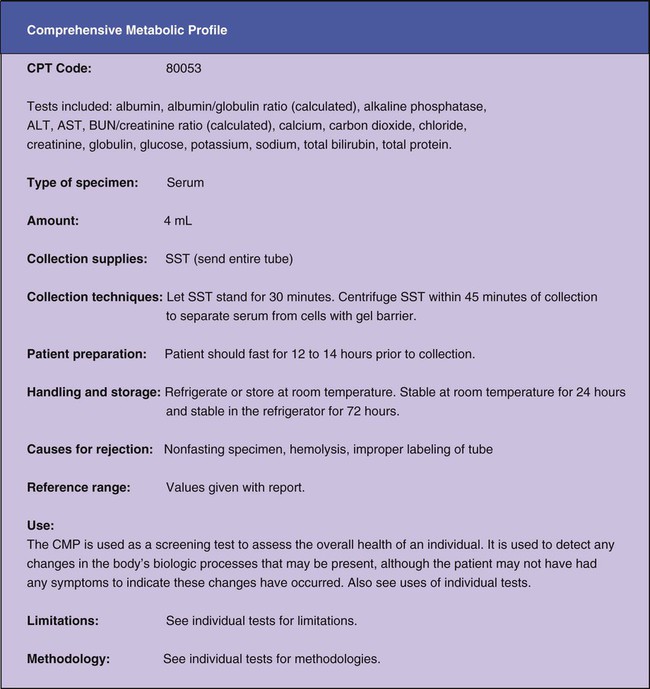
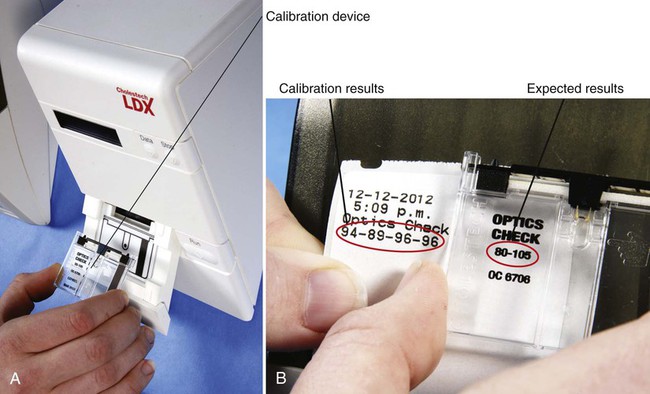
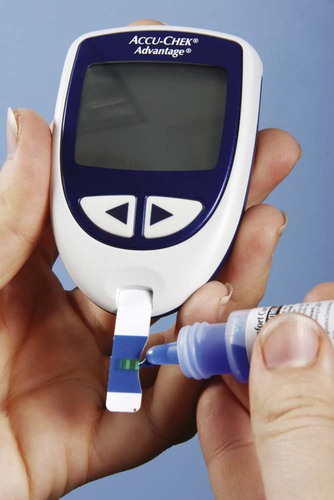
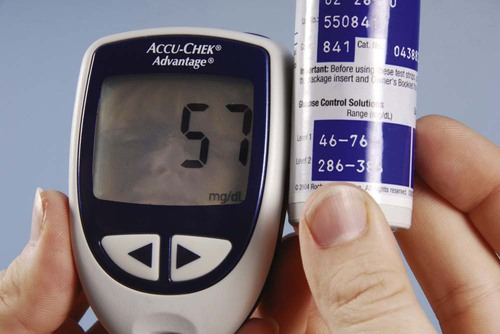
Most blood chemistry tests performed at an outside laboratory require a serum specimen for analysis (Figure 33-1). If the specimen is collected at the medical office, the medical assistant will need to perform a venipuncture using a serum separator tube (SST) or a red-stoppered tube. An example of a blood chemistry profile frequently ordered on patients in the medical office is a comprehensive metabolic profile (CMP). A CMP contains numerous blood chemistry tests and is used primarily in routine health screening of a patient. It is frequently used to detect any changes in the body’s biologic processes that may be present, although the patient may not have had any symptoms to indicate that these changes have occurred. A comprehensive metabolic profile is also used when the patient’s symptoms are so vague that the physician does not have enough concrete evidence to support a clinical diagnosis of a specific organ or disease state. Specimen collection and handling requirements for a comprehensive metabolic profile as presented in a laboratory directory are outlined in Figure 33-2. Most physicians consider it too expensive in terms of equipment, supplies, and medical laboratory personnel to perform blood chemistry tests that are any more complex than waived tests in the medical office. These tests are usually performed at an outside laboratory. However, if moderately complex blood chemistry tests are performed in the medical office, a “benchtop” blood chemistry analyzer is typically used to run them; examples of these nonwaived “benchtop” analyzers include the ATAC lab system (Clinical Data, Inc., Newton, Mass) (Figure 33-3) and the Reflotron Chemistry Analyzer (Roche Diagnostics, Branchburg, NJ). Examples of CLIA-waived blood chemistry analyzers that are more commonly used to run blood chemistry tests in the medical office include the Accu-Check Advantage blood glucose meter (Roche Diagnostics), A1c Now (Bayer Corporation, Morrisville, NJ), and the Cholestech LDX cholesterol system (Cholestech Corporation, Hayward, Calif). It is important that the medical assistant become completely familiar with all aspects of the CLIA-waived analyzer used to perform blood chemistry testing in his or her medical office. Medical offices running CLIA-waived tests are required to follow the manufacturer’s instructions exactly for each testing procedure. Instructions include information on the quality control procedures that must be performed when running the test. Quality control procedures are of particular importance to ensure that the analyzer is functioning properly, and that the test results are reliable and accurate. Additional information on quality control procedures is presented below (also refer to the quality control section in Chapter 29). Calibration is a mechanism used to check the precision and accuracy of a blood chemistry analyzer, to determine if the system is providing accurate results. A calibration check detects errors caused by laboratory equipment that is not working properly. Calibration is typically performed using a calibration device, often called a standard. The calibration device may come in the form of a calibration strip or cassette. The device is inserted into the analyzer (Figure 33-4, A) and the calibration results are displayed on the LCD screen of the analyzer. The calibration results are then compared with the expected results provided in the product insert or on the calibration device (Figure 33-4, B). If the calibration procedure does not perform as expected, patient testing should not be conducted until the problem has been identified and resolved. The frequency of performing a calibration check is indicated in the manufacturer’s instructions. At a minimum, a calibration check should be performed when a new lot number of testing reagents is put into use. A blood chemistry control consists of a solution that is used to monitor a blood chemistry analyzer to ensure the reliability and accuracy of the test results. Controls consist of commercially available solutions with known values. Controls usually come with a product insert, which lists the expected ranges for control results (Figure 33-5); however, expected ranges may sometimes be printed on the containers of the testing reagents. Controls are used to determine if the testing reagents are performing properly and to detect any errors in technique by the individual performing the test. Generally, two levels of controls must be performed on a blood chemistry analyzer. A low-level control (also known as a Level 1 control) produces results that fall below the reference range for the test; a high-level control (also known as a Level 2 control) produces results that fall above the reference range for the test. The control procedure is performed in a similar manner to the procedure for performing the test on a specimen collected from a patient. Instead of the patient specimen being added to the testing device, however, the control is added to it (Figure 33-6). The control results are compared with expected results provided in the product insert (see Figure 33-5) or on the container of the testing reagents (Figure 33-7). Diabetic patients who take insulin (insulin-dependent) must monitor their blood glucose levels each day. Based on the results of SMBG, decisions can be made regarding insulin and dietary adjustments that may be necessary to maintain normal glucose levels and to avoid the extremes of hypoglycemia and hyperglycemia (see Chapter 35). Satisfactory control of the blood glucose level on a day-to-day basis through SMBG reduces symptoms of the disease and helps delay or prevent long-term complications that can occur with diabetes.
Blood Chemistry and Immunology
Blood Chemistry
Collection of A Blood Chemistry Specimen

Automated Blood Chemistry Analyzers

Quality Control
Calibration
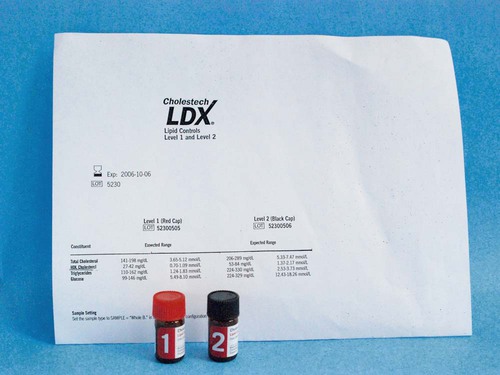
Controls
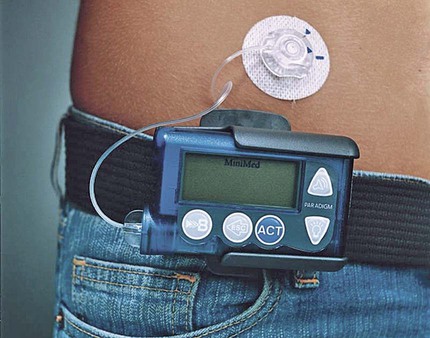
Blood Glucose
Blood Glucose Testing
Tests for Management of Diabetes
Self-Monitoring of Blood Glucose

Blood Chemistry and Immunology
Get Clinical Tree app for offline access
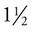 to 2 hours of glucose consumption, whereas the glucose level in a diabetic patient does not return to the fasting level. A postprandial glucose level of 140 g/dL or higher suggests diabetes and warrants further testing, such as the oral glucose tolerance test.
to 2 hours of glucose consumption, whereas the glucose level in a diabetic patient does not return to the fasting level. A postprandial glucose level of 140 g/dL or higher suggests diabetes and warrants further testing, such as the oral glucose tolerance test.