CHAPTER 86 The tetracyclines suppress bacterial growth by inhibiting protein synthesis. These drugs bind to the 30S ribosomal subunit, and thereby inhibit binding of transfer RNA to the messenger RNA–ribosome complex.* As a result, addition of amino acids to the growing peptide chain is prevented. At the concentrations achieved clinically, the tetracyclines are bacteriostatic. Tetracyclines are used topically and orally for severe acne vulgaris. Beneficial effects derive from suppressing the growth and metabolic activity of Propionibacterium acnes, an organism that secretes inflammatory chemicals. Oral doses for acne are relatively low. As a result, adverse effects are minimal. Acne is discussed at length in Chapter 105 (Drugs for the Skin). Helicobacter pylori, a bacterium that lives in the stomach, is a major contributing factor to peptic ulcer disease. Tetracyclines, in combination with metronidazole and bismuth subsalicylate, are a treatment of choice for eradicating this bug. The role of H. pylori in ulcer formation is discussed in Chapter 78 (Drugs for Peptic Ulcer Disease). Individual tetracyclines differ significantly in their pharmacokinetic properties. Of particular significance are differences in half-life and route of elimination. Also important is the degree to which food decreases absorption. The pharmacokinetic properties of individual tetracyclines are summarized in Table 86–1. TABLE 86–1 Pharmacokinetic Properties of the Tetracyclines *Percent absorbed when taken on an empty stomach. †Do not use in patients with renal impairment because the drug could accumulate to toxic levels. The tetracyclines can be divided into three groups: short acting, intermediate acting, and long acting (see Table 86–1). These differences are related to differences in lipid solubility: The only short-acting tetracycline (tetracycline) has relatively low lipid solubility, whereas the long-acting agents (doxycycline, minocycline) have relatively high lipid solubility. Ultimate elimination of short- and intermediate-acting tetracyclines—tetracycline and demeclocycline—is in the urine, largely as the unchanged drug (see Table 86–1). Because these agents undergo renal elimination, they can accumulate to toxic levels if the kidneys fail. Consequently, tetracycline and demeclocycline should not be given to patients with significant renal impairment. As discussed in Chapter 83, a suprainfection is an overgrowth with drug-resistant microbes, which occurs secondary to suppression of drug-sensitive organisms. Because the tetracyclines are broad-spectrum agents, and therefore can decrease viability of a wide variety of microbes, the risk of suprainfection is greater than with antibiotics that have a more narrow spectrum. For systemic therapy, tetracyclines may be administered orally, intravenously, and by IM injection. Oral administration is preferred, and all tetracyclines are available in oral formulations. As a rule, oral tetracyclines should be taken on an empty stomach (1 hour before meals or 2 hours after) and with a full glass of water. An interval of at least 2 hours should separate tetracycline ingestion and ingestion of products that can chelate these drugs (eg, milk, calcium or iron supplements, antacids). Three tetracyclines can be given IV (Table 86–2), but this route should be employed only when oral therapy cannot be tolerated or has proved inadequate. Intramuscular injection is extremely painful and used rarely. TABLE 86–2 Tetracyclines: Routes of Administration, Dosing Interval, and Dosage aDoses presented are for children over the age of 8 years. Use in children below this age may cause permanent staining of teeth. bThe intravenous route is used only if oral therapy cannot be tolerated or is inadequate. cIntramuscular injection is extremely painful and used only rarely. dFirst-day regimen is 100 mg initially, followed by 100 mg 12 hours later. eFirst-day regimen is 2.2 mg/kg initially, followed by 2.2 mg/kg 12 hours later. fFirst-day regimen is 200 mg in one or two slow infusions (1 to 4 hours). gFirst-day regimen is 4.4 mg/kg in one or two slow infusions (1 to 4 hours). hFirst-day regimen is 200 mg initially, followed by 100 mg 12 hours later. iFirst-day regimen is 4 mg/kg initially, followed by 2 mg/kg 12 hours later.
Bacteriostatic inhibitors of protein synthesis: tetracyclines, macrolides, and others
Tetracyclines
Mechanism of action
Therapeutic uses
Treatment of acne.
Peptic ulcer disease.
Pharmacokinetics

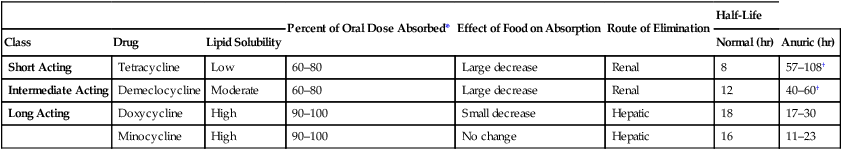
Percent of Oral Dose Absorbed*
Effect of Food on Absorption
Route of Elimination
Half-Life
Class
Drug
Lipid Solubility
Normal (hr)
Anuric (hr)
Short Acting
Tetracycline
Low
60–80
Large decrease
Renal
8
57–108†
Intermediate Acting
Demeclocycline
Moderate
60–80
Large decrease
Renal
12
40–60†
Long Acting
Doxycycline
High
90–100
Small decrease
Hepatic
18
17–30
Minocycline
High
90–100
No change
Hepatic
16
11–23
Duration of action.
Elimination.
Adverse effects
Suprainfection.
Dosage and administration
Administration.

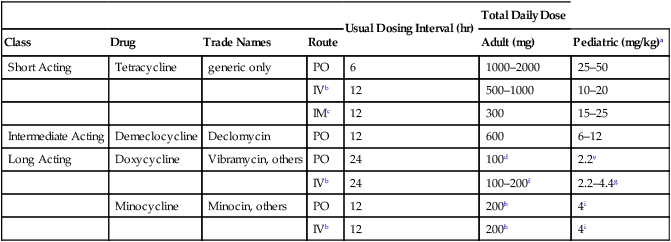
Usual Dosing Interval (hr)
Total Daily Dose
Class
Drug
Trade Names
Route
Adult (mg)
Pediatric (mg/kg)a
Short Acting
Tetracycline
generic only
PO
6
1000–2000
25–50
IVb
12
500–1000
10–20
IMc
12
300
15–25
Intermediate Acting
Demeclocycline
Declomycin
PO
12
600
6–12
Long Acting
Doxycycline
Vibramycin, others
PO
24
100d
2.2e
IVb
24
100–200f
2.2–4.4g
Minocycline
Minocin, others
PO
12
200h
4i
IVb
12
200h
4i
Bacteriostatic inhibitors of protein synthesis: tetracyclines, macrolides, and others
Get Clinical Tree app for offline access



