Chris Winkelman
Assessment of the Renal/Urinary System
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
11 Briefly review the relevant anatomy and physiology of the kidney and urinary system.
12 Describe age-related changes in the kidney and urinary system.
13 Describe the correct techniques to use in physically assessing the kidney and urinary system.
14 Use laboratory data to distinguish between dehydration and kidney impairment.
15 Describe how to obtain a sterile urine specimen from a urinary catheter.
16 Coordinate nursing care for the patient during the first 24 hours after IV urography.
17 Coordinate nursing care for the patient during the first 24 hours after a kidney biopsy.

http://evolve.elsevier.com/Iggy/
Animation: Filtration
Animation: Nephrons
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Key Points
Review Questions for the NCLEX® Examination
The kidneys help maintain health in many ways. Most important for urinary elimination, they maintain body fluid volume and composition and filter waste products for elimination. The kidneys also help regulate blood pressure and acid-base balance, produce erythropoietin for red blood cell (RBC) synthesis, and convert vitamin D to an active form.
The renal system includes the kidneys and the entire urinary tract. The ureters, bladder, and urethra are the drainage route for the excretion of urine. Structural or functional problems in the kidney or urinary tract may alter fluid, electrolyte, and acid-base balance.
Assessment of the patient at risk for or with actual problems of the kidney or urinary system begins with a history and physical assessment. Understanding the anatomy, physiology, and diagnostic tests of the renal system helps you in problem-solving about kidney function in the clinical setting. It also assists you in teaching the patient about the purpose of procedures and in physically and emotionally preparing the patient for assessment.
Anatomy and Physiology Review
Kidneys
Structure
Gross Anatomy.
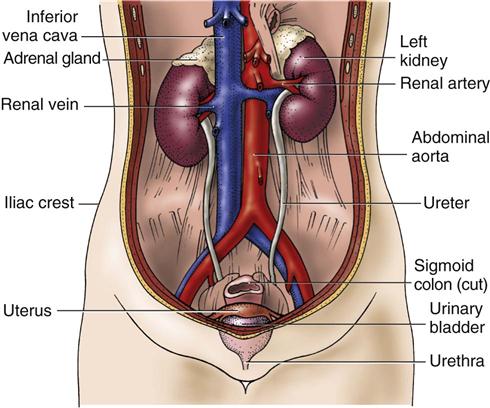
Normally, two kidneys are located behind the peritoneum, not really in the abdominal cavity, one on either side of the spine (Fig. 68-1). The adult kidney is 4 to 5 inches (10 to 13 cm) long, 2 to 3 inches (5 to 7 cm) wide, and about 1 inch (2.5 to 3 cm) thick. It weighs about 8 ounces (250 g). The left kidney is slightly longer and narrower than the right kidney. Larger-than-usual kidneys may indicate obstruction or polycystic disease. Smaller-than-usual kidneys may indicate chronic kidney disease (CKD).
Variation in kidney shape and number is not uncommon and does not necessarily mean there is also a problem in kidney function. Some people have more than two kidneys or may have only one, large, horseshoe-shaped kidney. As long as tests of kidney function are normal, these variations are of no significance.
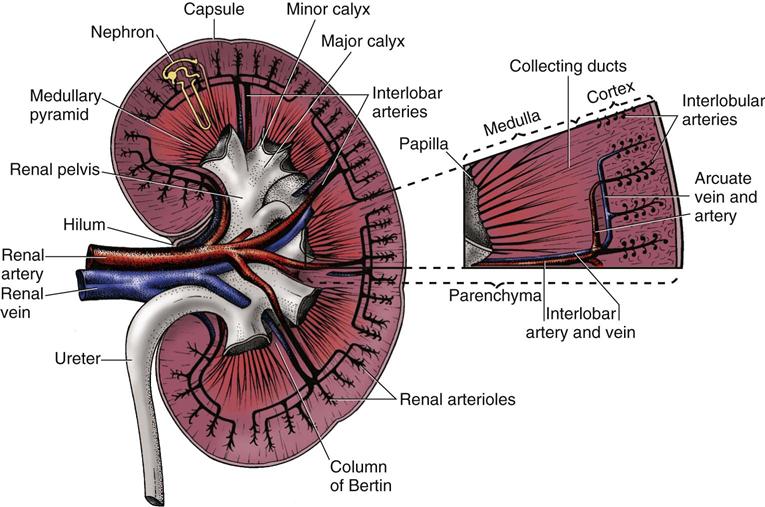
Several layers of tissue surround the kidney, providing protection and support. On the outer surface of the kidney is a layer of fibrous tissue called the capsule (Fig. 68-2). This capsule covers most of the kidney except the hilum, which is the area where the renal artery and nerve plexus enter and the renal vein and ureter exit. The renal capsule is surrounded by layers of fat and connective tissue.
Lying beneath the renal capsule are the two layers of functional kidney tissue—the cortex and the medulla. The renal cortex is the outer tissue layer and is covered by the renal capsule. The medulla is the medullary tissue lying below the cortex in the shape of many fans. Each “fan” is called a pyramid, and there are 12 to 18 pyramids per kidney. The renal columns are cortical tissue that dips down into the interior of the kidney and separates the pyramids.
The tip, or end, of each pyramid is called a papilla. The papillae drain urine into the collecting system. A cuplike structure called a calyx collects the urine at the end of each papilla. The calices join together to form the renal pelvis, which narrows to become the ureter.
The kidneys have a rich blood supply and receive 20% to 25% of the total cardiac output. Blood flow to the kidneys varies from about 600 to 1300 mL/min. The blood supply to each kidney comes from the renal artery, which branches from the abdominal aorta. The renal artery divides into progressively smaller arteries, supplying all blood to areas of the kidney tissue (parenchyma) and the nephrons. The smallest arteries (afferent arterioles) feed the nephrons directly to form urine.
Venous blood from the kidneys starts with the capillaries surrounding each nephron. These capillaries drain into progressively larger veins, with blood eventually returned to the inferior vena cava through the renal vein.
Microscopic Anatomy.
The nephron is the “working” or functional unit of the kidney, and it is here that urine is actually formed from blood. There are about 1 million nephrons per kidney, and each nephron separately makes urine from blood.
There are two types of nephrons: cortical nephrons and juxtamedullary nephrons. The cortical nephrons are short, with all parts located in the renal cortex. The juxtamedullary nephrons (about 20% of all nephrons) are longer, and their tubes and blood vessels dip deeply into the medulla. The purpose of the juxtamedullary nephrons is to concentrate urine during times of low fluid intake. The ability to concentrate urine allows for continued excretion of body wastes with less fluid loss.
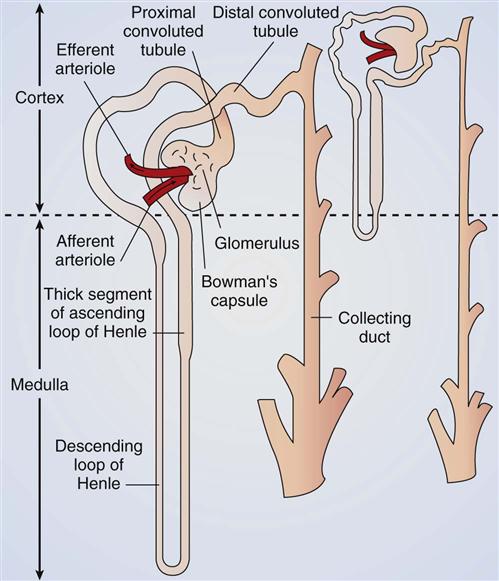
Blood supply to the nephron is delivered through the afferent arteriole—the smallest, most distal portion of the renal arterial system. From the afferent arteriole, blood flows into the glomerulus, which is a series of specialized capillary loops. It is through these capillaries that water and small particles are filtered from the blood to make urine. The remaining blood leaves the glomerulus through the efferent arteriole, which is the first vessel in the venous system of the kidney. From the efferent arteriole, blood exits into one of two additional capillary systems:
• The peritubular capillaries around the tubular part of the cortical nephrons
• The vasa recta around the tubular part of juxtamedullary nephrons
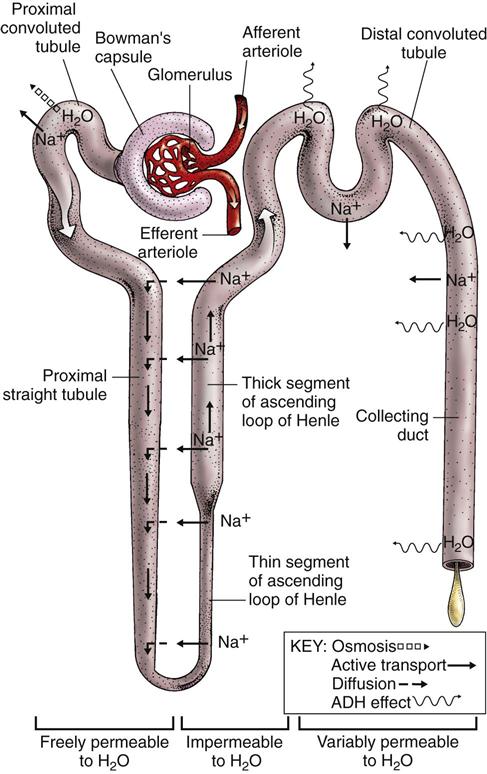
Each nephron is a tubelike structure with distinct parts (Fig. 68-3). The tube begins with Bowman’s capsule, a saclike structure that surrounds the glomerulus. The tubular tissue of Bowman’s capsule narrows into the proximal convoluted tubule (PCT). The PCT twists and turns, finally straightening into the descending limb of the loop of Henle. The descending loop of Henle dips in the direction of the medulla but forms a hairpin loop and comes back up into the cortex as the ascending loop of Henle.
There are two segments of the ascending limb of the loop of Henle: the thin segment and the thick segment. The distal convoluted tubule (DCT) forms from the thick segment of the ascending limb of the loop of Henle. The DCT ends in one of many collecting ducts located in the kidney tissue. The urine in the collecting ducts passes through the papillae and empties into the renal pelvis.
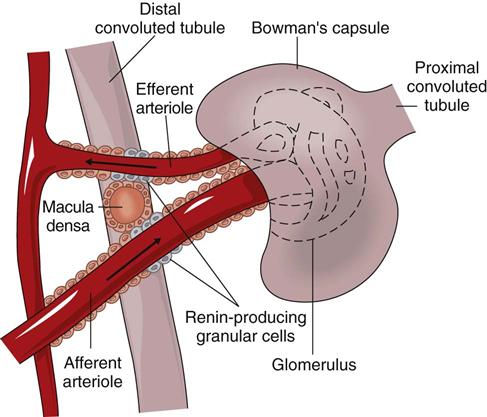
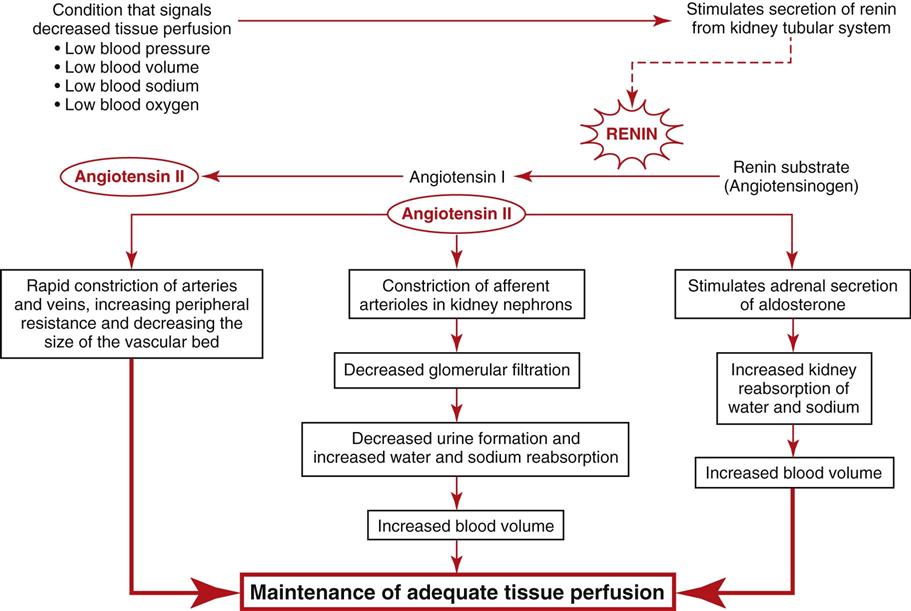
Special cells in the afferent arteriole, efferent arteriole, and DCT are known as the juxtaglomerular complex (Fig. 68-4). These specialized cells produce and store renin. Renin is a hormone that helps regulate blood flow, glomerular filtration rate (GFR), and blood pressure. Renin is secreted when sensing cells in the DCT (called the macula densa) sense changes in blood volume and pressure. The macula densa lies next to the renin-producing cells. Renin is produced when the macula densa cells sense that blood volume, blood pressure, or blood sodium level is low. Renin then converts renin substrate (angiotensinogen) into angiotensin I. This leads to a series of reactions that cause secretion of the hormone aldosterone (Fig. 68-5). Aldosterone increases kidney reabsorption of sodium and water, restoring blood pressure, blood volume, and blood sodium levels. It also promotes excretion of potassium. (See Chapter 13 for more discussion of the renin-angiotensin-aldosterone pathway.)
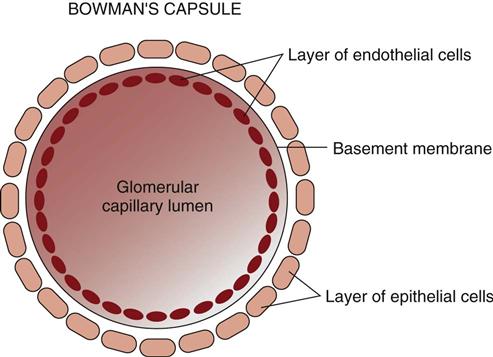
The glomerular capillary wall has three layers (Fig. 68-6): the endothelium, the basement membrane, and the epithelium. The endothelial and epithelial cells lining these capillaries are separated by pores that filter water and small particles from the blood into Bowman’s capsule. This fluid is called the “filtrate” or “early urine.”
Function
The kidneys have both regulatory and hormonal functions. The regulatory functions control fluid, electrolyte, and acid-base balance. The hormonal functions control red blood cell (RBC) formation, blood pressure, and vitamin D activation.
Regulatory Functions.
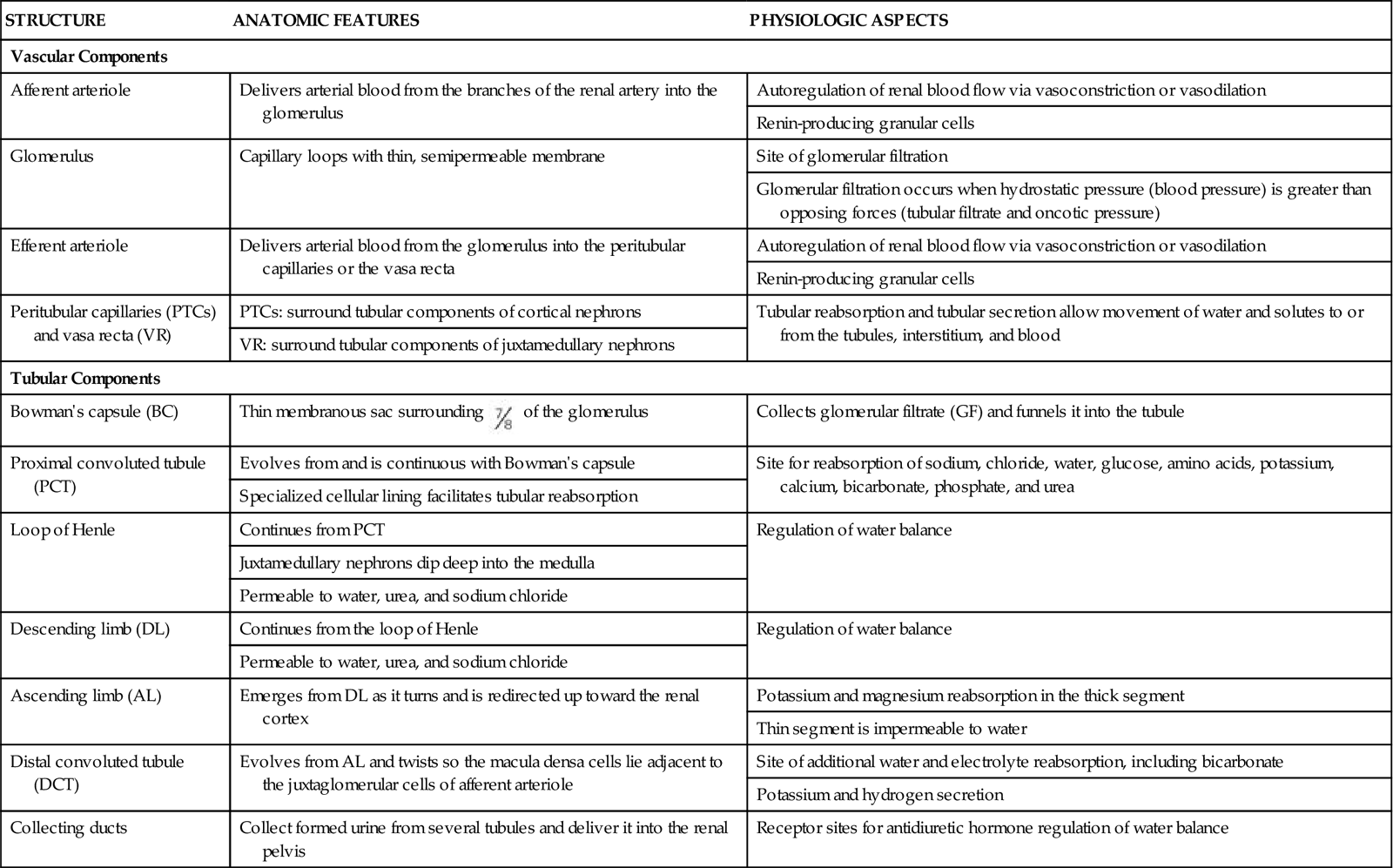
The kidney processes that maintain fluid, electrolyte, and acid-base balance are glomerular filtration, tubular reabsorption, and tubular secretion. These processes use filtration, diffusion, active transport, and osmosis. (See Chapter 13 for a review of these actions.) Table 68-1 lists the functions of nephron tubules and blood vessels.
TABLE 68-1
VASCULAR AND TUBULAR COMPONENTS OF THE NEPHRON
| STRUCTURE | ANATOMIC FEATURES | PHYSIOLOGIC ASPECTS |
| Vascular Components | ||
| Afferent arteriole | Delivers arterial blood from the branches of the renal artery into the glomerulus | Autoregulation of renal blood flow via vasoconstriction or vasodilation |
| Renin-producing granular cells | ||
| Glomerulus | Capillary loops with thin, semipermeable membrane | Site of glomerular filtration |
| Glomerular filtration occurs when hydrostatic pressure (blood pressure) is greater than opposing forces (tubular filtrate and oncotic pressure) | ||
| Efferent arteriole | Delivers arterial blood from the glomerulus into the peritubular capillaries or the vasa recta | Autoregulation of renal blood flow via vasoconstriction or vasodilation |
| Renin-producing granular cells | ||
| Peritubular capillaries (PTCs) and vasa recta (VR) | PTCs: surround tubular components of cortical nephrons | Tubular reabsorption and tubular secretion allow movement of water and solutes to or from the tubules, interstitium, and blood |
| VR: surround tubular components of juxtamedullary nephrons | ||
| Tubular Components | ||
| Bowman’s capsule (BC) | Thin membranous sac surrounding 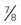 of the glomerulus of the glomerulus | Collects glomerular filtrate (GF) and funnels it into the tubule |
| Proximal convoluted tubule (PCT) | Evolves from and is continuous with Bowman’s capsule | Site for reabsorption of sodium, chloride, water, glucose, amino acids, potassium, calcium, bicarbonate, phosphate, and urea |
| Specialized cellular lining facilitates tubular reabsorption | ||
| Loop of Henle | Continues from PCT | Regulation of water balance |
| Juxtamedullary nephrons dip deep into the medulla | ||
| Permeable to water, urea, and sodium chloride | ||
| Descending limb (DL) | Continues from the loop of Henle | Regulation of water balance |
| Permeable to water, urea, and sodium chloride | ||
| Ascending limb (AL) | Emerges from DL as it turns and is redirected up toward the renal cortex | Potassium and magnesium reabsorption in the thick segment |
| Thin segment is impermeable to water | ||
| Distal convoluted tubule (DCT) | Evolves from AL and twists so the macula densa cells lie adjacent to the juxtaglomerular cells of afferent arteriole | Site of additional water and electrolyte reabsorption, including bicarbonate |
| Potassium and hydrogen secretion | ||
| Collecting ducts | Collect formed urine from several tubules and deliver it into the renal pelvis | Receptor sites for antidiuretic hormone regulation of water balance |
Glomerular filtration is the first process in urine formation. As blood passes from the afferent arteriole into the glomerulus, water, electrolytes, and other small particles (e.g., creatinine, urea nitrogen, glucose) are filtered across the glomerular membrane into the Bowman’s capsule to form glomerular filtrate. As the filtrate enters the proximal convoluted tubule (PCT), it is called tubular filtrate.
Large particles, such as blood cells, albumin, and other proteins, are too large to filter through the glomerular capillary walls. Therefore these substances are not normally present in the filtrate or in the final urine.
About 180 L of glomerular filtrate is formed from the blood each day. The rate of filtration is expressed in milliliters per minute. Normal glomerular filtration rate (GFR) averages 125 mL/min. If the entire amount of filtrate were excreted as urine, death would occur quickly from dehydration. Actually, only about 1 to 3 L is excreted each day as urine. The rest is reabsorbed back into the circulatory system.
The GFR is controlled by blood pressure and blood flow. The ability of the kidneys to self-regulate renal blood pressure and renal blood flow keeps GFR constant. GFR is controlled by selectively constricting and dilating the afferent and efferent arterioles. When the afferent arteriole is constricted or the efferent arteriole is dilated, pressure in the glomerular capillaries falls and filtration decreases. When the afferent arteriole is dilated or the efferent arteriole is constricted, pressure in the glomerular capillaries rises and filtration increases. Through this process, the kidney can maintain a constant GFR, even when systemic blood pressure changes. When systolic pressure drops below about 70 mm Hg, these processes cannot compensate and GFR stops.
Tubular reabsorption is the second process involved in urine formation. This reabsorption of most of the filtrate keeps normal urine output at 1 to 3 L/day and prevents dehydration. As the filtrate passes through the tubular parts of the nephron, most of the water and electrolytes is reabsorbed. Reabsorption returns particles (solutes) and water to the blood. Reabsorption occurs from the filtrate across the tubular lumen of the nephron and into the blood of the peritubular capillaries. The PCT reabsorbs about 65% of the total glomerular filtrate.
The tubules return about 99% of all filtered water back into the body (Fig. 68-7). Most water reabsorption occurs as the filtrate passes through the PCT. Water reabsorption continues as the filtrate flows down the descending loop of Henle. The thin and thick segments of the ascending loop of Henle are not permeable to water, and water reabsorption does not occur here.
The distal convoluted tubule (DCT) can be permeable to water, and some water reabsorption can occur as the filtrate continues to flow through the tubule. The membrane of the DCT may be made more permeable to water through the action of antidiuretic hormone (ADH) and aldosterone. ADH increases tubular permeability to water, allowing water to leave the tube and be reabsorbed into the capillaries. ADH is also known as vasopressin and affects arteriole constriction. Arteriole constriction alters blood pressure, which, in turn, affects the amount of fluid and solutes that exit glomeruli capillaries. Aldosterone promotes the reabsorption of sodium in the DCT. Water reabsorption occurs as a result of the movement of sodium (where sodium goes, water follows).
The ability of the kidneys to vary the volume or concentration of urine helps regulate water balance regardless of fluid intake. In this way, the healthy kidney can prevent dehydration when fluid intake is low and can prevent circulatory overload when fluid intake is excessive.
In addition to water, some particles in the tubular filtrate also are returned to the blood. This process is called tubular reabsorption and is selective. About 50% of all urea in the early urine is reabsorbed. On the other hand, no creatinine is reabsorbed.
Most sodium, chloride, and water reabsorption occurs in the PCT. The collecting ducts are the other site of sodium, chloride, and water reabsorption. Here reabsorption is caused by aldosterone. Potassium is also mostly reabsorbed in the PCT, with an additional 20% to 40% reabsorbed in the thick segment of the loop of Henle.
Bicarbonate, calcium, and phosphate are mostly reabsorbed in the PCT. Bicarbonate reabsorption helps balance acids and maintain a normal blood pH. Blood levels of calcitonin and parathyroid hormone (PTH) (see Chapters 13 and 66) control calcium balance.
The kidney reabsorbs some of the glucose filtered from the blood. However, there is a limit to how much glucose the kidney can reabsorb. This limit is called the renal threshold for glucose reabsorption or the transport maximum for glucose reabsorption. The usual renal threshold for glucose is about 220 mg/dL. This means that at a blood glucose level of 220 mg/dL or less, all glucose is reabsorbed and returned to the blood, with no glucose present in final urine. When blood glucose levels are greater than 220 mg/dL, some glucose stays in the filtrate and is present in the urine. Normally, almost all glucose and any amino acids or proteins are reabsorbed and are not present in the urine.
Tubular secretion is the third process involved in urine formation. Like glomerular filtration, it allows substances to move from the blood into the early urine. During tubular secretion, substances move from the peritubular capillaries in reverse, across capillary membranes, and into the cells that line the tubules. From the cells, these substances are moved into the urine and are excreted from the body. Potassium (K+) and hydrogen (H+) ions are some of the substances moved in this way to maintain homeostasis of electrolytes and pH.
Hormonal Functions.
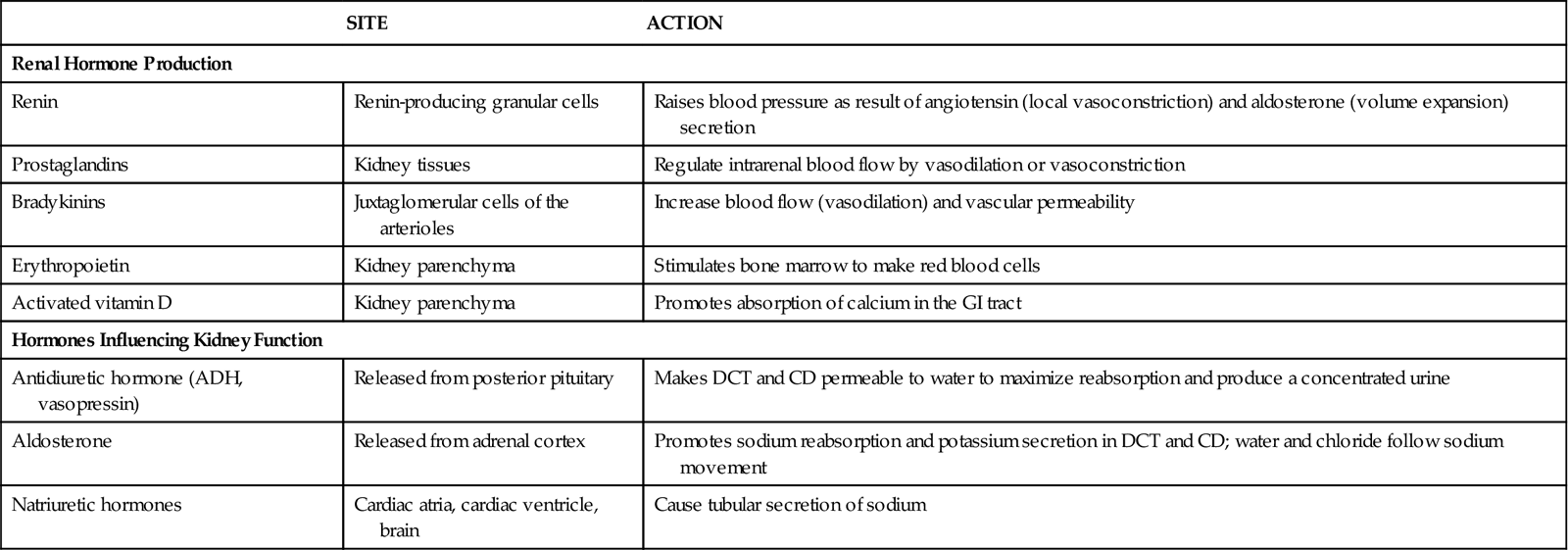
The kidneys produce renin, prostaglandins, bradykinin, erythropoietin, and activated vitamin D (Table 68-2). Other kidney products, such as the kinins, change kidney blood flow and capillary permeability. The kidneys also help break down and excrete insulin.
TABLE 68-2
RENAL HORMONE PRODUCTION AND HORMONES INFLUENCING RENAL FUNCTION
| SITE | ACTION | |
| Renal Hormone Production | ||
| Renin | Renin-producing granular cells | Raises blood pressure as result of angiotensin (local vasoconstriction) and aldosterone (volume expansion) secretion |
| Prostaglandins | Kidney tissues | Regulate intrarenal blood flow by vasodilation or vasoconstriction |
| Bradykinins | Juxtaglomerular cells of the arterioles | Increase blood flow (vasodilation) and vascular permeability |
| Erythropoietin | Kidney parenchyma | Stimulates bone marrow to make red blood cells |
| Activated vitamin D | Kidney parenchyma | Promotes absorption of calcium in the GI tract |
| Hormones Influencing Kidney Function | ||
| Antidiuretic hormone (ADH, vasopressin) | Released from posterior pituitary | Makes DCT and CD permeable to water to maximize reabsorption and produce a concentrated urine |
| Aldosterone | Released from adrenal cortex | Promotes sodium reabsorption and potassium secretion in DCT and CD; water and chloride follow sodium movement |
| Natriuretic hormones | Cardiac atria, cardiac ventricle, brain | Cause tubular secretion of sodium |
Renin, as discussed on p. 1468 in the Microscopic Anatomy section, assists in blood pressure control. It is formed and released when there is a decrease in blood flow, blood volume, or blood pressure through the renal arterioles or when too little sodium is present in kidney blood. These conditions are detected through the receptors of the juxtaglomerular complex.
Renin release causes the production of angiotensin II through a series of steps (see Fig. 68-5). Angiotensin II increases systemic blood pressure through powerful blood vessel constricting effects and triggers the release of aldosterone from the adrenal glands. Aldosterone increases the reabsorption of sodium in the distal tubule of the nephron. Therefore more water is reabsorbed and blood pressure is increased because of increases in blood volume. When blood flow to the kidney is reduced, this system also regulates pressures in the nephron to prevent fluid loss and maintain circulating blood volume (see also Chapter 13).
Prostaglandins are produced in the kidney and many other tissues. Those produced specifically in the kidney are prostaglandin E2 (PGE2) and prostacyclin (PGI2). These substances help regulate glomerular filtration, kidney vascular resistance, and renin production. PGE2 acts on the distal tubule and collecting duct to increase sodium and water excretion.
Bradykinin is released by the kidney in response to the presence of angiotensin II, prostaglandins, and ADH. It is a small hormone that dilates the afferent arteriole and increases capillary membrane permeability to some solutes. These actions maintain kidney blood flow and reabsorption even when other conditions cause systemic blood vessel constriction.
Erythropoietin is produced and released in response to decreased oxygen tension in the kidney’s blood supply. It triggers red blood cell (RBC) production in the bone marrow. When kidney function is poor, erythropoietin production decreases and the person becomes anemic.
Vitamin D activation occurs through a series of steps. Some of these steps take place in the skin when it is exposed to sunlight, and then more processing occurs in the liver. From there, vitamin D is converted to its active form (1,25-dihydroxy-cholecalciferol) in the kidney. It is needed to absorb calcium in the intestinal tract and to regulate calcium balance.
Ureters
Each kidney has a single ureter—a hollow tube that connects the renal pelvis with the urinary bladder. The ureter is about 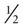 inch (1.25 cm) in diameter and about 12 to 18 inches (30 to 45 cm) in length. The diameter of the ureter narrows in three areas:
inch (1.25 cm) in diameter and about 12 to 18 inches (30 to 45 cm) in length. The diameter of the ureter narrows in three areas:
The ureter tunnels through bladder tissue for a short distance and then opens into the bladder at the trigone (Fig. 68-8).
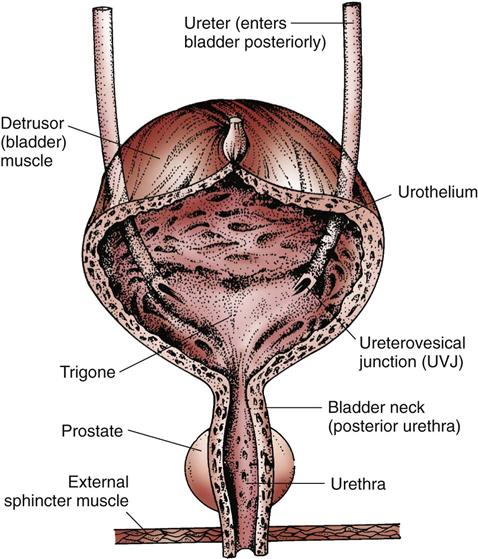
The ureter has three layers: an inner lining of mucous membrane (urothelium), a middle layer of smooth muscle fibers, and an outer layer of fibrous tissue. The outer layer contains the blood supply. The middle layer of muscle fibers is controlled by several nerve pathways from the lower spinal cord.
Contractions of the smooth muscle in the ureter move urine from the renal pelvis of the kidney to the bladder. Stretch receptors in the renal pelvis regulate this movement. For example, a large volume of urine in the renal pelvis triggers the stretch receptors, which respond by increasing ureteral contractions and ureter peristalsis.
Urinary Bladder
Structure
The urinary bladder is a muscular sac (see Fig. 68-8). The upper surface lies next to the peritoneal cavity. In men, the bladder is in front of the rectum. In women, it is in front of the vagina. The bladder lies directly behind the pubic bone.
The bladder is composed of the body (the rounded sac portion) and the bladder neck (posterior urethra), which connects to the bladder body. The bladder has three linings—an inner lining of epithelial cells (urothelium), middle layers of smooth muscle (detrusor muscle), and an outer lining. The trigone is an area on the posterior wall between the points of ureteral entry (ureterovesical junctions [UVJs]) and the urethra.
The internal urethral sphincter is the smooth detrusor muscle of the bladder neck and elastic tissue. The external urethral sphincter is skeletal muscle that surrounds the urethra. In men, the external sphincter surrounds the urethra at the base of the prostate gland. In women, the external sphincter is at the base of the bladder. The pudendal nerve from the spinal cord controls the external sphincter.
Function
The bladder is a temporary urine storage site. The bladder also provides continence and enables voiding. The secretions of the bladder lining resist bacteria.
Continence is the ability to voluntarily control bladder emptying. Bladder continence occurs during bladder filling through the combination of detrusor muscle relaxation, internal sphincter muscle tone, and external sphincter contraction. As the bladder fills with urine, stretch sensations are transmitted to spinal sacral nerves S2 and S3.
Maintaining continence occurs by the interaction of the nerves that control the muscles of the bladder, bladder neck, urethra, and pelvic floor, as well as by factors that close the urethra. During bladder filling, the sympathetic nervous system fibers prevent detrusor muscle contraction. These control centers are located in the cerebral cortex, the brainstem, and the sacral part of the spinal cord. For urethral closure to be adequate for continence, the mucosal surfaces must be in contact and must be adhesive. Contact depends on the presence and proper function of the involved nerves and muscles. Adhesion depends on the adequate secretion of mucus-like substances.
Micturition (voiding) is a reflex of autonomic control that triggers contraction of the detrusor muscle at the same time as relaxation of the external sphincter and the muscles of the pelvic floor. With detrusor muscle contraction, the UVJ of the ureter closes and the normally round bladder assumes the shape of a funnel. Voiding is a voluntary act as the result of a learned response and is controlled by the cerebral cortex and the brainstem. Contraction of the external sphincter inhibits the micturition reflex and prevents voiding.
Urethra
The urethra is a narrow tube lined with mucous membranes and epithelial cells. Its purpose is to eliminate urine from the bladder. The urethral meatus, or opening, is the endpoint of the urethra. In men, the urethra is about 6 to 8 inches (15 to 20 cm) long, with the meatus located at the tip of the penis. The male urethra has three sections:
• The prostatic urethra, which extends from the bladder to the prostate gland
• The membranous urethra, which extends to the wall of the pelvic floor
• The cavernous urethra, which is external and extends through the length of the penis
In women, the urethra is about 1 to 1.5 inches (2.5 to 3.75 cm) long and exits the bladder through the pelvic floor. The meatus lies slightly below the clitoris and directly in front of the vagina and rectum.
Kidney and Urinary System Changes Associated with Aging
Kidney Changes
Structural and functional changes occur in the kidney as a result of the aging process. These changes often affect health. The kidney loses cortical tissue and gets smaller by 80 years of age. This cortical loss is caused by reduced blood flow to the kidney. The medulla is not affected by aging, and the juxtamedullary nephron functions are preserved. However, the glomerular and tubular linings thicken. Both the number of glomeruli and their surface areas decrease with aging. Tubule length also decreases. These changes reduce the ability of the older adult to filter blood and excrete waste products.
Kidney size and function decrease with aging (Chart 68-1). Blood flow to the kidney declines by about 10% per decade as blood vessels thicken. This means that blood flow to the kidney is not as adaptive in older adults compared with younger adults, leaving nephrons more vulnerable to damage during episodes of either hypotension or hypertension.
Glomerular filtration rate (GFR) decreases with age, especially after 45 years of age. By age 65, the GFR is about 65 mL/min (half the rate of a young adult). This decline is more rapid in patients with diabetes, hypertension, or heart failure. As a result, the older patient is at greater risk for fluid overload. The combination of reduced kidney mass, reduced blood flow, and decreased GFR contributes to reduced drug clearance and a greater risk for drug reactions and kidney damage from drugs and contrast dyes in older adults.
Tubular changes with aging decrease the ability to concentrate urine, resulting in urgency (a sense of a nearly uncontrollable need to urinate) and nocturnal polyuria (increased urination at night). The regulation of sodium, acids, and bicarbonate remains effective but is less efficient. Along with an age-related impairment in the thirst mechanism, these changes increase the risk for dehydration and hypernatremia (increased blood sodium levels) in the older adult. Hormonal changes include a decrease in renin secretion, aldosterone levels, and activation of vitamin D.
Urinary Changes
Changes in the detrusor muscle elasticity lead to decreased bladder capacity and reduced ability to retain urine. The urge to void may cause immediate bladder emptying because the urinary sphincters lose tone and often become weaker with age. In women, weakened muscles shorten the urethra and promote incontinence. In men, an enlarged prostate gland makes starting the urine stream difficult and may cause urinary retention.
Assessment Methods
Patient History
One way to assess kidney and urologic function is to use Gordon’s Functional Health Patterns (Gordon, 2011). The patterns most related to the renal system are Nutritional/Metabolic and Elimination (Chart 68-2).
Demographic information, such as age, gender, race, and ethnicity, is important to consider as nonmodifiable risk factors in the patient with any kidney or urinary problem. A sudden onset of hypertension in patients older than 50 years suggests possible kidney disease. Clinical changes with adult polycystic kidney disease typically occur in patients in their 40s or 50s. In men older than 50 years, altered urinary patterns accompany prostate disease.
Anatomic gender differences make some disorders worse or more common. For example, men rarely have urinary tract infections unless there are abnormalities, such as ureteral reflux or prostatic enlargement. Women have a shorter urethra and more commonly develop cystitis (bladder infection) because bacteria pass more readily into the bladder.
Ask the patient about any previous kidney or urologic problems, including tumors, infections, stones, or urologic surgery. A history of any chronic health problems, especially diabetes mellitus or hypertension, increases the risk for development of kidney disease because these disorders damage kidney blood vessels.
Ask the patient about chemical exposures at the workplace or with hobbies. Exposure to hydrocarbons (e.g., gasoline, oil), heavy metals (especially mercury and lead), and some gases (e.g., chlorine, toluene) can impair kidney function. Use this opportunity to teach patients who come into contact with chemicals at work or during leisure time activities to avoid direct skin or mucous membrane contact with these chemicals. Use of heroin, cocaine, methamphetamine, ecstasy, and volatile solvents (inhalants) has also been associated with kidney damage.
Specifically ask the patient whether he or she has ever been told about the presence of protein or albumin in the urine. The question “Have you ever been told that your blood pressure is high?” may prompt a response different from the one to the question “Do you have high blood pressure?” Ask women about health problems during pregnancy (e.g., proteinuria, high blood pressure, gestational diabetes, urinary tract infections). Obtain information about:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree