Chapter 14 Assessment and Care of Patients with Acid-Base Imbalances
Safe and Effective Care Environment
1. Identify patients at risk for falls as a result of acid-base imbalances, especially older adults.
2. Describe the relationship between free hydrogen ion level and pH.
3. Explain the concept of compensation.
4. Compare the roles of the respiratory system and the kidneys in maintaining acid-base balance.
5. Use laboratory data and clinical manifestations to determine the presence of acid-base imbalances.
6. Interpret arterial blood gases to determine whether acidosis is respiratory or metabolic in origin.
7. Prioritize nursing care for the patient with an acid-base imbalance.
http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Review Questions for the NCLEX® Examination
The normal free hydrogen ion level of blood and other body fluids is quite low (less than 0.0001 mEq/L) compared with the levels of other electrolytes (see Chapter 13). Because it is so low, it is not measured directly but, instead, is a calculated value. The pH value is calculated as the negative logarithm of the concentration in milliequivalents per liter. Because it is calculated in negative logarithm units, the value of pH is inversely related (negatively related) to the level of free hydrogen ions. In other words, the lower the pH value of a fluid, the higher the level of free hydrogen ions in that fluid. The pH of a solution may range from 1 (as acidic as possible) to 14 (as alkaline as possible), with 7 being neutral. A change of 1 pH unit actually represents a tenfold change in free hydrogen ion level. Therefore any pH unit change (e.g., a change from 7.4 to 7.3) represents a very large increase in the free hydrogen ion level.
• Changing the shape and reducing the function of hormones and enzymes
• Changing the distribution of other electrolytes, causing fluid and electrolyte imbalances
• Changing excitable membranes, making the heart, nerves, muscles, and GI tract either less or more active than normal
Fortunately, the body has many mechanisms to ensure minimal changes in free hydrogen ion level.
Acid-Base Balance
As discussed in Chapter 13, body fluids are electrically neutral even though they contain ions with overall positive charges and ions with overall negative charges. When fluids contain an equal number of positive and negative charges, the electrical charge of the fluid is balanced and remains neutral. The body keeps blood pH between 7.35 and 7.45 in a similar manner; however, this value is not strictly neutral (7.0 is neutral) but, rather, is slightly alkaline. Normal body fluid pH remains at a near-neutral value when the acids and bases are nearly balanced, limiting the total number of free or unbalanced hydrogen ions. Acid-base balance occurs by matching the rate of hydrogen ion production (which is a continuous normal process) with hydrogen ion loss.
Acid-Base Chemistry
Acids
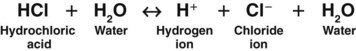
Acids are substances that release hydrogen ions when dissolved in water (H2O). An acid in solution increases the amount of free hydrogen ions in that solution. The strength of an acid is measured by how easily it releases a hydrogen ion in solution. A strong acid, such as hydrochloric acid (HCl), separates (dissociates) completely in water and readily releases all of its hydrogen ions (Fig. 14-1).
A weak acid does not completely separate in water; it releases only some of its hydrogen ions. In the following example, each molecule of acetic acid (CH3COOH), a weak acid, contains a total of four hydrogen molecules. When acetic acid combines with water (Fig. 14-2), it releases only one of its four hydrogen molecules. The other three hydrogen molecules remain bound to the acetic acid molecule (CH3COO−).
Buffers
Buffers are critical in keeping body fluid pH at normal levels because they can react in two ways: either as an acid (releasing a hydrogen ion) or as a base (binding a hydrogen ion). How a buffer reacts when dissolved in water depends on the existing acid-base balance of that fluid. Buffers always try to bring the fluid as close as possible to the normal body fluid pH of 7.35 to 7.45. If the fluid is basic (with few free hydrogen ions), the buffer releases hydrogen ions into the fluid (Fig. 14-3). If the fluid is acidic (with many free hydrogen ions), the buffer acts as a base, binding some of the excess hydrogen ions. In this way, buffers act like hydrogen ion “sponges,” soaking up hydrogen ions when too many are present and squeezing out hydrogen ions when too few are present. This flexibility allows buffers to keep body fluid pH in the normal range.
Liquids with a pH of 7.0 are neutral; they have a free hydrogen ion level in which the amount and strength of acids and bases are equal. Fig. 14-4 shows the concept of neutral pH. This figure shows that the combined strength and amount of all acids are equal to the combined strength and amount of all bases in a given solution. This is close to the actual case in human physiology. With human acid-base homeostasis, the relative amounts and strengths of acids and bases are nearly equal and, normally, hydrogen ion production is balanced with hydrogen ion loss so that the overall free hydrogen ion levels remain constant.
Liquids with a pH ranging from 1.0 to 6.99 have more or stronger (or both) acids compared with the amount or strength (or both) of bases. These liquids are acidic (see Fig. 14-4), which means that more free hydrogen ions are being released than bound, increasing the amount of free hydrogen ions in the liquid.
Liquids with a pH ranging from 7.01 to 14.0 have more or stronger (or both) bases compared with the amount or strength (or both) of acids. These liquids are basic, which means that more hydrogen ions are being bound than released, decreasing the amount of free hydrogen ions (Fig. 14-5).
Body Fluid Chemistry
Bicarbonate Ions
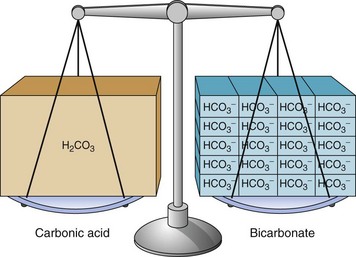
Body fluids contain many different types of acids and a few types of bases. The most common base in human body fluid is bicarbonate (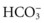 ); the most common acid is carbonic acid (H2CO3). In health, the body keeps these substances at a constant ratio of 1 molecule of carbonic acid to 20 free bicarbonate ions (1 : 20) (Fig. 14-6). To maintain this ratio, both carbonic acid and bicarbonate must be carefully controlled. This constant ratio is related to balancing the production and elimination of carbon dioxide (CO2) and hydrogen ions (H+).
); the most common acid is carbonic acid (H2CO3). In health, the body keeps these substances at a constant ratio of 1 molecule of carbonic acid to 20 free bicarbonate ions (1 : 20) (Fig. 14-6). To maintain this ratio, both carbonic acid and bicarbonate must be carefully controlled. This constant ratio is related to balancing the production and elimination of carbon dioxide (CO2) and hydrogen ions (H+).
Relationship Between Carbon Dioxide and Hydrogen Ions
A key concept in understanding acid-base balance is the carbonic anhydrase equation. This equation, driven by the enzyme carbonic anhydrase, shows how hydrogen ion levels and carbon dioxide levels are directly related to one another, so that an increase in one causes an equal increase in the other (Fig. 14-7).
When excess carbon dioxide is produced, the amount of carbon dioxide increases and the equation shifts to the right, causing an increase in hydrogen ions (and a decrease in pH), as shown in Fig. 14-8. When very little carbon dioxide is produced, no free hydrogen ions are created by this equation. When excess hydrogen ions are produced or brought into the body, the carbonic anhydrase equation shifts to the left, causing the creation of more carbon dioxide, as shown in Fig. 14-9. When the amount of free hydrogen ions in body fluids is low, no extra carbon dioxide is produced.
Because the kidneys control bicarbonate levels and the lungs control CO2 levels in the healthy person, pH is also described as the function of the kidneys divided by the function of the lungs (Fig. 14-10).
Acid-Base Regulatory Actions and Mechanisms
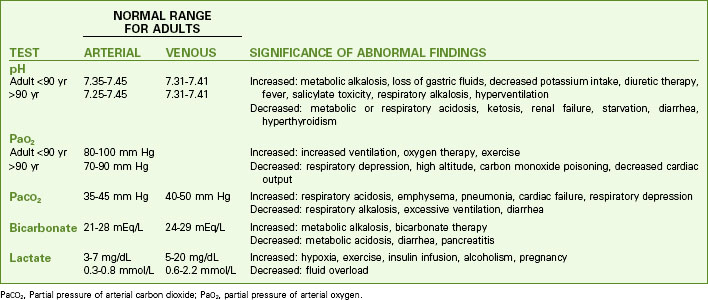
As long as body cells are healthy, they continuously produce acids, carbon dioxide, and free hydrogen ions. Despite this production, hydrogen ion, bicarbonate, oxygen, and carbon dioxide levels are kept within normal limits when acid-base controlling actions/mechanisms are normal. Chart 14-1 lists normal values for these substances in arterial and venous blood. This homeostasis depends on:
• Hydrogen ion production being consistent and not excessive
• CO2 loss from the body through breathing keeping pace with all forms of hydrogen ion production
Acid-Base Assessment
PaCO2, Partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen.
To keep the free hydrogen ion level (pH) of the ECF within the narrow range of normal, the body has chemical, respiratory, and kidney actions (mechanisms) for acid-base balance (Table 14-1).
TABLE 14-1 ACID-BASE REGULATORY MECHANISMS
| MECHANISM TYPE | KEY CHARACTERISTICS |
|---|---|
| Chemical | |
| Respiratory | |
| Primarily assist buffering systems when the fluctuation of hydrogen ion concentration is acute | |
| Kidney | |
Respiratory Acid-Base Control Actions and Mechanisms
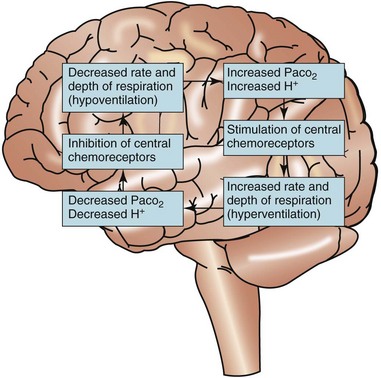
Respiratory regulation of acid-base balance is under the control of the central nervous system (Fig. 14-11). Special receptors in the respiratory areas of the brain are sensitive to changes in the amount of CO2 in brain tissues. As the amount of CO2 begins to rise above normal in brain blood and tissues, these central receptors trigger the neurons to increase the rate and depth of breathing (hyperventilation). As a result, more CO2 is exhaled (“blown off”) from the lungs and the amount of CO2 in the ECF decreases. When the amount of arterial CO2 returns back down to normal, the rate and depth of breathing return to levels that are normal for the person.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


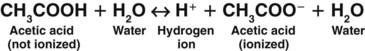
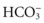 ). Although bicarbonate is a weak base, the many bicarbonate ions in the body are very important in preventing major changes in body fluid pH.
). Although bicarbonate is a weak base, the many bicarbonate ions in the body are very important in preventing major changes in body fluid pH.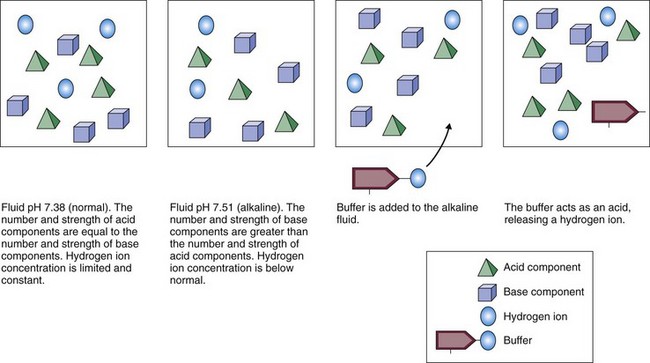

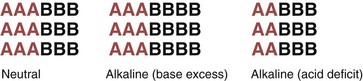
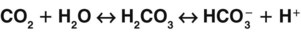
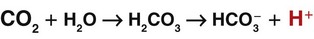
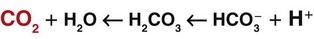

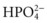 ). This negatively charged fluid draws hydrogen ions (which carry a positive charge) into the urine. Once the hydrogen ion is in the urine, it binds to phosphate ions, forming an acid, H2PO4, which is then excreted in the urine.
). This negatively charged fluid draws hydrogen ions (which carry a positive charge) into the urine. Once the hydrogen ion is in the urine, it binds to phosphate ions, forming an acid, H2PO4, which is then excreted in the urine.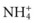 ). The ammonia is first secreted into the urine, where it can combine with excess hydrogen ions to form ammonium. The ammonium “traps” the hydrogen ions and then allows them to be excreted in the urine. The result is a loss of hydrogen ions and an increase in blood pH.
). The ammonia is first secreted into the urine, where it can combine with excess hydrogen ions to form ammonium. The ammonium “traps” the hydrogen ions and then allows them to be excreted in the urine. The result is a loss of hydrogen ions and an increase in blood pH.