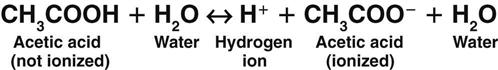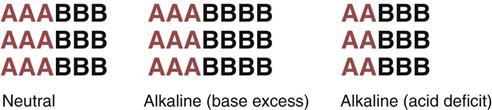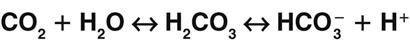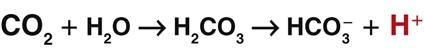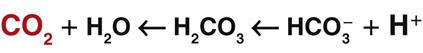M. Linda Workman
Assessment and Care of Patients with Acid-Base Imbalances
Learning Outcomes
Safe and Effective Care Environment
Physiological Integrity
2 Describe the relationship between free hydrogen ion level and pH.
3 Explain the concept of compensation.
4 Compare the roles of the respiratory system and the kidneys in maintaining acid-base balance.
5 Use laboratory data and clinical manifestations to determine the presence of acid-base imbalances.
6 Interpret arterial blood gases to determine whether acidosis is respiratory or metabolic in origin.
7 Prioritize nursing care for the patient with an acid-base imbalance.

http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Acid-base balance is the result of processes that carefully regulate hydrogen ion (H+) production and elimination. Body fluid pH is a measure of the body fluid’s free hydrogen ion level. This value has the narrowest range of normal and the tightest control mechanisms of all the electrolytes. The level of free hydrogen ions, formed from acids, must be rigidly controlled for proper function. Even small changes in the free hydrogen ion level, or pH, of body fluids can cause major problems in function. Keeping the pH within the normal range involves balancing acids and bases in body fluids. Normal pH ranges from 7.35 to 7.45 for arterial blood and from 7.31 to 7.41 for venous blood.
The normal free hydrogen ion level of blood and other body fluids is quite low (less than 0.0001 mEq/L) compared with the levels of other electrolytes (see Chapter 13). Because it is so low, it is not measured directly but, instead, is a calculated value. The pH value is calculated as the negative logarithm of the concentration in milliequivalents per liter. Because it is calculated in negative logarithm units, the value of pH is inversely related (negatively related) to the level of free hydrogen ions. In other words, the lower the pH value of a fluid, the higher the level of free hydrogen ions in that fluid. The pH of a solution may range from 1 (as acidic as possible) to 14 (as alkaline as possible), with 7 being neutral. A change of 1 pH unit actually represents a tenfold change in free hydrogen ion level. Therefore any pH unit change (e.g., a change from 7.4 to 7.3) represents a very large increase in the free hydrogen ion level.
Keeping the pH of the blood within the normal range is important because changes from normal interfere with many functions. These include:
• Changing the shape and reducing the function of hormones and enzymes
• Changing the distribution of other electrolytes, causing fluid and electrolyte imbalances
Fortunately, the body has many mechanisms to ensure minimal changes in free hydrogen ion level.
Acid-Base Balance
As discussed in Chapter 13, body fluids are electrically neutral even though they contain ions with overall positive charges and ions with overall negative charges. When fluids contain an equal number of positive and negative charges, the electrical charge of the fluid is balanced and remains neutral. The body keeps blood pH between 7.35 and 7.45 in a similar manner; however, this value is not strictly neutral (7.0 is neutral) but, rather, is slightly alkaline. Normal body fluid pH remains at a near-neutral value when the acids and bases are nearly balanced, limiting the total number of free or unbalanced hydrogen ions. Acid-base balance occurs by matching the rate of hydrogen ion production (which is a continuous normal process) with hydrogen ion loss.
Acid-Base Chemistry
Acids
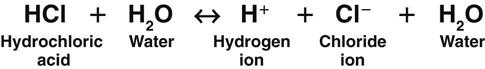
Acids are substances that release hydrogen ions when dissolved in water (H2O). An acid in solution increases the amount of free hydrogen ions in that solution. The strength of an acid is measured by how easily it releases a hydrogen ion in solution. A strong acid, such as hydrochloric acid (HCl), separates (dissociates) completely in water and readily releases all of its hydrogen ions (Fig. 14-1).
A weak acid does not completely separate in water; it releases only some of its hydrogen ions. In the following example, each molecule of acetic acid (CH3COOH), a weak acid, contains a total of four hydrogen molecules. When acetic acid combines with water (Fig. 14-2), it releases only one of its four hydrogen molecules. The other three hydrogen molecules remain bound to the acetic acid molecule (CH3COO−).
Bases
A base is a substance that binds free hydrogen ions in solution. Thus bases are “hydrogen acceptors” that lower the amount of free hydrogen ions in solution. Strong bases bind hydrogen ions easily. Examples of strong bases include sodium hydroxide (NaOH) and ammonia (NH3).
Weak bases bind hydrogen ions less readily. Examples of weak bases are aluminum hydroxide (AlOH3) and bicarbonate (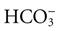 ). Although bicarbonate is a weak base, the many bicarbonate ions in the body are very important in preventing major changes in body fluid pH.
). Although bicarbonate is a weak base, the many bicarbonate ions in the body are very important in preventing major changes in body fluid pH.
Buffers
Buffers are critical in keeping body fluid pH at normal levels because they can react in two ways: either as an acid (releasing a hydrogen ion) or as a base (binding a hydrogen ion). How a buffer reacts when dissolved in water depends on the existing acid-base balance of that fluid. Buffers always try to bring the fluid as close as possible to the normal body fluid pH of 7.35 to 7.45. If the fluid is basic (with few free hydrogen ions), the buffer releases hydrogen ions into the fluid (Fig. 14-3). If the fluid is acidic (with many free hydrogen ions), the buffer acts as a base, binding some of the excess hydrogen ions. In this way, buffers act like hydrogen ion “sponges,” soaking up hydrogen ions when too many are present and squeezing out hydrogen ions when too few are present. This flexibility allows buffers to keep body fluid pH in the normal range.
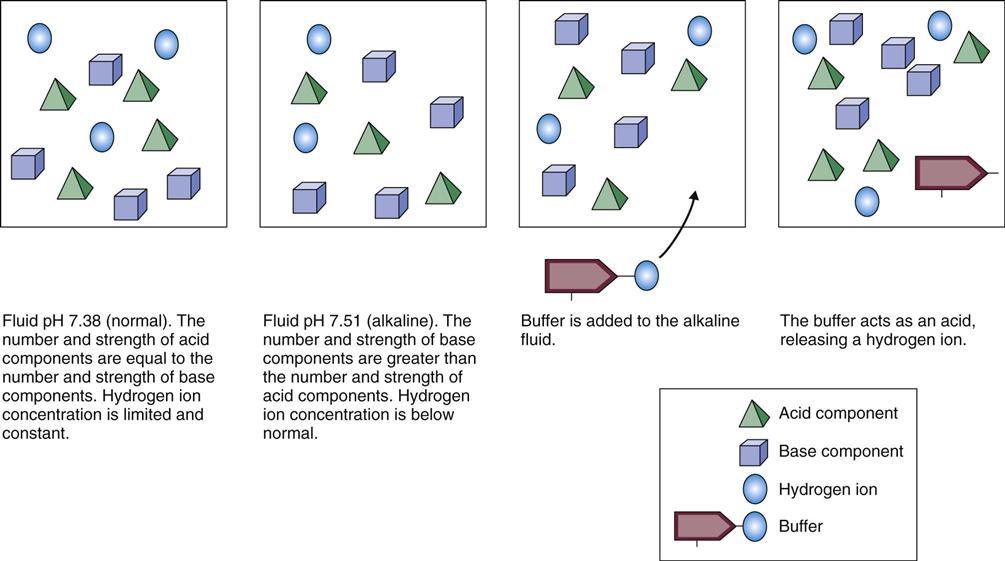
Liquids with a pH of 7.0 are neutral; they have a free hydrogen ion level in which the amount and strength of acids and bases are equal. Fig. 14-4 shows the concept of neutral pH. This figure shows that the combined strength and amount of all acids are equal to the combined strength and amount of all bases in a given solution. This is close to the actual case in human physiology. With human acid-base homeostasis, the relative amounts and strengths of acids and bases are nearly equal and, normally, hydrogen ion production is balanced with hydrogen ion loss so that the overall free hydrogen ion levels remain constant.
Liquids with a pH ranging from 1.0 to 6.99 have more or stronger (or both) acids compared with the amount or strength (or both) of bases. These liquids are acidic (see Fig. 14-4), which means that more free hydrogen ions are being released than bound, increasing the amount of free hydrogen ions in the liquid.
Liquids with a pH ranging from 7.01 to 14.0 have more or stronger (or both) bases compared with the amount or strength (or both) of acids. These liquids are basic, which means that more hydrogen ions are being bound than released, decreasing the amount of free hydrogen ions (Fig. 14-5).
Body Fluid Chemistry
Bicarbonate Ions
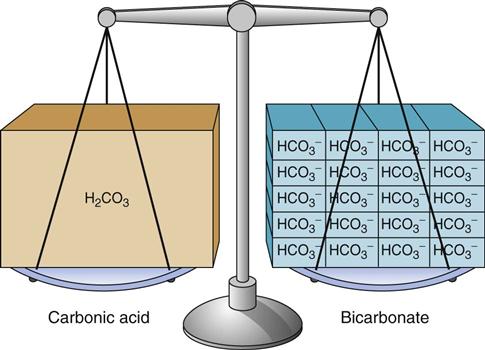
Body fluids contain many different types of acids and a few types of bases. The most common base in human body fluid is bicarbonate (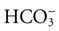 ); the most common acid is carbonic acid (H2CO3). In health, the body keeps these substances at a constant ratio of 1 molecule of carbonic acid to 20 free bicarbonate ions (1 : 20) (Fig. 14-6). To maintain this ratio, both carbonic acid and bicarbonate must be carefully controlled. This constant ratio is related to balancing the production and elimination of carbon dioxide (CO2) and hydrogen ions (H+).
); the most common acid is carbonic acid (H2CO3). In health, the body keeps these substances at a constant ratio of 1 molecule of carbonic acid to 20 free bicarbonate ions (1 : 20) (Fig. 14-6). To maintain this ratio, both carbonic acid and bicarbonate must be carefully controlled. This constant ratio is related to balancing the production and elimination of carbon dioxide (CO2) and hydrogen ions (H+).
Relationship Between Carbon Dioxide and Hydrogen Ions
A key concept in understanding acid-base balance is the carbonic anhydrase equation. This equation, driven by the enzyme carbonic anhydrase, shows how hydrogen ion levels and carbon dioxide levels are directly related to one another, so that an increase in one causes an equal increase in the other (Fig. 14-7).
Carbon dioxide is a gas that forms carbonic acid when combined with water, making carbon dioxide a part of carbonic acid. Carbonic acid is not stable, and the body needs to keep a 1 : 20 ratio of carbonic acid to bicarbonate. When carbonic acid is formed from water and carbon dioxide, it begins to separate into free hydrogen ions and bicarbonate ions. Therefore the carbon dioxide content of a fluid is directly related to the amount of hydrogen ions in that fluid. Whenever conditions cause carbon dioxide to increase, more free hydrogen ions are created. Likewise, whenever free hydrogen ion production increases, more carbon dioxide is produced.
When excess carbon dioxide is produced, the amount of carbon dioxide increases and the equation shifts to the right, causing an increase in hydrogen ions (and a decrease in pH), as shown in Fig. 14-8. When very little carbon dioxide is produced, no free hydrogen ions are created by this equation. When excess hydrogen ions are produced or brought into the body, the carbonic anhydrase equation shifts to the left, causing the creation of more carbon dioxide, as shown in Fig. 14-9. When the amount of free hydrogen ions in body fluids is low, no extra carbon dioxide is produced.
How is the relationship between free hydrogen ions and carbon dioxide helpful? Carbon dioxide is a gas that can be eliminated during exhalation, and this action is important for acid-base balance. When any condition causes the hydrogen ion concentration of body fluids to increase, extra CO2 is produced in the same proportion. This extra CO2 is eliminated during exhalation, helping to bring the hydrogen ion concentration down to normal. Whenever the CO2 level changes, the pH changes to the same degree, in the opposite direction. Thus when the CO2 level of a liquid increases, the pH drops, indicating more free hydrogen ions (more acidic). On the other hand, when the CO2 level of a liquid decreases, the pH rises, indicating fewer free hydrogen ions (more alkaline).
An increase in bicarbonate causes the amount of hydrogen ions to decrease and the pH to increase, or become more alkaline (basic). On the other hand, an increase in the CO2 level causes the free hydrogen ion level to increase and the pH to decrease, or become more acidic.
Because the kidneys control bicarbonate levels and the lungs control CO2 levels in the healthy person, pH is also described as the function of the kidneys divided by the function of the lungs (Fig. 14-10).
Sources of Acids and Bicarbonate
Whenever acids are present in body fluids, free hydrogen ions are dissociated and must be controlled for pH balance. How are acids and hydrogen ions produced? Remember that whole body human function is dynamic and continuously working. The processes that allow the body to perform its work continuously generate acids and hydrogen ions as waste products of metabolism.
Normal metabolism of carbohydrate, protein, and fat creates natural waste products. Carbohydrate metabolism forms carbon dioxide (CO2). Carbon dioxide is exhaled by the lungs during breathing. One factor that determines blood pH is how much CO2 is produced by body cells during metabolism versus how rapidly that CO2 is removed by breathing. Protein breakdown forms sulfuric acid. Fat breakdown forms fatty acids and ketoacids.
Incomplete breakdown of glucose, which occurs whenever cells metabolize under anaerobic (no oxygen) conditions, forms lactic acid. Anaerobic conditions occur with hypoxia, sepsis, and shock. Incomplete breakdown of fatty acids, occurring when large amounts of fatty acids are being metabolized, forms ketoacids.
Destruction of cells allows cell contents to be released. Some cell structures (e.g., lysosomes) contain acids. When these acids are released into the extracellular fluid (ECF), free hydrogen ions are dissociated.
Bicarbonate, a weak base, is the main buffer of the ECF. It comes from the intestinal absorption of ingested bicarbonate, pancreatic production of bicarbonate, movement of cellular bicarbonate into the ECF, kidney reabsorption of filtered bicarbonate, and the breakdown of carbonic acid. Once bicarbonate is in the ECF, it is kept at a level 20 times greater than that of carbonic acid.
Acid-Base Regulatory Actions and Mechanisms
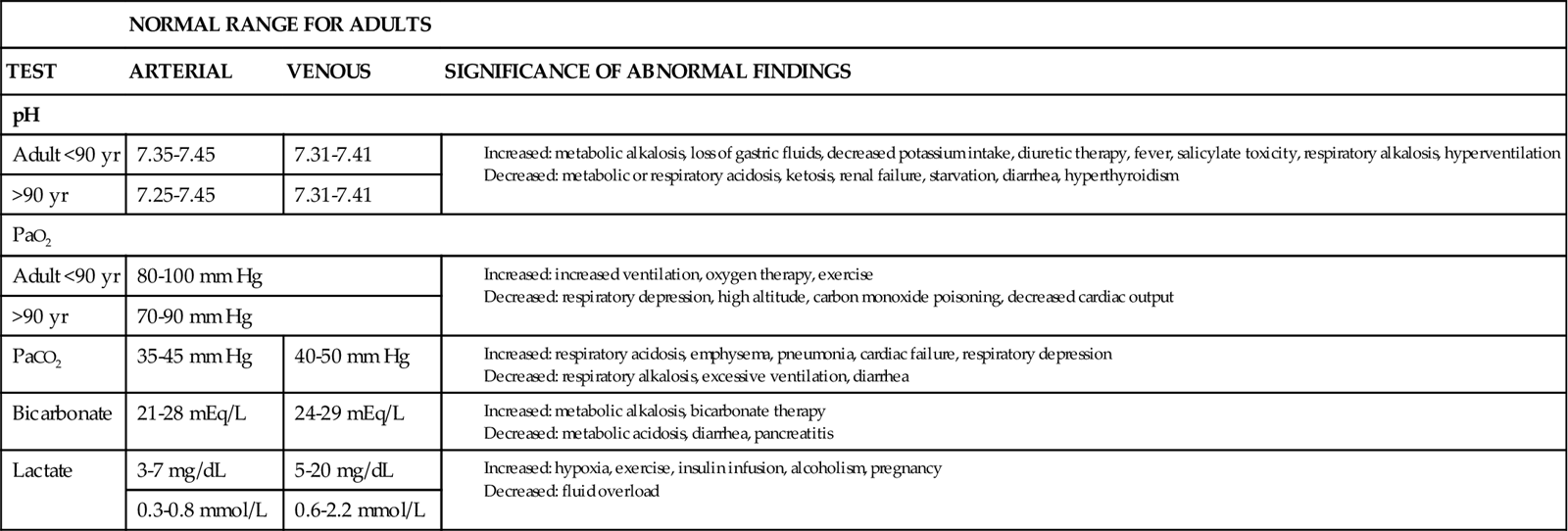
As long as body cells are healthy, they continuously produce acids, carbon dioxide, and free hydrogen ions. Despite this production, hydrogen ion, bicarbonate, oxygen, and carbon dioxide levels are kept within normal limits when acid-base controlling actions/mechanisms are normal. Chart 14-1 lists normal values for these substances in arterial and venous blood. This homeostasis depends on:
• Hydrogen ion production being consistent and not excessive
• CO2 loss from the body through breathing keeping pace with all forms of hydrogen ion production
To keep the free hydrogen ion level (pH) of the ECF within the narrow range of normal, the body has chemical, respiratory, and kidney actions (mechanisms) for acid-base balance (Table 14-1).
TABLE 14-1
ACID-BASE REGULATORY MECHANISMS
| MECHANISM TYPE | KEY CHARACTERISTICS |
| Chemical | |
| Respiratory | |
| Primarily assist buffering systems when the fluctuation of hydrogen ion concentration is acute | |
| Kidney | |
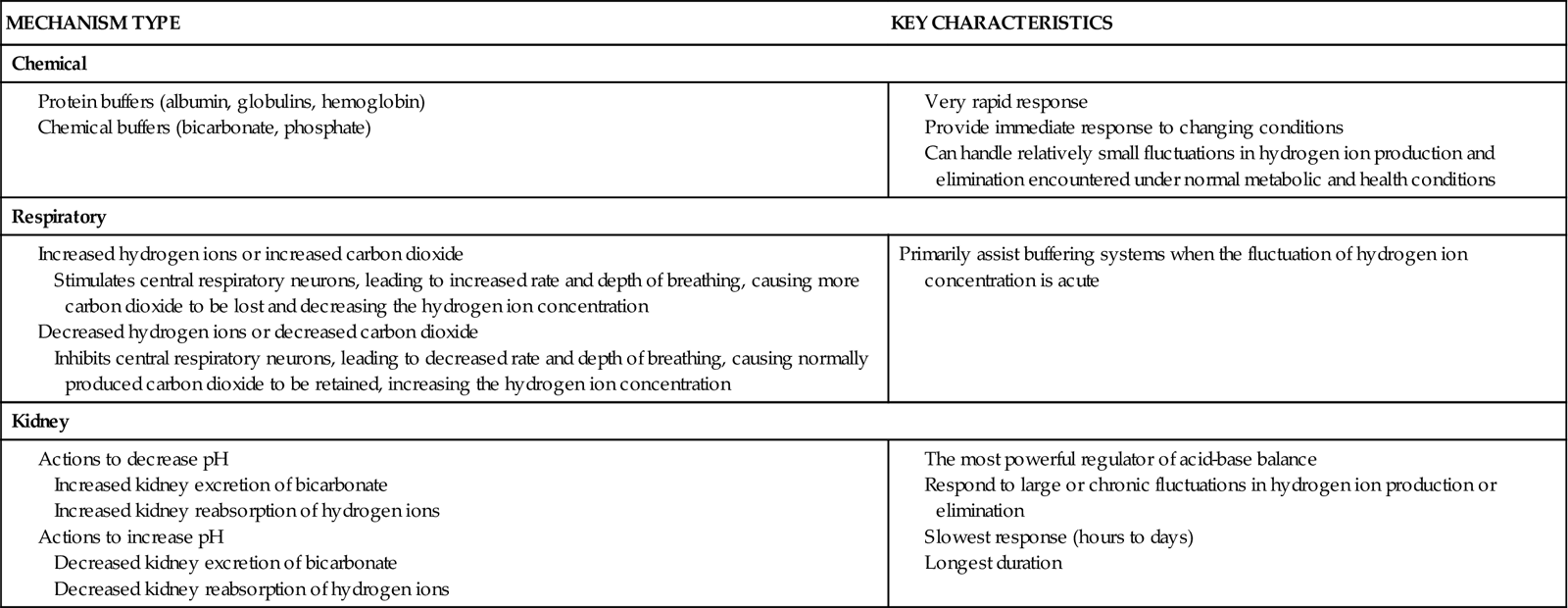
Chemical Acid-Base Control Actions and Mechanisms
Buffers are the first line of defense against changes in the amount of free hydrogen ions. Because they are always present in body fluids, buffers act fast to reduce or raise the amount of free hydrogen ions to normal. By acting as hydrogen ion “sponges,” buffers can bind hydrogen ions when too many are present or release hydrogen ions when not enough are present. Buffers are composed of chemicals or proteins.
Chemical buffers are paired mixtures—usually a weak base and an acid salt. The two most common chemical buffers are bicarbonate (which is active in both the extracellular fluid [ECF] and intracellular fluid [ICF]) and phosphate (which is active in the ICF).
Protein buffers are the most common buffers. Proteins in body fluids can either bind or release free hydrogen ions as needed. Both ICF and ECF proteins serve as buffers. Extracellular protein buffers are albumin and globulins. A major cell protein buffer is hemoglobin. Hemoglobin buffers hydrogen ions directly and also buffers acids formed during the production of carbon dioxide. When the amount of free hydrogen ions in the blood increases, some of the excess hydrogen ions cross the membranes of red blood cells and bind to the large numbers of hemoglobin molecules in each red blood cell. This binding of hydrogen ions to hemoglobin results in fewer hydrogen ions remaining in the blood, bringing blood pH back up toward normal.
Respiratory Acid-Base Control Actions and Mechanisms
When chemical buffers alone cannot prevent changes in blood pH, the respiratory system is the second line of defense against changes. Breathing controls the amount of free hydrogen ions by controlling the amount of carbon dioxide (CO2) in arterial blood. Remember, because CO2 is converted into hydrogen ions through the carbonic anhydrase reaction, the CO2 level is directly related to the hydrogen ion level. Breathing rids the body of the excess CO2 created through metabolism.
The amount of CO2 in venous blood increases during normal metabolism. This CO2 is moved in the blood to the lung capillaries. Because the amount (pressure) of CO2 is far higher in capillary blood than in the air in the alveoli, CO2 diffuses freely from the blood into the alveolar air. Once in the alveoli, CO2 is exhaled during breathing and is lost from the body. Because the amount (pressure) of CO2 in atmospheric air is nearly zero, CO2 usually continues to be exhaled even when breathing is impaired.
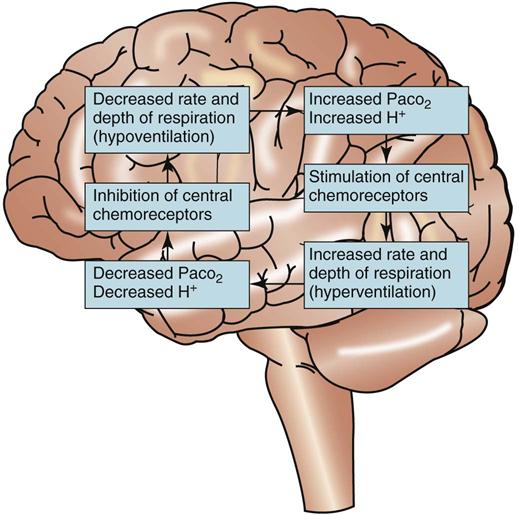
Respiratory regulation of acid-base balance is under the control of the central nervous system (Fig. 14-11). Special receptors in the respiratory areas of the brain are sensitive to changes in the amount of CO2 in brain tissues. As the amount of CO2 begins to rise above normal in brain blood and tissues, these central receptors trigger the neurons to increase the rate and depth of breathing (hyperventilation). As a result, more CO2 is exhaled (“blown off”) from the lungs and the amount of CO2 in the ECF decreases. When the amount of arterial CO2 returns back down to normal, the rate and depth of breathing return to levels that are normal for the person.
If the amount of ECF free hydrogen ions is too low, then the amount of CO2 also is too low. Central receptors sense these low CO2 levels and stop or slow the neuron activity in the respiratory centers of the brain, decreasing the rate and depth of breathing (hypoventilation). As a result, less CO2 is lost through the lungs and more CO2 is retained in arterial blood. This retention of already-formed CO2, together with the normal production of CO2 from metabolism, results in a rapid return of the arterial CO2 levels (and hydrogen ion levels) back up to normal. When these levels are normal, the rate and depth of breathing also return to normal levels.
The respiratory system’s response in acid-base balance is rapid. Changes in the rate and depth of breathing occur within minutes after changes in the hydrogen ion level or CO2 level of the ECF occur.
Kidney Acid-Base Control Actions and Mechanisms
The kidneys are the third line of defense against wide changes in body fluid pH. Kidney actions are stronger for regulating acid-base balance but take longer than chemical and respiratory actions to completely respond. (They take 24 to 48 hours to respond.) When blood pH changes are persistent, kidney actions that increase excretion and reabsorption rates of acids or bases (depending on the direction of the pH changes) begin to operate. These actions are kidney movement of bicarbonate, formation of acids, and formation of ammonium.
Kidney movement of bicarbonate is the first kidney pH control action. It occurs in the kidney tubules in two ways: (1) kidney movement of bicarbonate produced elsewhere in the body and (2) kidney movement of bicarbonate produced in the kidneys. Much of the bicarbonate made in other body areas is excreted in the urine. When blood hydrogen ion levels are high, this bicarbonate is reabsorbed from the kidneys back into circulation, where it can help buffer excess hydrogen ions. In this situation, the kidney tubules also can make additional bicarbonate and reabsorb it for an increased buffer effect. When blood hydrogen ion levels are low, the bicarbonate remains in the urine and is excreted.
Formation of acids is the second kidney pH control action. It occurs through the phosphate-buffering system inside the cells of the kidney tubules. When the newly created bicarbonate made in the kidney cells is reabsorbed into the blood along with sodium, the urine has an excess of anions, including phosphate (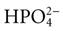 ). This negatively charged fluid draws hydrogen ions (which carry a positive charge) into the urine. Once the hydrogen ion is in the urine, it binds to phosphate ions, forming an acid, H2PO4, which is then excreted in the urine.
). This negatively charged fluid draws hydrogen ions (which carry a positive charge) into the urine. Once the hydrogen ion is in the urine, it binds to phosphate ions, forming an acid, H2PO4, which is then excreted in the urine.
Formation of ammonium is the third kidney pH control action. Ammonia (NH3), which is formed during normal protein breakdown, is converted into ammonium (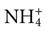 ). The ammonia is first secreted into the urine, where it can combine with excess hydrogen ions to form ammonium. The ammonium “traps” the hydrogen ions and then allows them to be excreted in the urine. The result is a loss of hydrogen ions and an increase in blood pH.
). The ammonia is first secreted into the urine, where it can combine with excess hydrogen ions to form ammonium. The ammonium “traps” the hydrogen ions and then allows them to be excreted in the urine. The result is a loss of hydrogen ions and an increase in blood pH.
Compensation
In the process of compensation, the body adapts to attempt to correct changes in blood pH. A pH below 6.9 or above 7.8 is usually fatal. The normal pH range for human extracellular fluid (ECF) is 7.35 to 7.45. Both the kidneys and the lungs can compensate for acid-base imbalances, but they are not equal in their final responses. The respiratory system is much more sensitive to acid-base changes and can begin compensation efforts within seconds to minutes after a change in pH. However, these efforts are limited and can be overwhelmed easily. The kidney compensatory actions are much more powerful and result in rapid changes in ECF composition. However, these more powerful actions are not fully triggered unless the acid-base imbalance continues for several hours to several days.
Respiratory compensation occurs through the lungs, usually to correct for acid-base imbalances from metabolic problems. For example, when prolonged running causes buildup of lactic acid, hydrogen ion levels in the ECF increase and the pH drops. To bring the pH back to normal, breathing is triggered in response to increased carbon dioxide levels. Both the rate and depth of respiration increase. These respiratory efforts cause the blood to lose carbon dioxide with each exhalation, so ECF levels of carbon dioxide and free hydrogen ions gradually decrease. When the lungs can fully compensate, the pH returns to normal.
Kidney compensation results when a healthy kidney works to correct for changes in blood pH that occur when the respiratory system either is overwhelmed or is not healthy. For example, in a person with chronic obstructive pulmonary disease (COPD), the respiratory system cannot exchange gases adequately. Carbon dioxide is retained continuously, hydrogen ion levels increase, and the blood pH falls (becomes more acidic). To oppose this process, the kidney excretes more hydrogen ions and increases the reabsorption of bicarbonate back into the blood. As a result, the blood pH remains either within or closer to the normal range. When these backup actions are completely effective, acid-base problems are fully compensated and the pH of the blood returns to normal even though the levels of oxygen and bicarbonate may be abnormal.
Sometimes, however, the respiratory problem causing the acid-base imbalance is so severe that kidney actions can only partially compensate and the pH is not quite normal. Partial compensation is helpful because it prevents the acid-base imbalance from becoming severe or life threatening.
Acid-Base Imbalances
Acid-base imbalances are changes in the blood hydrogen ion level or pH. These changes are caused by problems with the acid-base regulatory actions and mechanisms of the body or by exposure to dangerous conditions. Imbalances in which blood pH is below normal reflect acidosis, and imbalances in which blood pH is above normal reflect alkalosis. Acid-base imbalances impair the function of many organs and can be life threatening.
Acidosis
Pathophysiology
In acidosis, the acid-base balance of the blood and other extracellular fluid (ECF) is upset by an excess of hydrogen ions (H+). This problem is reflected as an arterial blood pH below 7.35. The amount or strength (or both) of acids is greater than normal compared with the amount or strength of bases.
Acidosis is not a disease; it is a condition caused by a disorder or pathologic process. Acidosis can be caused by metabolic problems, respiratory problems, or both. Patients at greatest risk for acute acidosis are those with problems that impair breathing. Older adults with chronic health problems are at greater risk for developing acidosis (Chart 14-2).