CHAPTER 94 In this chapter we discuss drug therapy of infection with the human immunodeficiency virus (HIV), the microbe that causes acquired immunodeficiency syndrome (AIDS). HIV promotes immunodeficiency by killing CD4 T lymphocytes (CD4 T cells), which are key components of the immune system (see Chapter 67). As a result of HIV-induced immunodeficiency, patients are at risk of opportunistic infections and certain neoplasms. Understanding this chapter requires a basic understanding of the immune system. Accordingly, you may find it helpful to read Chapter 67 before proceeding. The principal cells attacked by HIV are CD4 T cells (helper T lymphocytes). As discussed in Chapter 67, these cells are essential components of the immune system. They are required for production of antibodies by B lymphocytes and for activation of cytolytic T lymphocytes. Accordingly, as HIV kills CD4 T cells, the immune system undergoes progressive decline. As a result, infected individuals become increasingly vulnerable to opportunistic infections, a major cause of death among people with AIDS. HIV targets CD4 T cells because the CD4 proteins on the surface of these cells provide points of attachment for HIV (see below). Without such a receptor, HIV would be unable to connect with and penetrate these cells. Once HIV has infected a CD4 T cell, the cell dies in about 1.25 days. It is important to appreciate that only a few percent of CD4 T cells circulate in the blood; the vast majority reside in lymph nodes and other lymphoid tissues. The structure of HIV is very simple. As shown in Figure 94–1, the HIV virion (ie, the entire virus particle) consists of nucleic acid (RNA) surrounded by core proteins, which in turn are surrounded by a capsid (protein shell), which in turn is surrounded by a lipid bilayer envelope (derived from the membrane of the host cell). The replication cycle of HIV is depicted in Figure 94–2. The numbered steps below correspond to the numbers in the figure. • Step 1—The cycle begins with attachment of HIV to the host cell. The primary connection takes place between gp120 on the HIV envelope and a CD4 protein on the host cell membrane. Other host proteins, known as co-receptors, act in concert with CD4 to tighten the bond with HIV. Two of these co-receptors—known as CCR5 and CXCR4—are of particular importance. One drug—maraviroc—blocks HIV entry by binding CCR5. • Step 2—The lipid bilayer envelope of HIV fuses with the lipid bilayer of the host cell membrane. Fusion is followed by release of HIV RNA into the host cell. One drug—–enfuvirtide—–works by blocking the fusion process. • Step 3—HIV RNA is transcribed into single-stranded DNA by HIV reverse transcriptase. Twelve antiretroviral drugs inhibit this enzyme. • Step 4—Reverse transcriptase converts the single strand of HIV DNA into double-stranded HIV DNA. • Step 5—Double-stranded HIV DNA becomes integrated into the host’s DNA, under the direction of a viral enzyme known (aptly) as integrase. One drug—raltegravir—inhibits this enzyme. • Step 6—HIV DNA undergoes transcription into RNA. Some of the resulting RNA becomes the genome for daughter HIV virions (step 6a). The rest of the RNA is messenger RNA that codes for HIV proteins (step 6b). • Step 7—Messenger RNA is translated into HIV glycoproteins (step 7a) and HIV enzymes and structural proteins (step 7b). • Step 8—The components of HIV migrate to the cell surface and assemble into a new virus. Prior to assembly, HIV glycoproteins become incorporated into the host cell membrane (step 8a). In steps 8b and 8c, the other components of the virion migrate to the cell surface, where they undergo assembly into the new virus. • Step 9—The newly formed virus buds off from the host cell. As indicated, the outer envelope of the virion is derived from the cell membrane of the host. • Step 10—In this step, which occurs either during or immediately after budding off, HIV undergoes final maturation under the influence of protease, an enzyme that cleaves certain large polyproteins into their smaller, functional forms. If protease fails to cleave these proteins, HIV will remain immature and noninfectious. HIV protease is the target of several important drugs. HIV mutates rapidly. Why? Because HIV reverse transcriptase is an error-prone enzyme. Hence, whenever it transcribes HIV RNA into single-stranded DNA and then into double-stranded DNA, there is a high probability of introducing base-pair errors. In fact, according to one estimate, up to 10 incorrect bases may be incorporated into HIV DNA during each round of replication. Because of these errors, HIV can rapidly mutate from a drug-sensitive form into a drug-resistant form. The probability of developing resistance in the individual patient is directly related to the total viral load. Hence, the more virions the patient harbors, the greater the likelihood that at least one will become resistant. To minimize the emergence of resistance, patients must be treated with a combination of antiretroviral drugs. This is the same strategy we employ to prevent emergence of resistance when treating tuberculosis (see Chapter 90). HIV infection follows a triphasic clinical course. During the initial phase, HIV undergoes massive replication, causing blood levels of HIV to rise very high. As a result, between 50% and 90% of patients experience a flu-like acute retroviral syndrome. Signs and symptoms include fever, lymphadenopathy, pharyngitis, rash, myalgia, and headache (Table 94–1). Soon, however, the immune system mounts a counterattack, causing HIV levels to fall. As a result, symptoms of the acute syndrome fade. Very often, the acute retroviral syndrome is perceived as influenza, and hence goes unrecognized for what it really is. At this time, we have five types of antiretroviral drugs. Three types—reverse transcriptase inhibitors, integrase strand transfer inhibitors (INSTIs), and protease inhibitors (PIs)—inhibit enzymes required for HIV replication. The other two types—fusion inhibitors and chemokine receptor 5 (CCR5) antagonists—block viral entry into cells. As discussed below, the reverse transcriptase inhibitors are subdivided into two groups: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), which are structural analogs of nucleosides or nucleotides, and (2) non-nucleoside reverse transcriptase inhibitors (NNRTIs). Drugs that belong to these groups are listed in Table 94–2. All NRTIs, NNRTIs, PIs, INSTIs, and CCR5 antagonists are administered orally, and one NRTI—zidovudine—may also be given IV. The one fusion inhibitor available—enfuvirtide [Fuzeon]—is administered subQ. TABLE 94–2 Classification of Antiretroviral Drugs *Efavirenz is an NNRTI, not an NRTI. The nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) were the first drugs used against HIV infection, and remain mainstays of therapy today. In fact, these drugs constitute the backbone of all treatment regimens. As their name suggests, the NRTIs are chemical relatives of naturally occurring nucleosides or nucleotides, the building blocks of DNA. Antiretroviral effects derive from suppressing synthesis of viral DNA by reverse transcriptase. To be effective, all of the NRTIs must first undergo intracellular conversion to their active (triphosphate) forms. The NRTIs have few drug interactions, and most can be taken without regard to meals. Rarely, these agents cause a potentially fatal syndrome characterized by lactic acidosis and hepatomegaly with steatosis; pregnant women taking two NRTIs may be at increased risk. At this time, seven NRTIs are available. Major properties are summarized in Table 94–3. TABLE 94–3 Properties of Nucleoside/Nucleotide Reverse Transcriptase Inhibitors *Lactic acidosis with hepatic steatosis is a rare but potentially fatal toxicity associated with all NRTIs. Adapted from Guidelines for the Use of Antiretroviral Agents in HIV-1–Infected Adults and Adolescents, prepared by the Panel on Clinical Practices for Treatment of HIV Infection, convened by the DHHS, as updated on October 14, 2011. Granulocyte colony-stimulating factors may be given to reverse zidovudine-induced neutropenia. Also, if erythropoietin levels are not already elevated, epoetin alfa (recombinant erythropoietin) can be given to reduce transfusion requirements in patients with anemia. Granulocyte colony-stimulating factors and epoetin alfa are discussed in Chapter 56.
Antiviral agents II: drugs for HIV infection and related opportunistic infections
Pathophysiology
Characteristics of HIV
Target cells
Structure of HIV
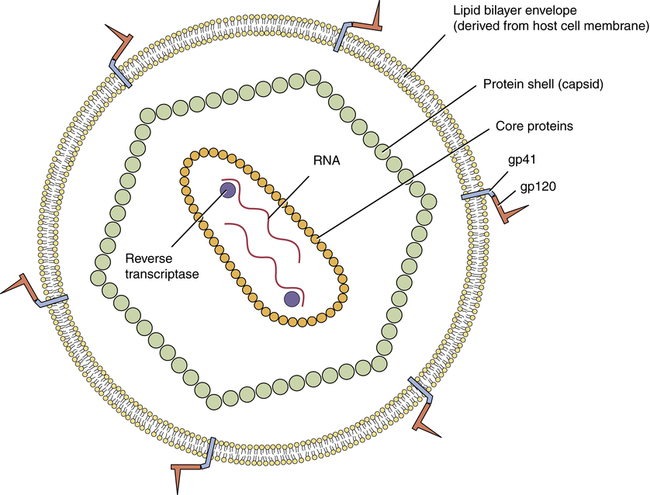
 Structure of the human immunodeficiency virus.
Structure of the human immunodeficiency virus.
Note that HIV has two single strands of RNA, and that each strand is associated with a molecule of reverse transcriptase. (gp41 = glycoprotein 41, gp120 = glycoprotein 120.)
Replication cycle of HIV
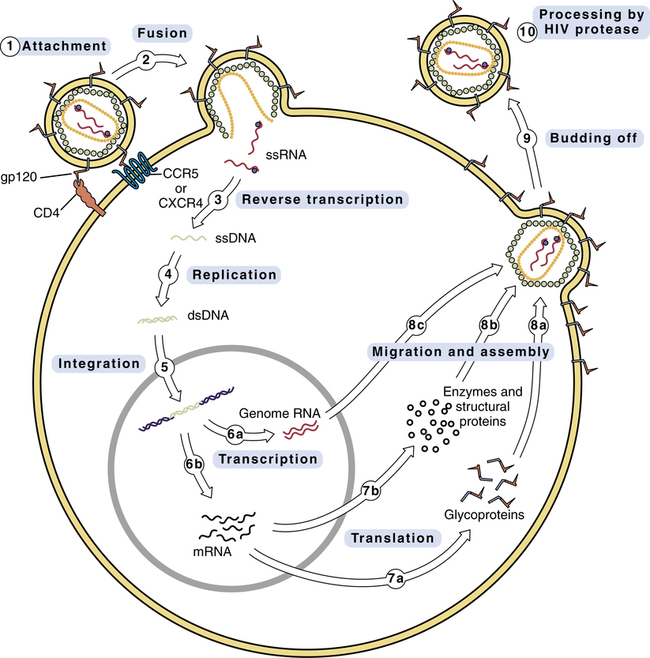
 Replication cycle of the human immunodeficiency virus.
Replication cycle of the human immunodeficiency virus.
See text for description of events. (CCR5 = CCR5 co-receptor, CD4 = CD4 receptor, CXCR4 = CXCR4 co-receptor, dsDNA = double-stranded DNA, gp120 = glycoprotein 120, mRNA = messenger RNA, ssDNA = single-stranded DNA, ssRNA = single-stranded RNA.)
Mutation and drug resistance
Clinical course of HIV infection
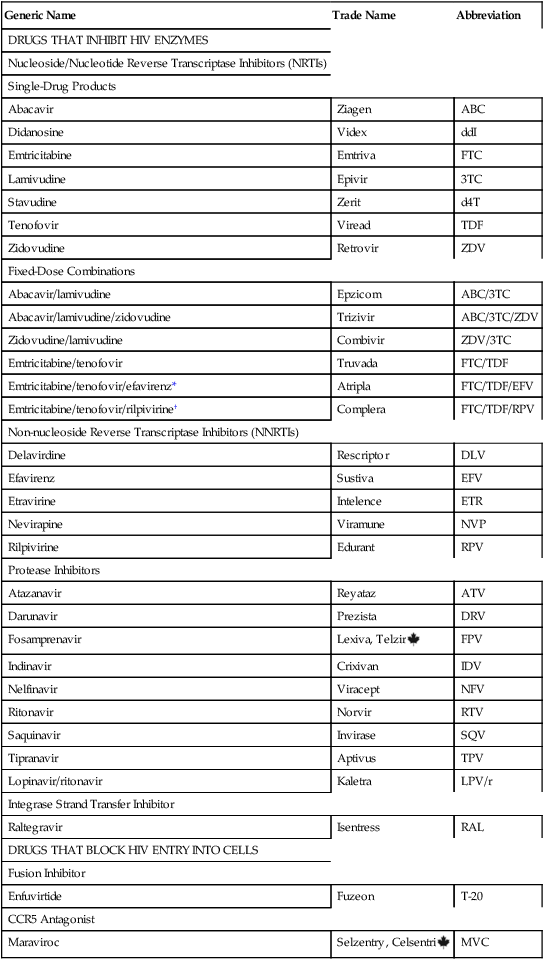
Classification of antiretroviral drugs

Generic Name
Trade Name
Abbreviation
DRUGS THAT INHIBIT HIV ENZYMES
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs)
Single-Drug Products
Abacavir
Ziagen
ABC
Didanosine
Videx
ddI
Emtricitabine
Emtriva
FTC
Lamivudine
Epivir
3TC
Stavudine
Zerit
d4T
Tenofovir
Viread
TDF
Zidovudine
Retrovir
ZDV
Fixed-Dose Combinations
Abacavir/lamivudine
Epzicom
ABC/3TC
Abacavir/lamivudine/zidovudine
Trizivir
ABC/3TC/ZDV
Zidovudine/lamivudine
Combivir
ZDV/3TC
Emtricitabine/tenofovir
Truvada
FTC/TDF
Emtricitabine/tenofovir/efavirenz*
Atripla
FTC/TDF/EFV
Emtricitabine/tenofovir/rilpivirine†
Complera
FTC/TDF/RPV
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
Delavirdine
Rescriptor
DLV
Efavirenz
Sustiva
EFV
Etravirine
Intelence
ETR
Nevirapine
Viramune
NVP
Rilpivirine
Edurant
RPV
Protease Inhibitors
Atazanavir
Reyataz
ATV
Darunavir
Prezista
DRV
Fosamprenavir
Lexiva, Telzir ![]()
FPV
Indinavir
Crixivan
IDV
Nelfinavir
Viracept
NFV
Ritonavir
Norvir
RTV
Saquinavir
Invirase
SQV
Tipranavir
Aptivus
TPV
Lopinavir/ritonavir
Kaletra
LPV/r
Integrase Strand Transfer Inhibitor
Raltegravir
Isentress
RAL
DRUGS THAT BLOCK HIV ENTRY INTO CELLS
Fusion Inhibitor
Enfuvirtide
Fuzeon
T-20
CCR5 Antagonist
Maraviroc
Selzentry, Celsentri ![]()
MVC
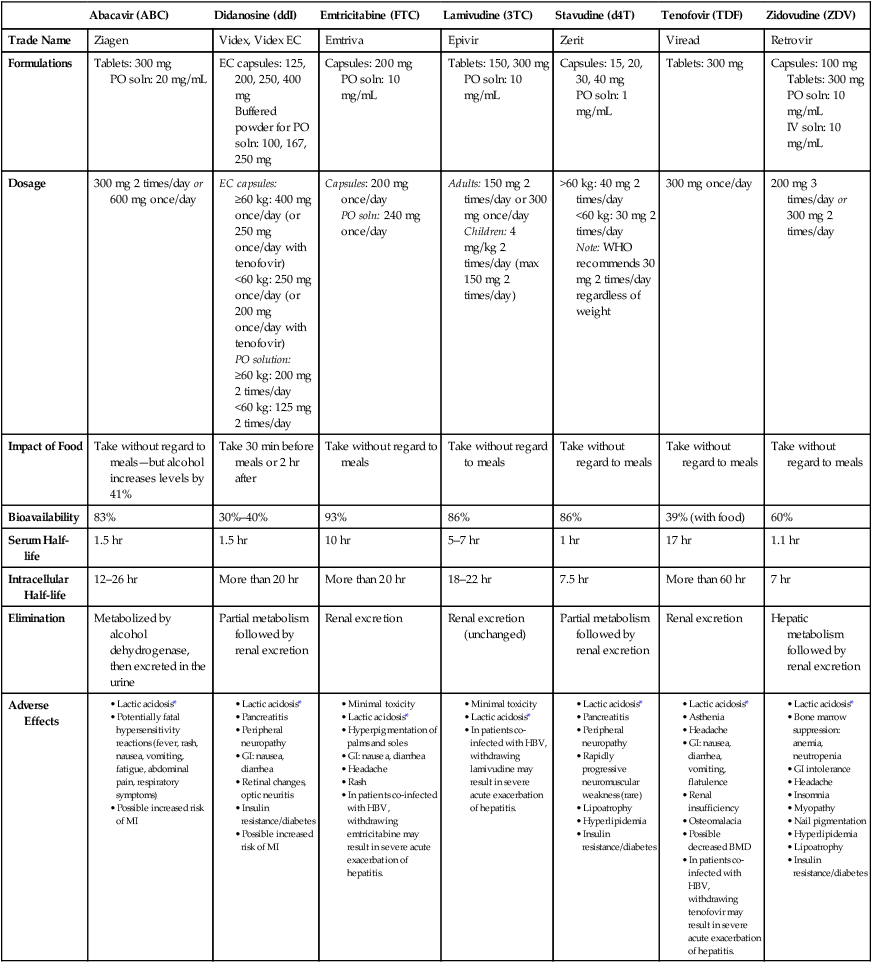
Nucleoside/nucleotide reverse transcriptase inhibitors

Abacavir (ABC)
Didanosine (ddI)
Emtricitabine (FTC)
Lamivudine (3TC)
Stavudine (d4T)
Tenofovir (TDF)
Zidovudine (ZDV)
Trade Name
Ziagen
Videx, Videx EC
Emtriva
Epivir
Zerit
Viread
Retrovir
Formulations
Tablets: 300 mg
PO soln: 20 mg/mL
EC capsules: 125, 200, 250, 400 mg
Buffered powder for PO soln: 100, 167, 250 mg
Capsules: 200 mg
PO soln: 10 mg/mL
Tablets: 150, 300 mg
PO soln: 10 mg/mL
Capsules: 15, 20, 30, 40 mg
PO soln: 1 mg/mL
Tablets: 300 mg
Capsules: 100 mg
Tablets: 300 mg
PO soln: 10 mg/mL
IV soln: 10 mg/mL
Dosage
300 mg 2 times/day or 600 mg once/day
EC capsules:
≥60 kg: 400 mg once/day (or 250 mg once/day with tenofovir)
<60 kg: 250 mg once/day (or 200 mg once/day with tenofovir)
PO solution:
≥60 kg: 200 mg 2 times/day
<60 kg: 125 mg 2 times/day
Capsules: 200 mg once/day
PO soln: 240 mg once/day
Adults: 150 mg 2 times/day or 300 mg once/day
Children: 4 mg/kg 2 times/day (max 150 mg 2 times/day)
>60 kg: 40 mg 2 times/day
<60 kg: 30 mg 2 times/day
Note: WHO recommends 30 mg 2 times/day regardless of weight
300 mg once/day
200 mg 3 times/day or 300 mg 2 times/day
Impact of Food
Take without regard to meals—but alcohol increases levels by 41%
Take 30 min before meals or 2 hr after
Take without regard to meals
Take without regard to meals
Take without regard to meals
Take without regard to meals
Take without regard to meals
Bioavailability
83%
30%–40%
93%
86%
86%
39% (with food)
60%
Serum Half-life
1.5 hr
1.5 hr
10 hr
5–7 hr
1 hr
17 hr
1.1 hr
Intracellular Half-life
12–26 hr
More than 20 hr
More than 20 hr
18–22 hr
7.5 hr
More than 60 hr
7 hr
Elimination
Metabolized by alcohol dehydrogenase, then excreted in the urine
Partial metabolism followed by renal excretion
Renal excretion
Renal excretion (unchanged)
Partial metabolism followed by renal excretion
Renal excretion
Hepatic metabolism followed by renal excretion
Adverse Effects
Zidovudine
Adverse effects
Anemia and neutropenia from bone marrow suppression.
Get Clinical Tree app for offline access

Antiviral agents II: drugs for HIV infection and related opportunistic infections
Get Clinical Tree app for offline access