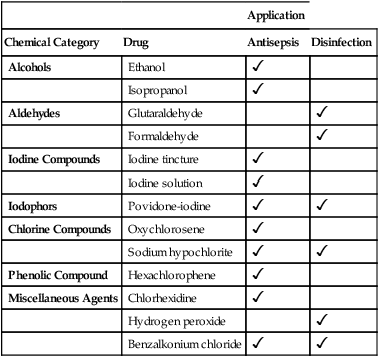
CHAPTER 96 Antiseptics and disinfectants derive from a variety of chemical families, ranging from alcohols to iodine compounds to phenols. The various antiseptics and disinfectants differ from one another with respect to mechanism of action, time course, and antimicrobial spectrum. In almost all cases, the drugs employed as disinfectants are not used for antisepsis and vice versa. The more commonly employed antiseptics and disinfectants are listed in Table 96–1. For each drug, the table indicates chemical family and clinical use (antisepsis, disinfection, or both). TABLE 96–1 Antiseptics and Disinfectants: Chemical Category and Application
Antiseptics and disinfectants
Properties of individual antiseptics and disinfectants

Application
Chemical Category
Drug
Antisepsis
Disinfection
Alcohols
Ethanol
Isopropanol
Aldehydes
Glutaraldehyde
Formaldehyde
Iodine Compounds
Iodine tincture
Iodine solution
Iodophors
Povidone-iodine
Chlorine Compounds
Oxychlorosene
Sodium hypochlorite
Phenolic Compound
Hexachlorophene
Miscellaneous Agents
Chlorhexidine
Hydrogen peroxide
Benzalkonium chloride

Antiseptics and disinfectants
Get Clinical Tree app for offline access


