Chapter 31 Maternal and fetal responses to pregnancy
After reading this chapter, you will be able to:
Introduction
Detailed information on the time course of specific changes and improved understanding of the coordinating factors have increased the potential number of tools to measure key adaptations and assess maternal health. For example, higher plasma concentrations of progesterone and relaxin in early pregnancy are associated with lower mean systolic blood pressure in the second and third trimester, and a positive correlation exists between the extent of plasma volume expansion and fetal growth, reduced risk of pregnancy complications, and pre-term labour (Khraibi et al 2003, Kristiansson & Wang 2001, Steer 2000). Some pre-existing and potential maternal health problems in early pregnancy may be identified through assessment of different hormone levels; and raised body mass index (BMI) (Duvekot et al 1995, Lain et al 2008, Maccarrone & Finazzi-Agro 2004).
This chapter will outline the time course and regulation of adaptive responses of maternal cardiovascular, renal and haemodynamic systems to pregnancy; adaptive responses of maternal hypothalamic–pituitary regulation of prolactin and growth hormone, and adaptive responses of maternal hypothalamic–pituitary–adrenal (HPA) axis to early and late pregnancy (Brunton et al 2008, Douglas 2010). It will conclude by examining the time course and regulation of the adaptive response of the uterus and mammary gland during pregnancy. See also website for more information.
Pulmonary and cardiovascular adaptations
During the infertile cycle, regulation of fluid balance, cardiovascular volume and blood pressure varies during different phases (Chapman et al 1997). This includes some degree of hyperventilation and changing alveolar and arterial tensions of carbon dioxide (CO2) levels influenced by oestrogens and progesterone (see website).
When conception occurs, hyperventilation increases and the magnitude of decline in PCO2 correlates with arterial concentrations of progesterone (Chapman et al 1998, Jensen et al 2005).
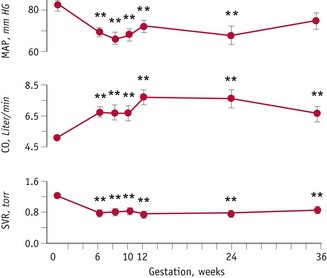
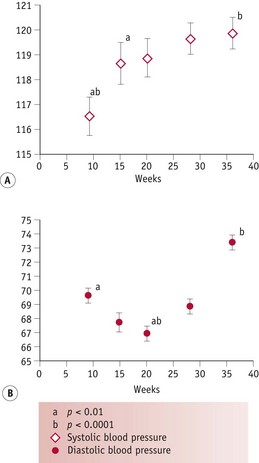
During the luteal phase, mean arterial pressure (MAP) declines significantly, leading to a reflexive rise in cardiac output, while blood volume remains constant (Chapman et al 1997, Williams et al 2001). In most studies, systolic blood pressure is slightly raised compared to the follicular phase, while diastolic pressure is 5% lower. Following conception, the largest change in blood pressure occurs before 8 weeks’ gestation. At this point, 80–90% of the total pregnancy-related fall of around 10 mmHg in MAP has already taken place (Duvekot & Peeters 1998). After 8 weeks, MAP continues to fall, reaching its lowest level by 24 weeks (Robson et al 1989). This pregnancy-related decline in MAP is largely due to the fall in diastolic pressure that begins during the luteal phase (Duvekot & Peeters 1998). Systolic blood pressure remains constant until 20 weeks, rising significantly during the second half of pregnancy, while diastolic pressure reaches its lowest point at 24 weeks and rises significantly for the remainder of pregnancy (Mabie et al 1994, Volman et al 2007) (see Figs 31.1 and 31.2).
Adaptations in fluid regulation
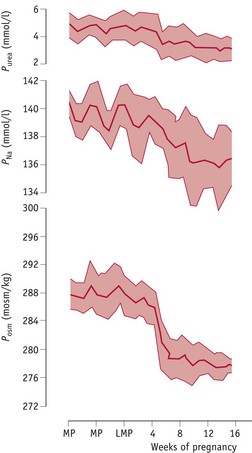
Osmotic thresholds for thirst and vasopressin secretion decline during the luteal phase (see website) leading to a comparable reduction in plasma osmolality, through a fall in plasma sodium and its associated anions, primarily chloride and bicarbonate (Chapman et al 1997, Vokes et al 1988). When conception occurs, plasma osmolality continues to decline to 8–10 mOsmol/kg below mid-follicular values by 10 weeks’ gestation, and this new setpoint for thirst and vasopressin secretion is maintained throughout pregnancy and labour (Chapman et al 1998, Davison et al 1981). These changes are stimulated by a primary renal and systemic vasodilation, which creates a fall in total vascular resistance (Fig. 31.3).
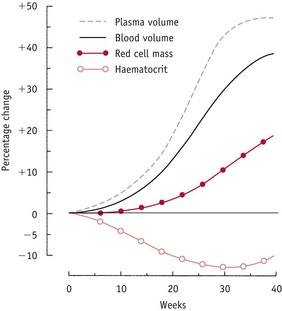
In the absence of any increase in plasma volume, the post-ovulatory fall in renal and systemic vascular resistance initiates a decline in ventricular afterload. This stimulates a reflex rise in cardiac output followed by a significant expansion in plasma volume by 6 weeks’ gestation, increasing rapidly to around 45–50% of non-pregnant values by 36 weeks (Chapman et al 1998). The fall in systemic vascular resistance from the luteal phase stimulates renal sodium and water retention, by activating fluid-retaining components of the renin–angiotensin–aldosterone system (RAAS) while blunting its vasoconstrictive effects (Chapman et al 1997, Chidambaram et al 2002, Sealey et al 1987).
When conception occurs, plasma renin activity, aldosterone and atrial natriuretic peptide (ANP) increase significantly by 6 weeks’ gestation (Chapman et al 1997, 1998, Sealey et al 1985). Despite higher basal activity of the RAAS during pregnancy, the early fall in plasma sodium sets a lower response threshold, and renal reactions to natriuretic stimuli like ANP and vascular responses to vasoconstrictor components, notably vasopressin and angiotensin II, are significantly blunted, as occurs during the luteal phase.
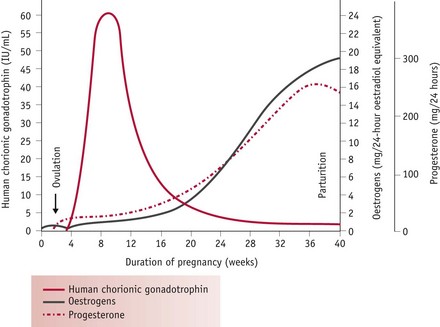
These responses are largely mediated by rising levels of hCG, oestrogens and progesterone (Fig. 31.4). Oestrogens stimulate hepatic synthesis of angiotensinogen (renin substrate) and promote formation of angiotensin-(1-7) [ANG-(1-7)] over angiotensin II (ANG II). ANG-(1-7) opposes the pressor effects of ANG II by releasing nitric oxide, bradykinin and prostacyclin (Valdes et al 2001, Zhang et al 2001). Rising levels of hCG in early pregnancy simultaneously attenuate the pressor effects of ANG II while progesterone counteracts its vasoconstrictive actions by decreasing mean arterial pressure. Progesterone also stimulates aldosterone synthesis, promoting water retention, while the natriuretic actions of progesterone are inactivated in the kidneys (Hermsteiner et al 2002, Quinkler et al 2001, Szmuilowicz et al 2006). Together, these modifications of the RAAS sustain volume expansion without an attendant increase in blood pressure (Duvekot & Peeters 1998, Nakamura et al 1988, Sudhir et al 1995).
Renal haemodynamic adaptations
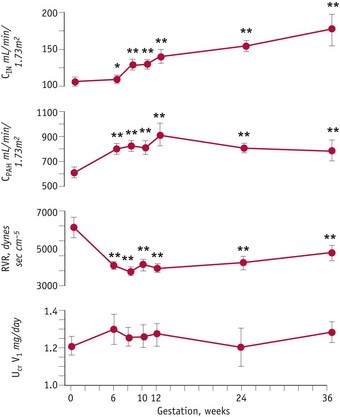
Although the kidneys constitute less than 0.5% of total body weight, in resting non-pregnant adults, blood flow is equal to 25% of cardiac output, reflecting their key role in regulating fluid and electrolyte balance (Stanton & Koeppen 1993). From the mid-luteal phase of the cycle, significant changes occur in renal haemodynamics, as vascular resistance declines, while plasma flow and glomerular filtration rate increase significantly, by 6 weeks’ gestation, compared to values obtained during the mid-follicular phase of the cycle. Minimal renal vascular resistance occurs at 8 weeks’ gestation and this coincides with a peak rise in plasma flow of around 70%, which remains at slightly lower values for the remainder of pregnancy (Baylis & Davison 1998, Chapman et al 1998, Conrad & Novak 2004) (Fig. 31.5).
These findings suggest that hormonal changes in the corpus luteum, before and after conception, stimulate a primary fall in renal and systemic vascular resistance that initiates a chain of events leading to an early rise in cardiac output, renal sodium and water retention, despite the increase in glomerular filtration rate, leading to an expansion in plasma volume, before any increase in basal metabolic rate (Spaanderman et al 2000). A primary reduction in vascular resistance of non-reproductive organs may be initiated in preparation for the dramatic increase in uteroplacental blood flow during the second and third trimesters.
Considerable evidence suggests that the luteal decline in vascular resistance and plasma osmolality and the subsequent rise in plasma volume and total body water are positive indicators of maternal adaptations to pregnancy and long-term health, because of their strong association with increased fetal growth, reduced perinatal mortality and risk of cardiovascular disease in later life (Churchill et al 1997, Conrad et al 2001, Duvekot et al 1995, Steer et al 1995) (see website).
Hormonal regulation
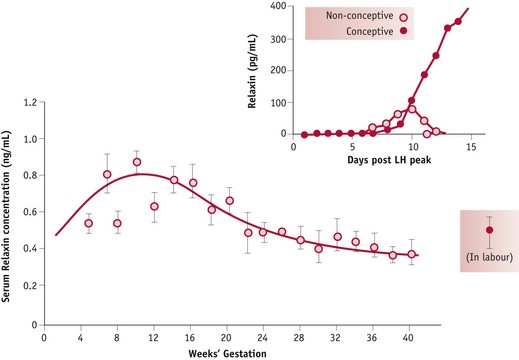
Current findings suggest that the renal and systemic fall in vascular resistance and the rise in cardiac output are stimulated by the post-ovulatory increase in relaxin, oestrogens and progesterone from the corpus luteum followed by a significant rise in adrenomedullin, a long-lasting vasorelaxant, from a variety of tissues by 8 weeks’ gestation (Conrad et al 2001, Di Iorio et al 1999, Hermsteiner et al 2002, Nakamura et al 1988, Novak et al 2001, Sudhir et al 1995).
In pregnancy, relaxin is detectable in the peripheral circulation 6 days after the midcycle LH/FSH surge. By 11 days, concentrations are significantly higher in fertile than in non-fertile cycles and concentrations increase rapidly up to 20 weeks’ gestation (Johnson et al 1991, Stewart et al 1993). Limited human studies have demonstrated that higher plasma concentrations of relaxin and progesterone in early pregnancy are associated with lower mean systolic blood pressure in late pregnancy (Kristiansson & Wang 2001). Recent evidence suggests that while oestrogens have a stimulatory effect on cardiac output and oestrogens and progesterone stimulate systemic and uterine vasodilation, neither hormone seems to influence renal blood vessels, which show a marked degree of dilation following ovulation (Chapman et al 1997, Nakamura et al 1988, Sudhir et al 1995) (Fig. 31.6).
Cardiovascular adaptations
The maternal cardiovascular system undergoes an extensive expansion in response to pregnancy. The first event is decreased vascular resistance of non-reproductive organs, leading to a profound fall in systemic vascular resistance, which reaches its lowest point at the end of the first trimester (Poppas et al 1997). By reducing cardiac afterload, this primary adaptation stimulates an overall rise of approximately 40–50% in cardiac output (Duvekot et al 1995, Volman et al 2007). Longitudinal studies suggest that cardiac output rises significantly during the first trimester, and peaks at 20 to 32 weeks, with no significant changes thereafter, or continues to show further small rises until term (Chapman et al 1997, Desai et al 2004, Duvekot & Peeters 1998, Mabie et al 1994, Volman et al 2007). Measures of the pattern and relative contribution of heart rate (HR) and stroke volume (SV) to increased cardiac output suggest that HR rises progressively until 38 weeks while SV increases significantly by 8 weeks and rises further to reach maximum values between 20 and 37 weeks and declines slightly over the remainder of pregnancy (Desai et al 2004, Robson et al 1989, Volman et al 2007).
Stroke volume increases significantly by 8 weeks, peaks at 16–22 weeks and plateaus or shows further small increases during the third trimester (Volman et al 2007). This represents a rise of 21–22% over pre-pregnancy values. In different studies, HR has been found to increase significantly above pre-pregnancy values by 5 and 16 weeks’ gestation. Data on the remainder of pregnancy suggest that the increase peaks at 31–36 weeks and shows no significant change or a slight decline thereafter. Current evidence indicates that HR may increase by between 11% and 17% over pre-pregnancy values (Capeless & Clapp 1989, Duvekot et al 1993, Robson et al 1989, Volman et al 2007) (see website).
Peripheral arterial vasodilatation
Cardiac output rises in early pregnancy, in response to a fall in systemic vascular resistance that reduces afterload in the myocardial fibres during left ventricular ejection. Decreases in both MAP and total peripheral resistance are evident at 8 weeks’ gestation and reach their lowest point by the middle of pregnancy, before returning to similar or slightly above pre-pregnancy values at term (Desai et al 2004). The decline in peripheral vascular resistance is brought about by early reduced vasomotor tone; remodelling of resistance-sized arteries; relaxation of systemic, renal and pulmonary vascular tone and development of new vascular beds in the placenta during the second trimester (Conrad & Novak 2004, Kelly et al 2004, Schrier & Briner 1991).
The initial fall is induced during the luteal phase by rising levels of oestrogens and progesterone. Progesterone has been shown to reduce muscle tone in the vasculature (Magness & Rosenfeld 1989, Omar et al 1995). When fertilization occurs, the further fall in peripheral vascular resistance creates a state of relative hypovolaemia, causing a reflexive increase in stroke volume and heart rate (Clapp et al 1988, Davison & Noble 1981, Duvekot et al 1993, Schrier & Briner 1991).
The compensatory rise in cardiac output produces a rise in vascular filling state characterized by a rise in left atrial diameter, a rise in glomerular filtration rate and little change in plasma renin, between 5 and 8 weeks’ gestation (Duvekot et al 1995). Studies have demonstrated that the fall in vascular resistance precedes the rise in circulating blood volume during pregnancy. This suggests that systemic vasodilatation is a primary adaptation to pregnancy that initiates a rise in cardiac output and maintains overall tissue perfusion and blood pressure, prior to significant increases in circulating blood volume (Capeless & Clapp 1989, Phippard et al 1986) (Fig. 31.7).