Chapter 45 Respiratory and cardiac disorders
After reading this chapter, you will be able to:
Introduction
Developments in the organization of the maternity services means that midwives are taking on greater responsibility for the care of the newborn at birth and in the postnatal period. Midwives need to be able to respond to the challenge of resuscitating newborn babies, examining babies to confirm normality and detect abnormality, and stabilizing sick infants while awaiting transfer to tertiary facilities. This chapter provides an overview of fetal anatomy and physiology and relates this to resuscitation of the newborn (see Ch. 29). The chapter also offers an overview of some of the common respiratory and cardiac disorders midwives may encounter in practice.
Respiratory and cardiac development in the fetus
Respiratory development (Table 45.1)
Fetal lungs are filled with fluid secreted by the lungs – rather than amniotic fluid. This fluid is important in facilitating the maturation and development of the fetal lungs. Approximately 300–350 mL of fetal lung fluid is produced daily by the fetus at term. In utero, it can move up the trachea, where some is swallowed by the fetus and some escapes into the amniotic fluid. At birth, a small amount of this fluid drains from the nose but most is moved out of the alveoli into the lymphatic system with the first breaths (Greenough & Milner 2005).
Table 45.1 Respiratory development in the fetus
| Post conception | |
|---|---|
| 3–6 weeks | Fetal lungs start to develop from the foregut The division of the foregut and the respiratory system is complete by the end of this period. Disruption at this time can lead to abnormalities such as tracheo-oesophageal fistula (Blackburn 2007) |
| 7–16 weeks | Respiratory system continues to grow and differentiate |
| 16 weeks | Tracheobronchial tree is formed Cilia and mucus-producing glands are present |
| 16–26 weeks | Primitive bronchioles start to develop rich vascular network required for gaseous exchange in extrauterine life |
| 20–24 weeks | Lung is lined with epithelium composed of Type I and Type II pneumatocytes Type II pneumatocytes start to appear Type II pneumatocytes produce surfactant, a pulmonary lipoprotein which decreases surface tension, thus reducing the work of breathing As gestational age increases, more surfactant is synthesized (Blackburn 2007) |
| 24 weeks | Vascular system proliferates Leads to thinning of the vascular epithelium and the capillaries come into close contact with the developing airways – eventually becomes the blood–gas barrier |
| 26 weeks onwards | Terminal air sacs appear, which then develop into the alveoli (Note: Despite the lack of alveoli in babies born between 24 and 26 weeks’ gestation, the vascular bed is sufficient to allow some gaseous exchange and this can, with support, sustain extrauterine life [Hodson 1998]) |
| 29–35 weeks | Proliferation of alveoli starts and increases dramatically (Hodson 1998) Development of alveoli continues after birth |
| 30 weeks onwards | Significant increase of total lung surface and lung volume |
| 35 weeks | Fetus has sufficient surfactant and functional alveoli to support extrauterine life |
Cardiac development
The cardiovascular system is the first system to develop in the embryo. The rapidly developing embryo requires an efficient and effective way of transporting oxygen and nutrients and excreting waste products (Blackburn 2007). The heart begins to develop from the neural plate at around 3 weeks post conception, at first appearing like two long strands. The cords undergo a process known as canalization to become two hollow endocardial tubes which fold back on themselves and fuse to become a single tube. This becomes the endocardium. The tissue around the outside of the endocardial tube becomes thicker and eventually becomes the myocardium.
The fetal circulation
The placenta provides the fetus with oxygen and nutrients and also disposes of waste products. In order to achieve this, the fetal circulation has a number of unique features, including temporary structures, to allow shunting of blood, allowing the mixing of oxygenated and deoxygenated blood, high pulmonary vascular resistance and a low systemic circulation (see Ch. 29 and website).
Transition to extrauterine life
At birth, the newborn infant is exposed to changes in temperature and tactile stimulation, which with the hypoxic and hypercapnic changes that take place as labour progresses, stimulates the first breath. This breath inflates the lungs and forces the fetal lung fluid out into the lymphatic system (Strang 1977). Pulmonary vascular resistance decreases dramatically and the pressure in the right side of the heart falls. Because gas exchange now occurs in the lungs, alveolar oxygenation concentration levels increase.
The changes in the temporary structures may take some time to become permanent. It is recommended that auscultation of the fetal heart to elicit cardiac murmurs should be delayed until at least 6 hours after birth to allow for the closure of the ductus arteriosus and the foramen ovale (Onuzo 2006).
The changes may also be reversed in adverse conditions – hypoxia can cause the ductus arteriosus to remain patent, particularly in preterm infants. Some infants are less likely to make a successful transition to extrauterine life. Preterm infants may experience difficulty in establishing adequate lung volume or oxygenation because of poor muscle tone or lack of surfactant. Babies born at term by elective caesarean section are more likely to encounter problems clearing fetal lung fluid because of the lack of stress response associated with labour, leading to the development of transient tachypnoea of the newborn (Morrison et al 1995).
Resuscitation of the newborn
The need for resuscitation may be anticipated in certain situations (Box 45.1), but is not always predictable; therefore, all midwives must have skills in resuscitation of the newborn. ‘Fire drills’ and participation at courses, including the Newborn Life Support (NLS) course, are useful ways of maintaining skills in resuscitation of the newborn.
Thermal control
Babies are born into an environment considerably cooler than the intrauterine environment, are wet and have a large surface area (see Chs 42, 44 and website). Prior to resuscitation, term babies should be dried and wrapped in warm towels to prevent heat loss, and their thermoregulation needs to be monitored during resuscitation.
Induced hypothermia as a possible method of reducing neurological damage in babies with birth asphyxia is currently under investigation, and results are awaited (Edwards & Azzopardi 2006).
Airway management
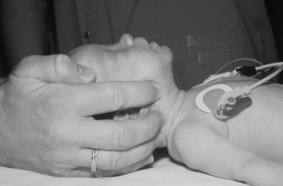
The baby is placed on his back on a flat surface. The prominent occiput tends to encourage the neck to flex and this can lead to airway obstruction. To correct this, the baby’s head is placed in the neutral position (Fig. 45.1). To help maintain this position, a small roll may be placed under the shoulders.
If the baby has poor tone, the tongue may fall back and block the airway. To overcome this, jaw thrust can be employed. This involves placing the fingers under the angle of the jaw to move it forward. This can be done with one hand or two (Fig. 45.2).
Breathing
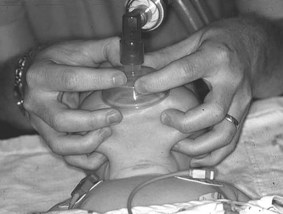
A face mask which covers the nose and mouth without leaving gaps is selected and attached to either a T-piece or 500 mL self-inflating bag. If using a T-piece, the pressure setting should be checked and the blow-off valve on the self-inflating bag should be checked to ensure it works. There is debate as to whether resuscitation of the newborn requires oxygen or if air can be just as effective. Current evidence suggests that oxygen is required for preterm babies but there is a lack of good-quality information in relation to term babies (Wang et al 2008). If oxygen is available, then it should be used. If a T-piece is used, the pressure limiter should be set to a maximum of 30 cmH2O initially.
Circulation
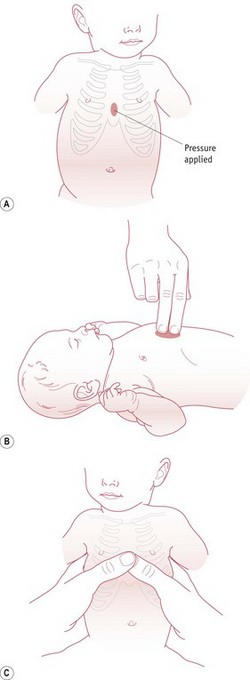
The most effective method of chest compressions is to encircle the chest with both hands, placing the thumbs just below the nipples on the sternum (David 1988). Alternatively, though less effective, a two finger technique can be used (Fig. 45.3). The chest is then compressed by about a third. Three chest compressions should be carried out for every ventilation breath. The heart rate should be assessed after 30 seconds. Once the heart rate is above 60 bpm, cardiac compressions can be stopped.