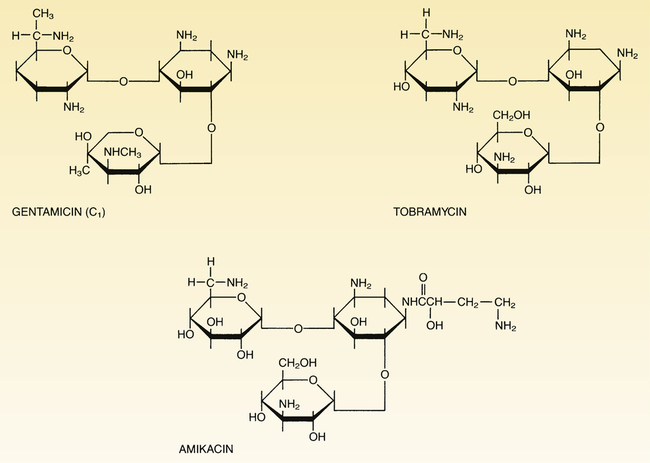
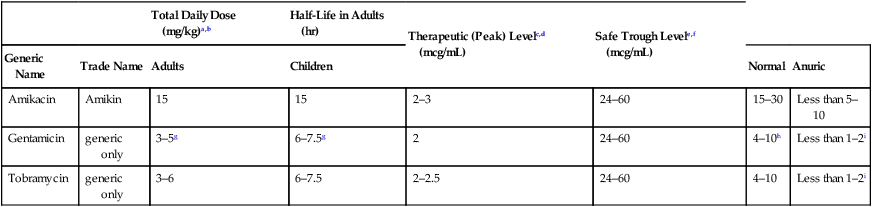
CHAPTER 87 The aminoglycosides are composed of two or more amino sugars connected by a glycoside linkage, hence the family name. At physiologic pH, these drugs are highly polar polycations (ie, they carry several positive charges), and therefore cannot readily cross membranes. As a result, aminoglycosides are not absorbed from the GI tract, do not enter the cerebrospinal fluid, and are rapidly excreted by the kidneys. Structural formulas for the three major aminoglycosides are shown in Figure 87–1. The aminoglycosides disrupt bacterial protein synthesis. As indicated in Figure 87–2, these drugs bind to the 30S ribosomal subunit, and thereby cause (1) inhibition of protein synthesis, (2) premature termination of protein synthesis, and (3) production of abnormal proteins (secondary to misreading of the genetic code). All of the aminoglycosides have similar pharmacokinetic profiles. Pharmacokinetic properties of the principal aminoglycosides are summarized in Table 87–1. TABLE 87–1 Dosages and Pharmacokinetics of Systemic Aminoglycosides aThe total daily dose may be administered as one large dose each day, or as two or three divided doses given at equally spaced intervals around-the-clock. bBecause of interpatient variability, standard doses cannot be relied upon to produce appropriate serum drug levels, and hence dosage should be adjusted on the basis of serum drug measurements. cMeasured 30 minutes after IM injection or completing a 30-minute IV infusion. dThe peak values presented refer to levels obtained when the total daily dosage is given in divided doses, rather than as a single large daily dose. eMeasured just prior to the next dose. fTo minimize ototoxicity and nephrotoxicity, drug levels should drop below the listed values between doses. gWhen gentamicin is combined with either vancomycin or a beta-lactam antibiotic to treat certain gram-positive infections, the total daily dose is much lower (eg, about 1 mg/kg for adults). hThese peak values apply when gentamicin is used to treat gram-negative infections, not when gentamicin is combined with vancomycin or a beta-lactam antibiotic to treat gram-positive infections. iFor severe infections, the trough may be higher (eg, less than 2-4 mcg/mL). The aminoglycosides are eliminated primarily by the kidney. These drugs are not metabolized. In patients with normal renal function, half-lives of the aminoglycosides range from 2 to 3 hours. However, because elimination is almost exclusively renal, half-lives increase dramatically in patients with renal impairment (see Table 87–1). Accordingly, to avoid serious toxicity, we must reduce dosage size or increase the dosing interval in patients with kidney disease.
Aminoglycosides: bactericidal inhibitors of protein synthesis
Basic pharmacology of the aminoglycosides
Chemistry
Mechanism of action
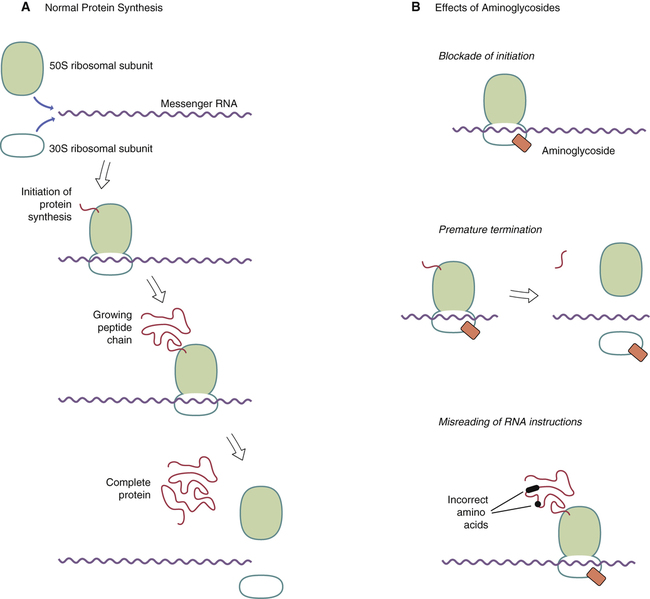
 Mechanism of action of aminoglycosides.
Mechanism of action of aminoglycosides.
A, Protein synthesis begins with binding of the 50S and 30S ribosomal subunits to messenger RNA (mRNA), followed by attachment of the first amino acid of the new protein to the 50S subunit. As the ribosome moves down the mRNA strand, additional amino acids are added to the growing peptide chain. When the new protein is complete, it separates from the ribosome, and then the ribosomal subunits separate from the mRNA. B, Aminoglycosides bind to the 30S ribosomal subunit and can thereby (1) block initiation, (2) terminate synthesis before the new protein is complete, and (3) cause misreading of the genetic code, which causes synthesis of faulty proteins.
Pharmacokinetics

Total Daily Dose (mg/kg)a,b
Half-Life in Adults (hr)
Therapeutic (Peak) Levelc,d (mcg/mL)
Safe Trough Levele,f (mcg/mL)
Generic Name
Trade Name
Adults
Children
Normal
Anuric
Amikacin
Amikin
15
15
2–3
24–60
15–30
Less than 5–10
Gentamicin
generic only
3–5g
6–7.5g
2
24–60
4–10h
Less than 1–2i
Tobramycin
generic only
3–6
6–7.5
2–2.5
24–60
4–10
Less than 1–2i
Elimination.
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aminoglycosides: bactericidal inhibitors of protein synthesis
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access