Barbara Lauritsen Christensen
Care of the patient with a cardiovascular or a peripheral vascular disorder
Objectives
1. Discuss the location, size, and position of the heart.
2. Identify the chambers of the heart and their functions.
3. Identify the valves of the heart and their locations.
4. Discuss the electrical conduction system that causes the cardiac muscle fibers to contract.
5. Explain what produces the two main heart sounds.
6. Trace the path of blood through the coronary circulation.
7. List diagnostic tests used to evaluate cardiovascular function.
9. Describe five cardiac dysrhythmias.
12. Discuss the purposes of cardiac rehabilitation.
15. Identify 10 conditions which may result in the development of secondary cardiomyopathy.
16. Discuss the indications and contraindications for cardiac transplant.
17. Describe the effects of aging on the peripheral vascular system.
18. Identify risk factors associated with peripheral vascular disorders.
19. Compare and contrast signs and symptoms associated with arterial and venous disorders.
20. Discuss nursing interventions for arterial and venous disorders.
21. Compare essential (primary) hypertension, secondary hypertension, and malignant hypertension.
23. Discuss the importance of patient education for hypertension.
26. Discuss appropriate patient education for thrombophlebitis.
Key terms
angina pectoris (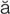 n-J
n-J -n
-n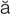 P
P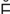 K-t
K-t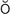 r-
r- s, p. 321)
s, p. 321)
arteriosclerosis ( r-t
r-t -r
-r –
– -skl
-skl -R
-R -s
-s s, p. 355)
s, p. 355)
atherosclerosis ( th-
th- r-
r- -skl
-skl -R
-R -s
-s s, p. 320)
s, p. 320)
bradycardia (br d-
d- -K
-K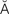 R-d
R-d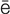 –
–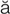 , p. 315)
, p. 315)
B-type natriuretic peptide (BNP) (n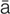 ’tr
’tr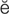 -y-r
-y-r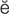 t-
t-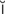 k, p. 311)
k, p. 311)
cardioversion (k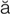 r-d
r-d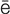 –
– -V
-V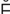 R-zh
R-zh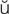 n, p. 310)
n, p. 310)
coronary artery disease (CAD) (p. 320)
defibrillation (d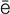 -f
-f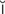 b-r
b-r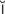 -L
-L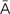 -sh
-sh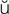 n, p. 317)
n, p. 317)
dysrhythmia (d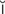 s-R
s-R TH-m
TH-m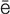 –
–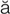 , p. 314)
, p. 314)
endarterectomy (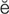 nd-
nd-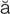 r-t
r-t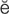 r-
r-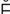 K-t
K-t -m
-m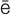 , p. 358)
, p. 358)
hypoxemia (h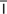 -p
-p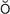 k-S
k-S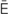 -m
-m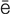 –
–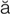 , p. 310)
, p. 310)
intermittent claudication (kl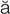 w-d
w-d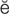 -K
-K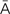 -sh
-sh n, p. 349)
n, p. 349)
myocardial infarction (MI) (m –
– -K
-K R-d
R-d –
–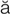 l
l 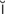 n-F
n-F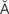 RK-sh
RK-sh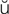 n, p. 326)
n, p. 326)
orthopnea (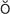 r-th
r-th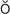 p-N
p-N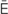 –
–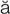 , p. 334)
, p. 334)
peripheral (p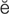 -R
-R F-
F-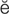 r-
r-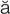 l, p. 348)
l, p. 348)
pleural effusion (PLR-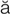 l
l  -F
-F -zh
-zh n, p. 333)
n, p. 333)
polycythemia (p l-
l- -s
-s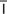 -TH
-TH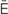 -m
-m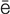 –
–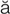 , p. 310)
, p. 310)
pulmonary edema (P L-m
L-m -n
-n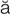 -r
-r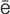
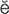 -D
-D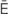 -m
-m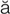 , p. 340)
, p. 340)
tachycardia (t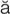 k-
k-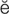 -K
-K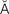 R-d
R-d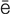 –
–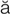 , p. 314)
, p. 314)
Anatomy and physiology of the cardiovascular system
The cardiovascular (circulatory) system is the transportation system of the body. It delivers oxygen and nutrients to the cells to support their individual activities and transports the cells’ waste products to the appropriate organs for disposal. This chapter discusses the structure and function of the blood vessels and the heart.
Heart
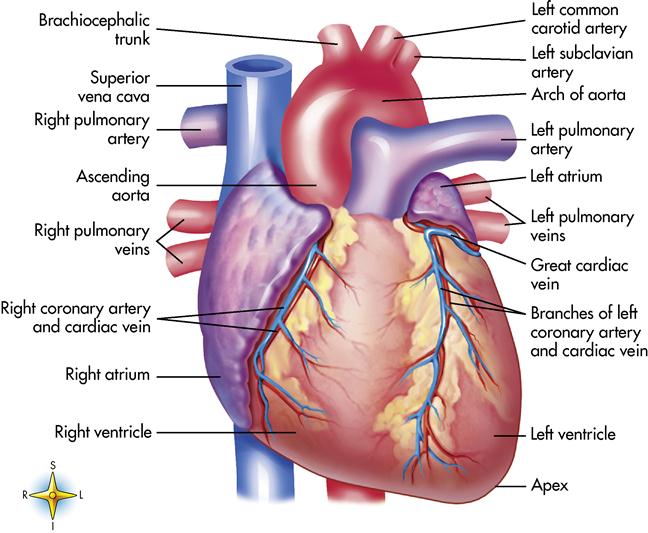
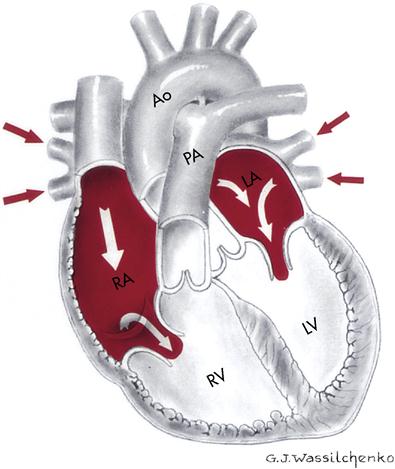
The heart is a remarkable organ, not much bigger than a fist (Figure 8-1). It is responsible for pumping 1000 gallons of blood every day through the closed circuit of blood vessels. It beats 100,000 times a day and transports the blood 60,000 miles through a network of blood vessels. The heart is a hollow organ composed mainly of muscle tissue with a series of one-way valves.
The heart is located in the chest cavity between the lungs in a region called the mediastinum (the mass of organs and tissues separating the lungs; in addition to the heart and its greater vessels, the mediastinum contains the trachea and the esophagus). Two thirds of the heart lies left of the midline. The wider base of the heart lies superior and beneath the second rib. The apex, or narrow part, of the heart lies inferiorly, slightly to the left between the fifth and sixth ribs near the diaphragm.
Heart wall
The heart is composed of three layers: pericardium, myocardium, and endocardium. The pericardium is a two-layered, serous membrane that covers the entire structure. Between the two thin membranes is a serous fluid that allows friction-free movement of the heart as it contracts and relaxes. The pericardium is the outermost layer of the heart. The myocardium forms the bulk of the heart wall and is the thickest and strongest layer of the heart. It is composed of cardiac muscle tissue. Contraction of this tissue is responsible for pumping blood. The endocardium (innermost layer) is composed of a thin layer of connective tissue. This structure lines the interior of the heart, the valves, and the larger vessels of the heart.
Heart chambers
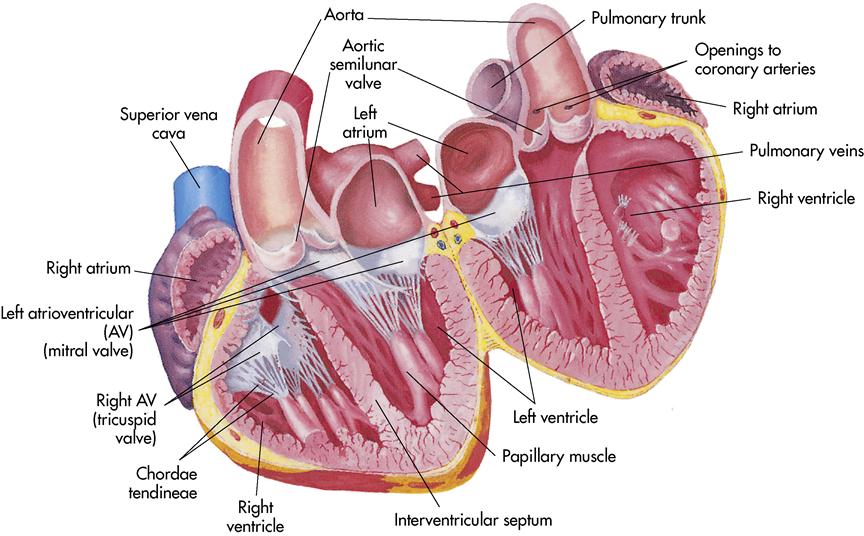
The heart is divided into a right and left half by a muscular partition called the septum (Figure 8-2). The heart has the following four chambers:
The heart actually functions as two separate pumps: (1) the right side receives deoxygenated blood and pumps it to the lungs, and (2) the left side receives oxygenated blood from the lungs and pumps it throughout the body.
Heart valves
Located within the heart are four valves that keep the blood moving forward and prevent backflow. The heart has two atrioventricular (AV) valves. They are located between the atrium and the ventricles. The right AV valve, located between the right atrium and the right ventricle, is called the tricuspid valve because it contains three flaps, or cusps. The left AV valve is composed of two cusps (bicuspid) and is commonly called the mitral valve. It is located between the left atrium and the left ventricle. Both of these valves rapidly close to prevent backflow of blood. Small cordlike structures, chordae tendineae, connect the AV valves to the walls of the heart and work with the papillary muscles located in the walls of the ventricles to make a tight seal to prevent backflow when the ventricles contract.
The two remaining valves, the semilunar valves, are located at the points where the blood exits the ventricles. The pulmonary semilunar valve is located between the right ventricle and the pulmonary artery. Blood is pushed out of the right ventricle and travels to the lung via the pulmonary artery. The aortic semilunar valve is located between the left ventricle and the aorta. When the left ventricle contracts, the blood is forced into the aorta and the aortic semilunar valve closes. Both of the semilunar valves have three cusps that resemble a half moon, hence the name semilunar (see Figure 8-2).
Electrical conduction system
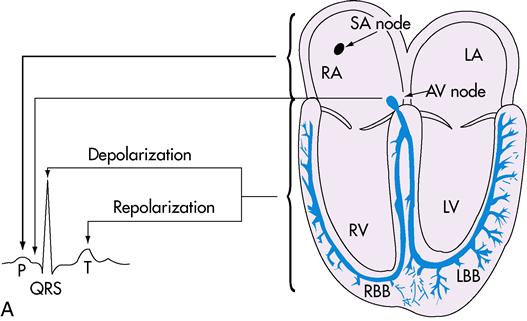
Heart muscle tissue contains an inherent ability to contract in a rhythmic pattern. This ability is called automaticity. If heart muscle cells are removed and placed under a microscope, they continue to beat. In addition, they can respond to a stimulus in the same way that nerve cells do. This unique property is called irritability. Automaticity and irritability are two characteristics that affect the functions of the conduction system. Hormones, ion concentration, and changes in body temperature also affect the conduction of messages around the heart, initiation of heartbeat, and coordination of beating patterns between the atria and the ventricles.
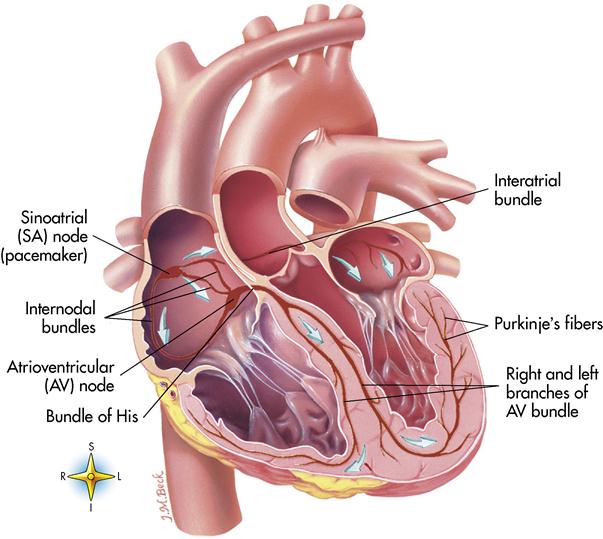
The heartbeat is initiated in the sinoatrial (SA) node, which is located in the upper part of the right atrium, just beneath the opening of the superior vena cava (Figure 8-3). Because it regulates the heartbeat, the SA node is known as the pacemaker. Impulses are passed to the AV node, which is located in the base of the right atrium. The AV node slows the impulses to allow the atrium to complete contraction and to allow the ventricles to fill. The impulses then pass to a group of conduction fibers called the bundle of His and divides into right and left branches of AV bundle to travel to smaller branches called the Purkinje fibers, which surround the ventricles. The message travels rapidly through the ventricles and causes contractions, which empty the ventricles.
IMPULSE PATTERN: SA node → AV node → bundle of His → right and left bundle branches of AV bundle → Purkinje fibers
Cardiac cycle
The cardiac cycle refers to a complete heartbeat. The two atria contract while the two ventricles relax. When the ventricles contract, the two atria relax. The phase of contraction is called systole (Figure 8-4), and the phase of relaxation is called diastole (the period between contraction of the atria or the ventricles during which blood enters the relaxed chambers from the systemic circulation and the lungs [Figure 8-5]). Complete diastole and systole of both atria and ventricles constitute a cardiac cycle; this takes an average of 0.8 second.
The heart sounds, lubb and dubb, are produced by closure of the valves. The first sound, lubb (long duration and low pitch), is heard when the AV valves close. The second sound, dubb (short duration, sharp sound), is heard when the semilunar valves close. Occasionally a murmur (swishing sound) can be heard. This can be a normal functional phenomenon produced by rapid filling of the ventricles, or it can be an abnormal condition produced by ineffective closure of the valves.
Blood vessels
Three main types of blood vessels are organized to carry blood to and from the heart. Capillaries (tiny blood vessels joining arterioles and venules) connect the arteries (large vessels carrying blood away from the heart) to the veins (vessels that convey blood from the capillaries and return it to the heart). The heart delivers the blood to the arteries, which branch into tiny vessels called arterioles (blood vessels of the smallest branch of the arterial circulation), which deliver the blood to the tissues. Within the tissues, microscopic vessels (capillaries) form an extensive (50,000 miles) network that allows exchanges of products and by-products between the tissues and blood. The capillaries then join with tiny veins, or venules, that link with the larger veins and return to the heart. The pattern is as follows:
Artery → arteriole → capillary → venule → vein
Circulation
Coronary blood supply
To sustain life, the heart must pump blood throughout the body on a continuous basis. As a result, the heart muscle (or myocardium) requires a constant supply of blood containing nutrients and oxygen to function effectively. The delivery of oxygen and nutrient-rich arterial blood to cardiac muscle tissue and the return of oxygen-poor blood from this active tissue to the venous system are called the coronary circulation (Figure 8-6).
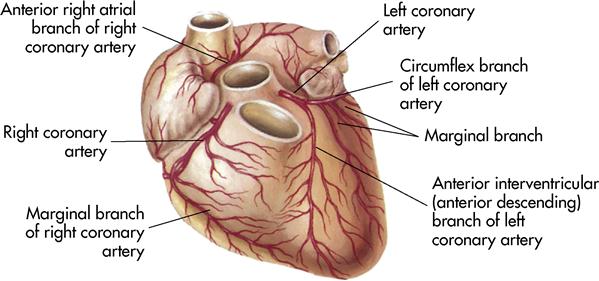
Blood flows into the heart muscle by way of two small vessels, the right and left coronary arteries, which are the best known of all the blood vessels. The coronary arteries form a crown around the myocardium (see Figures 8-1 and 8-6). The openings into these vessels lie behind the flaps of the aortic semilunar valves (see Figure 8-6). The coronary arteries bring oxygen and nutrition to the myocardium. Once the circulation is completed and the carbon dioxide and waste products have been collected, the blood flows into a large coronary vein and finally into the coronary sinus, which empties into the right atrium. These two main arteries have many tiny branches that serve the heart muscle. If an artery becomes occluded, these branches provide collateral circulation (alternate routes) to nourish the heart muscle. If the occlusion is severe, surgery and other procedures may be needed. These treatments are discussed later in this chapter.
Systemic circulation
Systemic circulation occurs when blood is pumped from the left ventricle of the heart through all parts of the body and returns to the right atrium. When the oxygenated blood leaves the left ventricle, it enters the largest artery (1 inch [2.5 cm] in diameter) of the body, the aorta. This is the main trunk of the systemic arterial circulation and is composed of four parts: the ascending aorta, the arch, the thoracic portion of the descending aorta, and the abdominal portion of the descending aorta. As the blood flows through the artery branches, the branches become smaller in diameter (arterioles). The blood continues to flow into the capillaries. The capillaries surround the cells and exchange oxygen, nutrients, carbon dioxide, and other waste products. The blood proceeds to the tiny venules, then to the larger veins, and finally returns to the right atrium via the largest vein, the vena cava (one of two large veins returning blood from the peripheral circulation to the right atrium of the heart).
The blood is now deoxygenated and needs to be replenished with oxygen. Note that the upper portion of the vena cava (superior vena cava) returns deoxygenated blood from the head, neck, chest, and upper extremities. The inferior vena cava returns deoxygenated blood from parts of the body below the diaphragm.
Pulmonary circulation
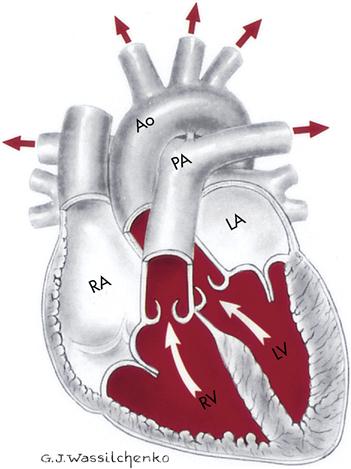
The deoxygenated blood now passes through the pulmonary circulation to pick up the needed oxygen. Blood is pumped from the right atrium to the right ventricle, where it leaves the heart to travel via the pulmonary artery to the lungs. Once the blood reaches the lungs, it travels through arterioles to the capillaries. The microscopic capillaries surround the alveoli (air sacs), where oxygen diffuses into the bloodstream. The capillaries then connect with the venules and finally with the four pulmonary veins, which return the oxygenated blood to the left atrium of the heart. It is then pumped to the left ventricle and to the aorta, and systemic circulation is then repeated. The blood circulation pattern is as follows:
Superior or inferior vena cava → right atrium → tricuspid valve → right ventricle → pulmonary semilunar valve → pulmonary artery → capillaries in the lungs → pulmonary veins → left atrium → bicuspid valve → left ventricle → aortic semilunar valve → aorta
Laboratory and diagnostic examinations
A number of diagnostic tests are used to evaluate cardiovascular function. The nursing responsibilities are to physically prepare the patient for diagnostic procedures and to explain the examination to the patient.
Diagnostic imaging
Radiographic examination of the chest provides a film record of heart size, shape, and position and outline of shadows. Lung congestion is also shown, indicating heart failure (HF), perhaps in the earliest stages. Pleural effusion may be noted in left-sided HF.
Fluoroscopy, the action-picture radiograph, allows observation of movement. It is invaluable in pacemaker or intracardial catheter placement.
An angiogram is a series of radiographs taken after injection of a contrast medium into an artery or vein. Picturing the circulatory process aids in diagnosis of vessel occlusion, pooling in various heart chambers, and congenital anomalies. Angiography allows x-ray visualization of the heart, aorta, inferior vena cava, pulmonary artery and vein, and coronary arteries.
In an aortogram, the abdominal aorta and the major leg arteries are viewed by x-ray visualization after a contrast medium is injected through the femoral artery and into the aorta. Aneurysms and many other abnormalities can be diagnosed. Contrast media to visualize the aortic arch and branches may also be used.
Cardiac catheterization and angiography
Cardiac catheterization is an invasive procedure used to visualize the heart’s chambers, valves, great vessels, and coronary arteries. This procedure aids in diagnosis, in prevention of progression of cardiac conditions, and in accurate evaluation and treatment of the critically ill patient.
The passage of a catheter into the heart chambers through a peripheral vessel is used to measure (1) pressure within the heart and (2) blood-volume relationship to cardiac competence. Valvular defects, arterial occlusion, and congenital anomalies are determined. Blood samples are obtained. Contrast dye may be injected to allow better heart and vessel visualization (angiography). Cardiac catheterization is performed under sterile surgical conditions; its invasive nature requires a prior signed consent. Because iodine is in the contrast medium, determine sensitivity to iodine before injection to avoid an allergic reaction. After the procedure, assess circulation to the extremity used for catheter insertion. Check peripheral pulses, color, and sensation of the extremity every 15 minutes for 1 hour and then with decreasing frequency. Observe the puncture site for hematoma and bleeding. Monitor vital signs. Assess for abnormal heart rate, dysrhythmias, and signs of pulmonary emboli (respiratory difficulty). The patient lies supine for a designated period with a compression device over the pressure dressing at the insertion site to prevent hemorrhage.
Electrocardiography
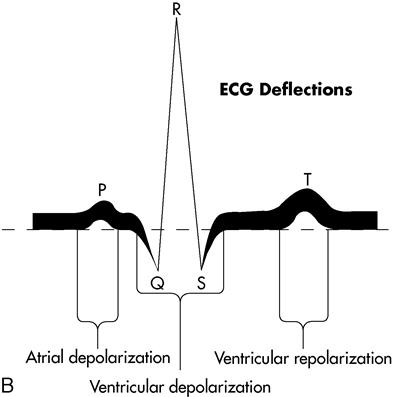
The electrocardiogram (ECG, or EKG) is a graphic study of the electrical activities of the myocardium to determine transmission of cardiac impulses through the muscles and conduction tissue. Each ECG has three distinct waves, or deflections: the P wave, the QRS complex, and the T wave. When the heart contracts, the electrical activity is called depolarization. Repolarization is the relaxation phase. The P wave represents the depolarization of the atria. The QRS complex represents the depolarization of the ventricles. The T wave represents the repolarization of the ventricles. Atrial repolarization is not represented but does occur; it is covered by the large QRS complex and cannot be seen on the ECG tracing.
A standard ECG has 12 electrodes attached to the skin surface to measure the total electrical activity of the heart. Each lead records the electrical potential between the limbs or between the heart and limbs. A conductive gel enhances the contact and transmission. The patient is in a supine position. However, ambulatory ECGs and exercise stress test ECGs require position variation. The machine, an electrocardiograph or galvanometer, records the energy wave of each heartbeat through a vibrating needle on graph paper, which feeds through the machine at a standard rate. Each ECG waveform represents a single electrical impulse as it travels through the heart (Figure 8-7).
The ECG tracing is read or interpreted by a cardiac specialist (cardiologist) or by internal medicine specialists, family practitioners, pediatricians, and emergency department practitioners. The reading can also be displayed on the fluorescent screen (oscilloscope) of a cardiac monitor. A graphic tracing may be printed out by the monitor.
Ambulatory ECGs can be used to monitor heart rhythm over prolonged periods—12, 24, or 48 hours—and compared with various activities or symptoms recorded in a diary kept by the patient. A Holter monitor (small portable recorder) is attached to the patient by leads, with a 2-pound tape recorder carried on a belt or shoulder strap. The monitor operates continuously to record the patterns and rhythms of the patient’s heartbeat. In conjunction with the diary, the physician can note various events, times, and medication peaks that affect or precipitate dysrhythmias. An ambulatory ECG is particularly useful for patients whose clinical symptoms indicate heart disorders but who may have normal ECG tracings on a resting test.
Cardiac monitors
It is common practice to continuously assess the cardiac electrical activity of patients who are known or suspected to have dysrhythmias or who are prone to develop dysrhythmias or acute cardiovascular symptoms. A cardiac monitor displays information on the electrical activity of the heart transferred via conductive electrodes placed on the chest.
Most monitors provide a visual display of cardiac electrical activity and the correct heart rate. Preset alarms warn of heart rates that exceed or drop below limits considered acceptable for each patient and also warn of dysrhythmias.
Ambulatory patients are increasingly monitored by battery-powered ECG transmitters that do not directly connect the patient to the oscilloscope. This monitoring is called telemetry, which is the electronic transmission of data to a distant location. The electrodes placed on the patient’s chest are attached to a transmitter the patient carries in a pocket or pouch. The transmitter sends a radio signal to a receiver, usually located at the nurse’s station.
Patients need telemetry monitoring for various reasons, including a history of cardiac disease, angina pectoris, suspected dysrhythmias, a change in medications, an electrolyte abnormality, or unexplained syncope. Many of these patients are monitored in a centralized area such as an intermediate care or step-down unit (with monitors at the nurse’s station). Remote telemetry means the patient is on a medical-surgical unit and is monitored at a separate location, called the home unit, which is usually on a critical care unit. Remote telemetry patients are usually stable. But even a stable patient’s condition can change rapidly, and telemetry allows continuous heart monitoring to detect abnormalities.
Attachment to a cardiac monitor does not change a patient’s need for nursing interventions. Because the monitoring electrodes are on the anterior thorax (chest) rather than the extremities, the patient is relatively free to carry on usual activities. Pay special attention to the electrode site to ensure a constant tight seal between the electrode and the skin and to note the development of any skin impairment. The conduction gel dries out, even if the pad is sealed; changing electrodes regularly is recommended. Also check the telemetry pack for integrity of the lead wires and test the monitoring device’s battery with a battery tester. Inform the monitoring area whenever the patient is moved off the unit for a diagnostic test, since the patient may go outside the monitor’s range. Another important safety measure is to never remove the telemetry device and allow the patient to shower unless the physician has written the order to allow a shower. The patient could be subject to severe dysrhythmia, which would not be detected with the telemetry device removed.
Exercise-stress ECG is another form of monitoring the heart’s capability, this time in a laboratory setting while the patient performs a prescribed exercise. Tasks include use of treadmills, stair climbing, and aerobic exercise. While being monitored carefully, the patient is coaxed to a limit of exertion to evaluate ischemia, dysrhythmia, and cardiac capability under extreme circumstances. Thus the test sets the limit of exercise tolerance in cardiac disease. If the patient is unable to tolerate activity, a stress test can be done by administering dipyridamole (Persantine) or adenosine (Adenocard), which mimics the patient’s heart under stress or activity.
Thallium scanning
Thallium 201 is an intracellular ion that is actively transported into normal cells. If the cell is ischemic or infarcted, the thallium will not be picked up. Because the thallium concentrates in tissue with normal blood flow, tissue with inadequate perfusion appears as dark areas on scanning—a “cold spot.” The radioisotope is injected intravenously while the patient exercises on a treadmill. Breast tissue in the female can produce artifact, leading to a false-positive result. Using technetium 99m sestamibi instead of thallium can help minimize artifact and thus improve accuracy. In patients who cannot tolerate physical exercise, dipyridamole is given before the thallium to physiologically simulate exercise-induced stress.
Echocardiography
Echocardiography uses high-frequency ultrasound directed at the heart. The echo, or reflected sound, is graphically recorded, outlining size, shape, and position of cardiac structures. This test is used to detect pericardial effusion (collection of blood or other fluid in the pericardial sac), ventricular function, cardiac chamber size and contents, ventricular muscle and septal motion and thickness, cardiac output (ejection fraction), cardiac tumors, valvular function, and congenital heart disorder.
Ejection fraction (EF) of ventricles as demonstrated by an echocardiogram is as follows:
Positron emission tomography
Positron emission tomography (PET) is a computerized radiographic technique that uses radioactive substances to examine the metabolic activity of various body structures. In PET studies the patient either inhales or is injected with a biochemical radioactive substance. Specific color-coded images reveal organs’ metabolic functions. Used to study dementia, stroke, epilepsy, and tumors, PET is proving its merit in the diagnosis and treatment of cardiac disease. PET’s ability to distinguish between viable and nonviable myocardial tissue allows physicians to identify the most appropriate candidates for bypass surgery or angioplasty. PET is also able to accurately detect coronary artery disease (CAD), noninvasively, in an asymptomatic patient, prompting early intervention that can salvage potentially ischemic myocardium.
Laboratory tests
The history, the physical examination, and blood studies aid the health care provider in diagnosing and monitoring the cardiovascular disease process. The nurse’s responsibility is to prepare the patient by explaining the tests and the preparation required for each test.
Blood cultures to detect growth of bacteria in the blood are crucial to the diagnosis of infective endocarditis.
A complete blood count (CBC) is a determination of the number of red and white blood cells per cubic millimeter, as well as the white blood cell differential, platelets, hemoglobin, and hematocrit. Low hemoglobin indicates decreased ability to carry oxygen to the cells and anemia; an elevated white blood cell (leukocyte) count indicates infection or inflammation; and an elevated red blood cell (erythrocyte) count indicates that the body is compensating for chronic hypoxemia (an abnormal deficiency of oxygen in the arterial blood) by stimulating red blood cell production by the bone marrow, leading to secondary polycythemia (abnormal increase in the number of red blood cells in the blood). Chronic hypoxemia is often noted in HF.
Coagulation studies are useful in monitoring the patient receiving anticoagulant drug therapy, which is prescribed for patients with myocardial infarction (MI). Coagulation studies are also important in patients who have chronic atrial fibrillation or patients with atrial fibrillation who are undergoing cardioversion (the restoration of the heart’s normal sinus rhythm by delivery of a synchronized electric shock through two metal paddles placed on the patient’s chest). Coagulation studies are needed if an MI is diagnosed in case fibrinolytics are needed to dissolve the thrombus. These studies include prothrombin time (PT), International Normalized Ratio (INR), and partial thromboplastin time (PTT).
Erythrocyte sedimentation rate (ESR) is used to monitor or rule out inflammatory infective conditions. The ESR is elevated with MI and infective endocarditis and decreases when healing begins. The ESR also indicates the extent of inflammation and infection in rheumatic fever.
Serum electrolyte tests focus on the body’s balance of sodium, potassium, calcium, and magnesium, which are necessary for myocardial muscle function. Sodium (Na+) helps maintain fluid balance. Potassium (K+) is required for relaxation of cardiac muscle, and calcium (Ca++) is necessary for contraction of cardiac muscle. Magnesium (Mg++) helps maintain the correct level of electric excitability in the nerves and the muscles, including the myocardium and the cardiac conduction system. The health care provider compares serum electrolyte levels with ECG changes.
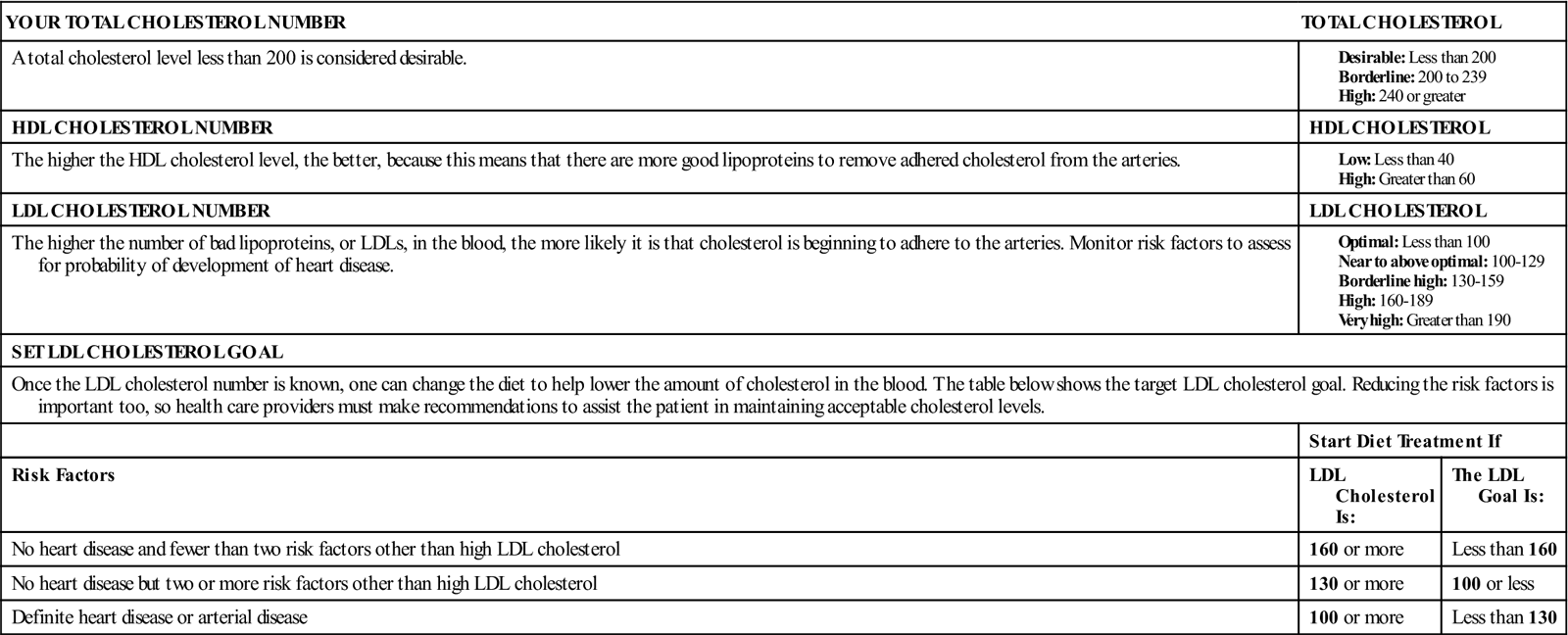
Serum lipids are associated with vascular disease, particularly CAD. Cholesterol and triglycerides bound to plasma proteins are found in the blood as lipoproteins. Density levels vary according to the protein-fat ratio. An elevated high-density lipoprotein (HDL) is desired, but low-density lipoprotein (LDL) or very-low-density lipoprotein (VLDL) increases the risk for cardiovascular disease (see Box 8-1, p. 313).
Arterial blood gases are measured to monitor oxygenation (PaO2, PaCO2) and acid-base balance (pH). This test is useful in patients with unstable cardiac conditions to determine the blood oxygenation process and in evaluation of patients in cardiac failure.
Serum cardiac markers are certain proteins that are released into the blood in large quantities from necrotic heart muscle after an MI. These markers, specifically cardiac serum enzymes and troponin I, are important screening diagnostic criteria for an acute MI. The cardiac enzyme creatine kinase (CK) and its isoenzyme, creatine phosphokinase (CK-MB), have been the gold standard for years. However, CK-MB is also found in skeletal muscle and can be elevated by surgery, muscle trauma, and muscular diseases, so it is not a specific indicator for MI. CK and CK-MB start to rise within 2 to 3 hours after the beginning of an MI, peak in 24 hours, and return to normal within 24 to 40 hours (Nagle, 2002). When cardiac cells die, their cellular enzymes are released into circulation. The increase in serum enzymes that occurs after cell death can demonstrate whether cardiac damage is present and the approximate extent of the damage. Other causes of increased serum enzymes may make the differential diagnosis more difficult.
Troponin I is a myocardial muscle protein released into circulation after a myocardial injury. In the heart there are two subtypes: cardiac-specific troponin T and troponin I. These are sensitive markers that identify very small amounts of myocardial damage. Troponin T appears in the blood 3 to 5 hours after an MI and may remain elevated for up to 21 days. Like CK-MB, troponin T is affected by skeletal muscle injury and renal disease. Troponin I is a sensitive and specific cardiac marker, not influenced by skeletal muscle trauma or renal failure. Troponin I rises 3 hours after MI, peaks at 14 to 18 hours, and returns to normal in 5 to 7 days. Troponin I is useful in diagnosing an MI (Nagle, 2002). The recent ability to measure myocardial contractile proteins (troponins) in serum is a milestone in the diagnosis of acute MI and acute myocardial damage resulting from other causes.
Myoglobin is released into circulation within a few hours after an MI. Although it is one of the first serum cardiac markers that increase after an MI, myoglobin is also present in skeletal muscle, so an increase can be associated with noncardiac causes. In addition, it is rapidly excreted in urine so that blood levels return to normal range within 24 hours after an MI.
B-type natriuretic peptide (BNP) is a neurohormone secreted by the heart in response to ventricular expansion. An elevated BNP of greater than 100 pg/mL indicates HF. BNP is present in the ventricle of the heart and correlates well to left ventricular pressure. The greater the BNP level, the more severe the HF (Pagana & Pagana, 2007).
Homocysteine is an amino acid produced during protein digestion. Normal values range from 4 to 14 µmol/L. Elevated blood levels of homocysteine may act as an independent risk factor for ischemic heart disease, cerebrovascular disease, peripheral arterial disease (PAD), and venous thrombosis. Homocysteine appears to promote the progression of atherosclerosis by causing endothelial damage, promoting LDL deposits, and promoting vascular smooth muscle growth. Homocysteine is an amino acid that plays an important role in blood clotting. An elevated level results in increased platelet aggregation. Screening for elevated homocysteine levels (more than 14 μmol/L) should be considered in patients who have progressive and unexplained atherosclerosis despite normal lipoproteins and who have no other risk factors. It is also recommended in patients with an unusual family history of atherosclerosis, especially at a young age.
Dietary deficiency of vitamins B6, B12, or folate is the most common cause of elevated homocysteine. Some researchers believe that elevated levels of homocysteine can be treated by administration of vitamins B6, B12, and folate. Whether this treatment will reduce the incidence of MI remains to be seen (Pagana & Pagana, 2007).
The liver produces C-reactive protein (CRP) during periods of acute inflammation. The presence of CRP is a predictor of cardiac events and is emerging as an independent risk factor for CAD. Persons who have diabetes mellitus are already at high risk for developing cardiovascular disease. If a patient has both an elevated CRP and diabetes, his or her risk for a cardiovascular disorder becomes even greater (Pagana & Pagana, 2007).
Disorders of the cardiovascular system
Cardiovascular disorders are a major health care problem in the United States. Public awareness, modifications in lifestyles, and improvements in medical treatment have contributed to a decline in overall deaths. The nurse’s role in caring for patients with cardiovascular disorders includes being aware of the prevalence of cardiac disease, risk factors, and the disease process; implementing nursing interventions; and patient teaching.
Normal aging patterns
By the time an individual reaches the age of 65 years, physiologic changes have reduced the efficiency of the heart as a pump. Yet the heart still is capable of functioning adequately unless there is underlying cardiac disease (see Life Span Considerations box).
Risk factors
Research has identified risk factors that indicate predispositions to developing cardiovascular disease. The presence of more than one risk factor is associated with an increasing risk of developing cardiovascular disease. Risk factors are classified as those that are nonmodifiable and those that are modifiable.
Nonmodifiable factors
An important aspect of caring for the patient with a cardiovascular disorder is understanding the risk factors for cardiovascular disease and incorporating them into patient teaching. The nonmodifiable risk factors associated with cardiovascular disorders include the following.
Family history
A family member such as a parent or sibling who has a cardiovascular problem before 50 years of age places the patient at greater risk for developing cardiovascular disease.
Age
Normal physiologic changes that occur with aging and past lifestyle habits increase the patient’s risk for developing cardiovascular disease with advancing age. CAD and MI occur most frequently among white, middle-aged men.
Gender
Middle-aged men are at a greater risk of developing cardiovascular disease than women. Although the incidence in men and women equalizes after age 65, cardiovascular disease is a greater cause of death in women than in men. Women develop CAD about 10 years later than men because natural estrogen is believed to have a cardioprotective effect before menopause. The incidence of cardiovascular disease in women 50 years of age and older is increasing. Factors believed to be responsible are increased social and economic pressures on women and changes in lifestyle. Ten times more women die from heart disease than die from breast cancer. The mortality rate for women with CAD has remained relatively constant even though cardiovascular disease remains the leading cause of death. Despite this statistic, only 15% of women consider CAD their greatest health risk. Recent research on CAD has shown that women often do not have the classic signs and symptoms of an acute coronary event (Lewis et al., 2007).
Cultural and ethnic considerations
Black men have a higher incidence of hypertension than do white men. Black women have a higher incidence of CAD, with greater severity and higher death rates, than white women (Lewis et al., 2007).
Modifiable factors
Smoking
Individuals who smoke have a two to three times greater risk of developing cardiovascular disease than nonsmokers. The degree of risk is proportional to the number of cigarettes smoked. Individuals who quit smoking decrease their risk. Tobacco smoke contains nicotine, which causes catecholamine (i.e., epinephrine, norepinephrine) release. Catecholamine release causes tachycardia, hypertension, and vasoconstriction of the peripheral arteries, which in turn increase the work of the heart and result in greater myocardial oxygen consumption (Lewis et al., 2007). Nicotine also increases platelet adhesion, which results in increased risk of embolism (Lewis et al., 2007). The nicotine content of cigarettes causes the production of carbon monoxide, which places a greater demand on the heart and interferes with oxygen supply.
Hyperlipidemia
Hyperlipidemia is elevated concentrations of any or all lipids in the plasma. The ratio of HDL to LDL is the best predictor for the development of cardiovascular disease. Density levels vary according to the protein-fat ratio:
| Less than 100 mg/dL | Optimal |
| 100 to 129 mg/dL | Near optimal to above optimal |
| 130 to 159 mg/dL | Borderline high |
| 160 to 189 mg/dL | High |
| More than 190 mg/dL | Very high |
• HDL contains more protein than fat (which serves a protective function, removing cholesterol from tissues). It is suspected that HDL also removes cholesterol from the peripheral tissues and transports it to the liver for excretion. Also, HDL may have a protective effect by preventing cellular uptake of cholesterol and lipids. Low levels (less than 40 mg/dL) are believed to increase a person’s risk for CAD, whereas high levels (more than 60 mg/dL) are considered protective (Box 8-1).
A diet high in saturated fat, cholesterol, and calories contributes to hyperlipidemia. Therefore dietary control is an important factor in modifying this risk factor. An overall serum cholesterol level of less than 200 mg/dL is desirable, 200 to 239 mg/dL is borderline high, and more than 239 mg/dL is high.
Change in diet is probably the most important method of lowering cholesterol level. Weight reduction, in overweight patients with abnormal lipid profiles, is an essential element of the dietary intervention. In addition to lowering LDL levels, weight reduction leads to decreases in triglyceride level and blood pressure. A combination of weight reduction and physical exercise improves the lipid profile, with a decrease in LDL level, an increase in HDL level, and a decrease in triglyceride levels. Low HDL levels are often familial and only somewhat modifiable.
Cholesterol-lowering drugs are often included in treatment of hyperlipidemia. Cholesterol-lowering drugs are divided into six classes: (1) bile acid sequestrants; (2) nicotinic acid (niacin); (3) statins such as simvastatin (Zocor), pravastatin (Pravachol), and rosuvastatin (Crestor); (4) fibric acid derivatives such as gemfibrozil (Lopid) and probucol (Lorelco); (5) the cholesterol absorption inhibitor ezetimibe [Zetia]; and (6) combination drugs such as ezetimibe and simvastatin (Vytorin) (Cuddy, 2006). Pravastatin reduces the risk of a first MI by about one third in hypercholesterolemic patients with no history of coronary disease. Simvastatin is now allowed by the U.S. Food and Drug Administration (FDA) to add a label statement that the drug can reduce deaths by lowering cholesterol.
Hypertension
Hypertension is blood pressure higher than 140/90 mm Hg, which increases an individual’s risk of developing cardiovascular disease. Adhering to medical therapy for control of elevated blood pressure helps to modify the individual’s risk.
Diabetes mellitus
Cardiac disease has been found to be more prevalent in individuals with diabetes mellitus. Diabetes mellitus poses a greater risk than other factors. This is thought to be related to elevated blood glucose levels, which damage the arterial intima and contribute to atherosclerosis. Diabetic patients also have alterations in lipid metabolism and tend to have high cholesterol and triglyceride levels. Medical therapy for regulating blood glucose levels helps to modify the individual’s risk.
Obesity
Excess body weight increases the workload of the heart. It also contributes to the severity of other risk factors. A weight-reduction program and maintenance of an ideal body weight help to modify the individual’s risk.
Sedentary lifestyle
Lack of regular exercise has been correlated with increased risk of developing cardiovascular disease. Regular aerobic exercise can improve the heart’s efficiency and help lower blood glucose levels, improving the ratio of HDLs to LDLs, reducing weight, lowering the blood pressure, reducing stress, and improving overall feelings of well-being. Some practitioners define regular physical exercise as exercising at least three to five times a week for at least 30 minutes, causing perspiration and an increase in heart rate by 30 to 50 bpm. Walking is one of the best forms of exercise.
Stress
The body’s stress response releases catecholamines that increase the heart rate. Catecholamines also affect myocardial cells and may result in cellular damage. The vasoconstriction that occurs may contribute to development of cardiovascular disease. Stress reduction measures may be important in modifying an individual’s risk.
Psychosocial factors
People who develop CAD are more likely to have the coronary-prone, or type A, personality. Type A personality traits include aggressiveness, competitiveness, perfectionism, compulsiveness, and an urgent sense of time. When the type A personality is combined with other risk factors such as age, high lipid levels, and smoking, the risk of heart disease increases.
Cardiac dysrhythmias
A dysrhythmia (or arrhythmia) refers to any cardiac rhythm that deviates from normal sinus rhythm. Normal sinus rhythm originates in the SA node and is characterized by the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 N-
N- r-
r- -z
-z m,
m,  M-b
M-b -l
-l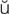 s,
s, 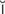 s-K
s-K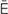 -m
-m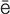 –
–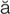 ,
,  -KL-zh
-KL-zh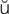 n,
n,