Hematology
Objectives
After completing this chapter you should be able to:
Fundamental Concepts
1. Define and match terms and abbreviations from the glossary.
2. Identify the proper specimen collection for hematology testing.
4. Describe and identify the basic formation of cells in the bone marrow.
5. Perform a blood smear and stain.
6. Identify typical blood cells from a stained blood smear.
7. Observe selected abnormal cells from visual aids.
CLIA-Waived Tests
Advanced Concepts
1. Describe the seven tests involved in the complete blood count.
2. Identify the red blood cell indices, and explain their significance in determining anemia.
3. Explain the significance of the white blood cell count and differential.
Key Terms
anemia condition in which the red blood cell or hemoglobin level is below normal
anisocytosis abnormal variances in red blood cell size
band immature neutrophil whose nucleus has not segmented (also called stab)
baso- prefix meaning alkaline
basophil white blood cell with large granules that stain dark blue
cytoplasm fluid within cells between the nucleus and the outer cell membrane
differential count procedure for determining the distribution of the five types of leukocytes based on their staining characteristics, shapes, and sizes
embolus traveling clot
eosino – prefix meaning acid
eosinophil white blood cell with large granules that stain red
erythroblasts immature red blood cells (also called rubriblasts)
erythrocyte sedimentation rate (ESR) rate at which red blood cells settle out of an anticoagulated blood specimen after 60 minutes
fibrinogen (factor I) one of two plasma proteins involved in clotting
formed elements cells and cell fragments that can be viewed under the microscope
granulocytes white blood cells that contain granules in their cytoplasm: neutrophils, basophils, and eosinophils
hematocrit test that measures percentage of packed red blood cells compared with total blood volume
hematologist one who evaluates the cellular elements of blood microscopically and analytically by using a variety of test methods
hematology study of the visible cellular components in the bloodstream and bone marrow
hematopoiesis blood production
hemocytoblast stem cell that differentiates (changes) and becomes any of the seven visible blood elements found in circulating blood
hemoglobin oxygen-carrying reddish pigment in red blood cells
hemolysis (hemolyzing) destruction of the red blood cells
hyperchromia increase in color (based on hemoglobin concentration)
hypochromic pertaining to less than normal color
hypoxemia lack of oxygen in the blood
immunoglobulins antibodies that destroy or render harmless foreign invaders containing antigens
leukemia various cancers of the white blood cells
leukocytosis abnormal increase in white blood cells
leukopenia abnormal decrease in white blood cells
lymphocyte small, nongranular white blood cell that develops from lymphoblasts in bone marrow
macrophages large, engulfing cells that come from monocytes when they enter the tissues
megakaryocyte large nuclear cell in the bone marrow that fragments its cytoplasm to become platelets
monocytes large, nongranular white blood cells that develop from monoblasts in bone marrow
myeloblasts stem cells that develop into the three kinds of granulocytes
neutro- prefix meaning neither acid nor alkaline
neutrophil white blood cells with fine granules that stain lavender/pink
nongranulocytes (agranulocytes) white blood cells that may have a few or no granules in their cytoplasm; lymphocytes and monocytes
normocytes young red blood cells that shed their nuclei before entering the bloodstream
nucleus central controlling structure in the cell
-phil suffix meaning attraction
poikilocytosis abnormal shapes in red blood cells
polychromia increase in color variation (based on hemoglobin concentration)
polycythemia abnormal condition of increased red blood cells
polymorphonuclear having a multishaped, segmented nucleus; sometimes abbreviated as PMN or seg
prothrombin (factor II) one of two plasma proteins involved in clotting
protime test test for monitoring coagulation times for patients taking anticoagulants
RBC indices mathematic ratios of the three red blood cell tests (hemoglobin, hematocrit, and red blood cell count)
reticulocytes newly released red blood cells in the blood that still contain some nuclear DNA
rouleaux formation arrangement of red blood cells resembling stacked chips
thrombosis abnormal condition of clotting
thrombus unwanted stationary clot
vitamin K critical element in the production of prothrombin
Abbreviations
ALL acute lymphocytic leukemia
CLL chronic lymphocytic leukemia
CML chronic myelocytic leukemia
ESR erythrocyte sedimentation rate
g/dL grams per deciliter (g/dL), which represents the weight of a substance (g) per volume (dL)
HCT hematocrit
Hgb hemoglobin
INR international normalized ratio (for protime test results)
MCHC mean (average) cell hemoglobin concentration
MCV mean cell volume
 Fundamental Concepts
Fundamental Concepts
Overview of Hematology and Blood
Hematology is the study of the visible cellular components in the bloodstream and bone marrow. The complete blood count (CBC) is one of the most commonly ordered “routine” tests for evaluating a person’s internal health status. Hematologists work in the hematology departments of hospitals, reference labs, and private practices, where they evaluate the blood’s cellular elements microscopically and analytically by using a variety of test methods. Hematologists may also evaluate hemostasis, the body’s ability to initiate a clotting response to stop bleeding and at the same time prevent the blood from forming an unwanted stationary clot.
In the ambulatory setting, basic CLIA-waived hematology tests and coagulation (clotting) tests are performed on capillary blood. If a reference laboratory does the testing, the requisition and laboratory protocol must be checked to determine the proper tubes to draw and how they are to be processed. The blood for hematology tests is usually collected in lavender-topped vacuum tubes containing the anticoagulant ethylenediaminetetraacetic acid (EDTA), which also preserves cellular elements in their natural state.
The blood for coagulation studies is usually collected in blue-topped vacuum tubes that must be allowed to fill accurately because the blood and the liquid anticoagulant in the tube must be in the proper ratio. This is why a “waste” tube is needed when using the butterfly needle and tubing.
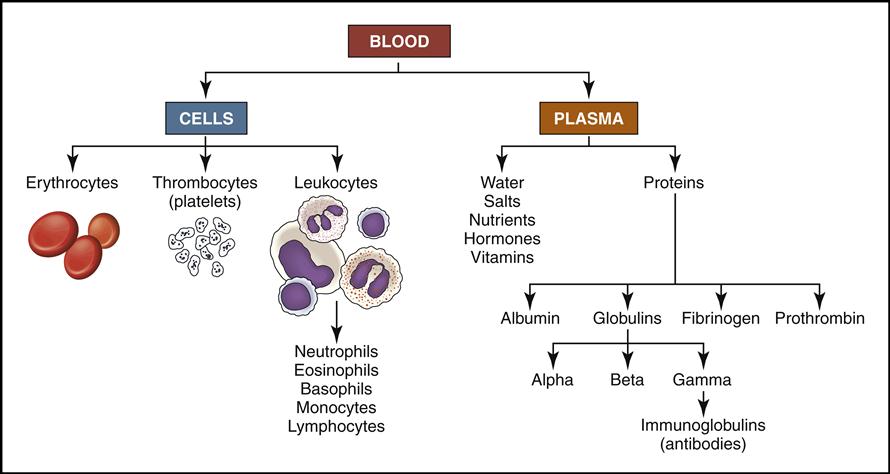
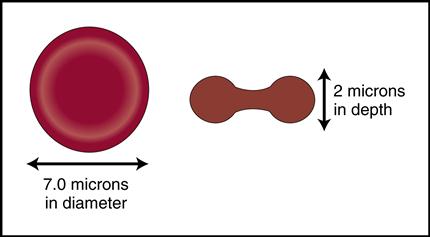
Blood is a complex liquid connective tissue that is constantly circulating through the blood vessels. The formed elements within blood can be viewed under the microscope as cells and cell fragments (Fig. 5-1, left). These formed elements are suspended in the watery liquid called plasma, which contains hundreds of dissolved biochemical substances (see Fig. 5-1, right). (NOTE: Chapters 6 and 7 will focus on the biochemical substances located in the plasma.)
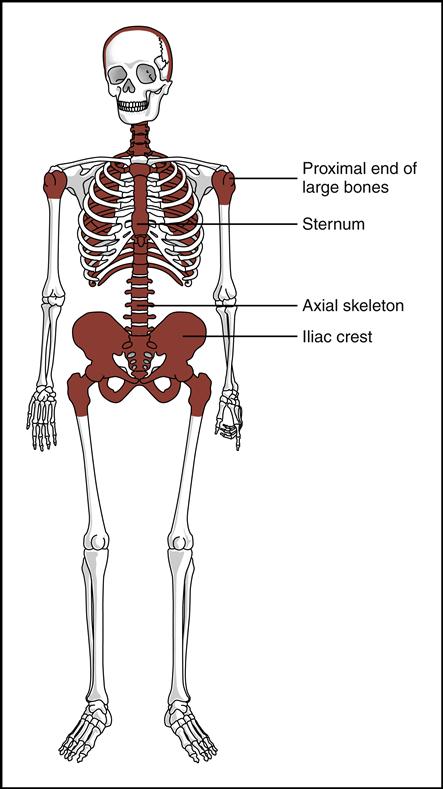
Blood cells are produced in the red bone marrow found in flat bones and at the ends of long bones (Fig. 5-2). All the cells originate from a stem cell, or hemocytoblast, that differentiates (changes) and becomes any of the following seven visible blood elements found in circulating blood (red blood cells, five types of white blood cells, and platelets):
• Red blood cells (RBCs, also called erythrocytes)
• White blood cells (WBCs, also called leukocytes):
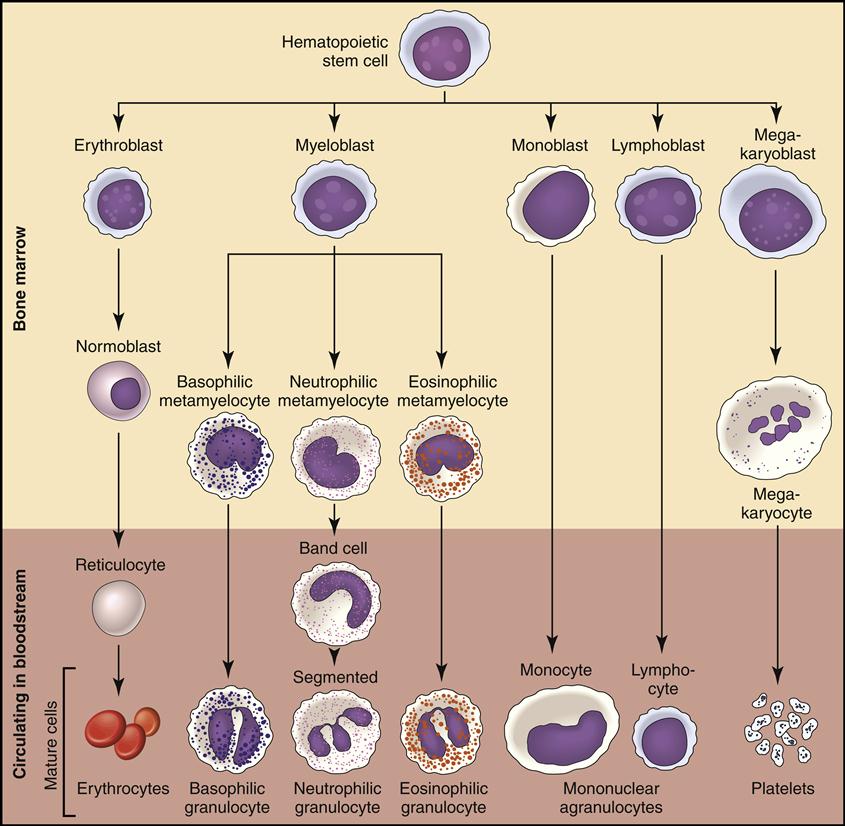
Fig. 5-3 is a simplified flow chart illustrating blood production, or hematopoiesis. Each of the seven formed elements is viewed and discussed as it is developed in the bone marrow and then released into the bloodstream or tissue, where it fulfills its function.
Red Blood Cells (Erythrocytes)
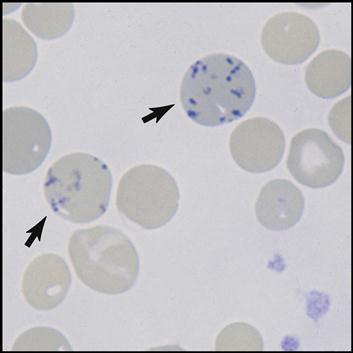
The bone marrow is constantly producing new RBCs. The beginning stages of RBC production in the bone marrow are shown in Fig. 5-4. Erythroblasts (immature RBCs), also called rubriblasts, become normocytes that shed their nuclei before entering the bloodstream. In the bloodstream the young RBCs, which still contain some nuclear deoxyribonucleic acid (DNA), are called reticulocytes (Fig. 5-5). The average amount of reticulocytes found in peripheral blood is approximately 1%. A percentage higher than 3% indicates that the individual is actively producing new red cells because of a loss of RBCs, such as when hemorrhaging occurs. As the reticulocytes continue to mature, they shed their remaining intracellular nuclear material while retaining millions of hemoglobin (Hgb) molecules (reddish pigment capable of carrying oxygen).
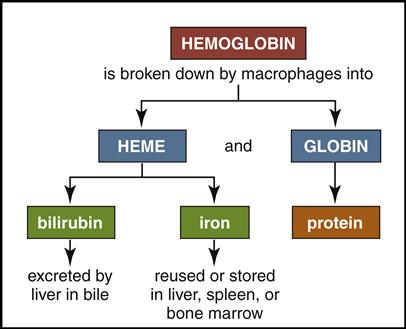
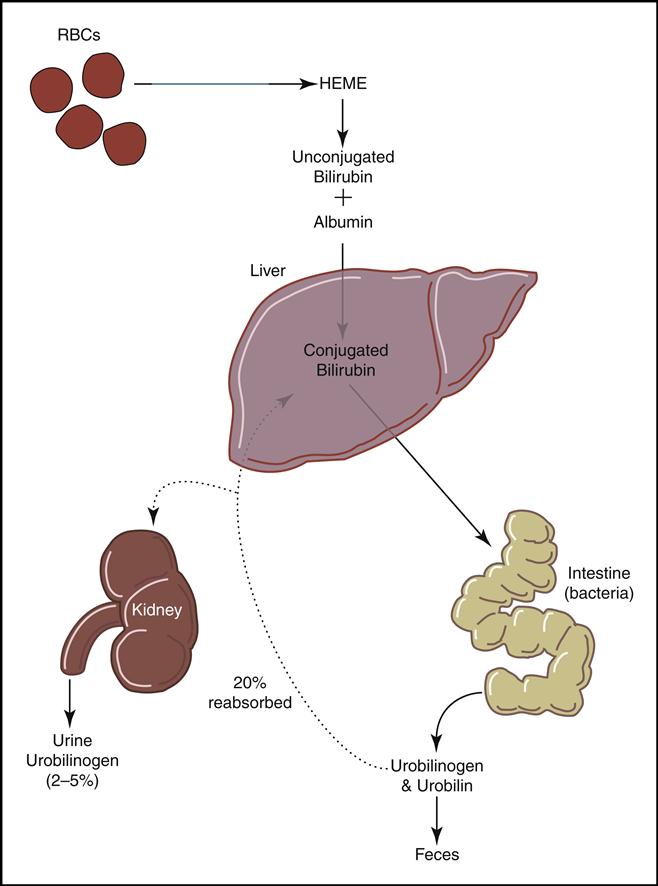
Mature red cells are biconcave disks filled with a reddish pigment called hemoglobin (Fig. 5-6). Hgb consists of an iron (heme) and a protein (globin) molecule. Hgb has a strong affinity for oxygen that it picks up and carries from the lungs to the cells of the body. This oxygen-carrying capacity of RBCs is critical to sustaining life energy throughout the body. Because of their critical role, RBCs account for almost half of the blood volume. After 80 to 120 days, RBCs disintegrate, releasing the iron (heme) portion, which goes back to the bone marrow to make new RBCs (Fig. 5-7). The remaining elements convert to bilirubin, which is further processed through various stages in the liver, intestines, and kidneys (Fig. 5-8).
White Blood Cells
WBCs fall into two general categories: granulocytes, consisting of neutrophils, basophils, and eosinophils; and nongranulocytes, consisting of lymphocytes and monocytes.
Granulocytes
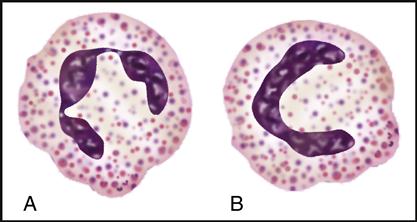
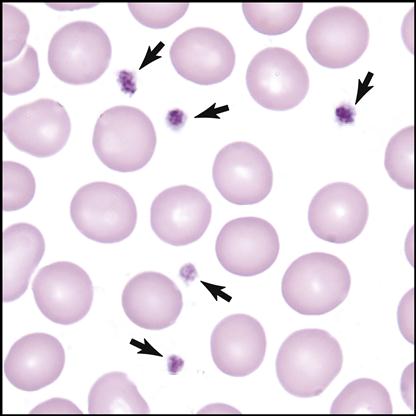
Myeloblasts are the stem cells in the bone marrow that become myelocytes (see Fig. 5-3). The myelocytes develop into the three kinds of granulocytes: neutrophils, eosinophils, and basophils. Fig. 5-9 illustrates a myeloblast becoming a myelocyte and metamyelocyte within the bone marrow. The granulocyte enters the bloodstream with its nucleus (the central controlling structure in the cell) in an elongated “band” shape. The maturing nuclei of the eosinophils and basophils become segmented into two lobes. The mature neutrophil’s multisegmented nucleus is referred to as polymorphonuclear (PMN) or as a seg (Fig. 5-10).
The three mature granulocytes in the bloodstream are distinguished from one another by the staining characteristics of the granules located in their cytoplasm (fluid within cells between the nucleus and the outer cell membrane). When a drop of whole blood is smeared onto a slide and then stained, the three different granulocytic WBCs show an affinity, or attraction, to either the red acid dye (eosino), the blue alkaline dye (baso), or both colors (neutro). The WBCs are named on the basis of their attraction to the dyes (indicated by the suffix –phil) and have distinct purposes in aiding the body during infection and inflammation.
Neutrophils
Neutrophils have small granules that stain lavender/pink {Leave the / in rather than “or”}. They are usually the most numerous WBCs in the blood because of their ability to engulf and digest foreign matter, especially pathogenic bacteria.
Eosinophils
Eosinophils have large granules that stain red. They increase in number during allergic reactions and parasitic infestations.
Basophils
Basophils have large granules that stain dark blue. They are the rarest WBCs in the blood and are involved in preventing blood from excessive clotting by producing the anticoagulant heparin. They also mediate the inflammatory response.
Nongranulocytes (Agranulocytes)
Bone marrow also produces the following two nongranulocytes, or agranulocytes, which may have a few or no granules in their gray and sky-blue cytoplasm:
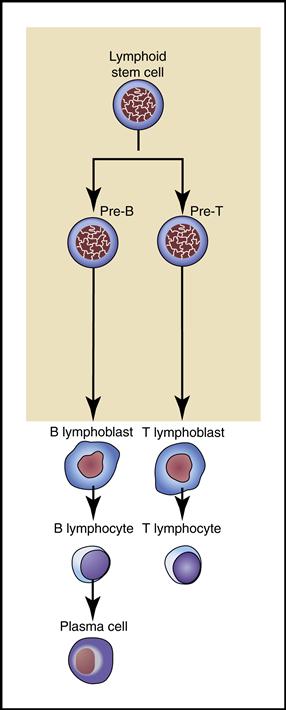
2. Lymphocytes are the smallest, nongranular WBCs that develop from lymphoblasts (immature lymphocytes) in the bone marrow. They further differentiate into B and T cells (Fig. 5-11).
• B lymphocytes become plasma cells capable of producing specific antibodies (immunoglobulins) that destroy or render harmless foreign invaders, especially viruses. (The immune response involving T and B lymphocytes is covered in Chapter 7.)
Lymphocytes are the smallest WBCs in the bloodstream. They move freely among the blood vessels, lymph vessels, and tissues as they constantly survey for and respond to foreign invaders.
Platelets (Thrombocytes)
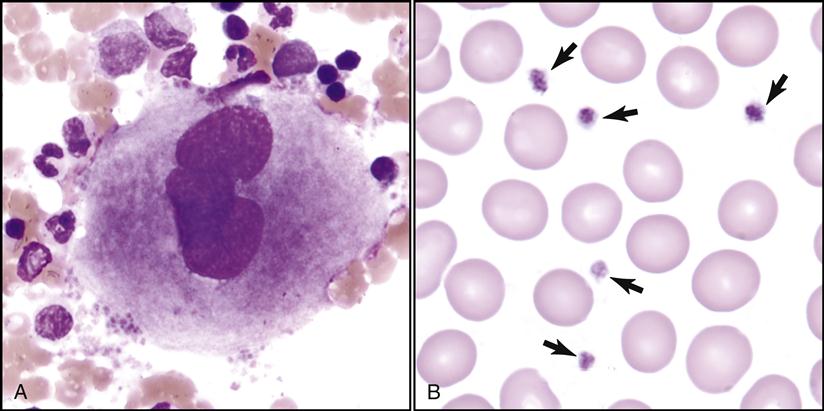
The seventh formed element also comes from the stem cell (hemocytoblast) after it differentiates into a megakaryoblast and then into a megakaryocyte (large nuclear cell) in the bone marrow (Fig. 5-12). This very large cell releases fragments of its cytoplasm into the bloodstream, which are seen as small thrombocytes, or platelets.
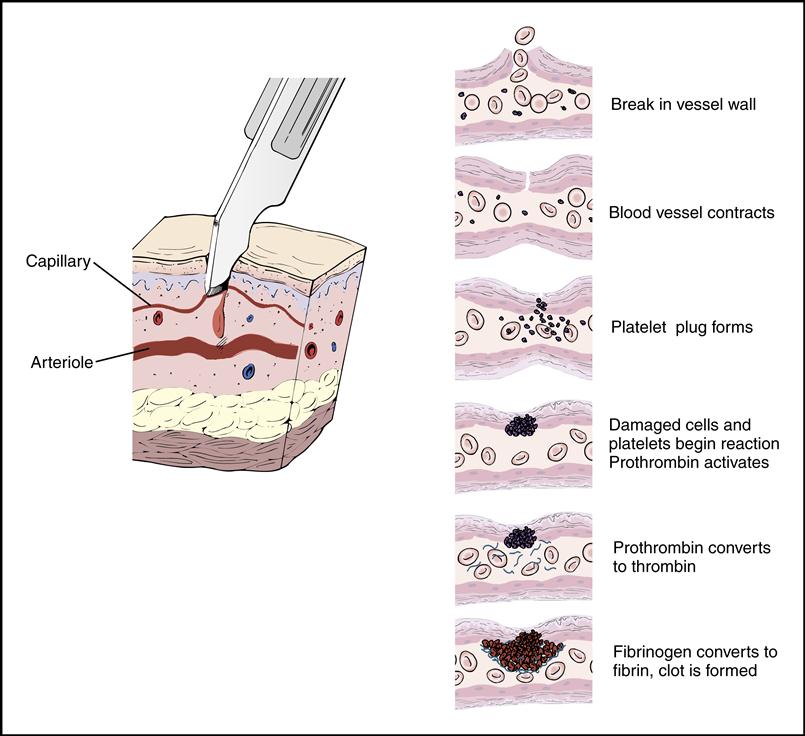
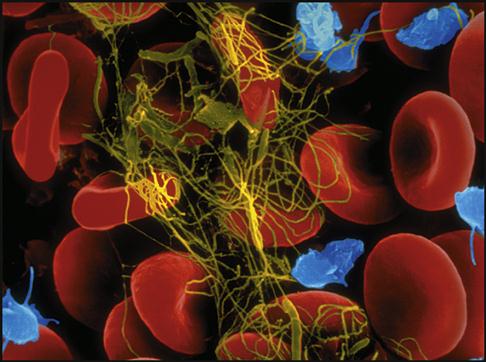
Platelets gather around the site of a damaged blood vessel in an effort to “plug” the leak (Fig. 5-13). They also release clotting chemicals that activate the formation of sticky fibrin strands that entangle the blood cells and form a clot (Fig. 5-14).
Preparing a Blood Smear for Observation by Physician or Laboratory Technician
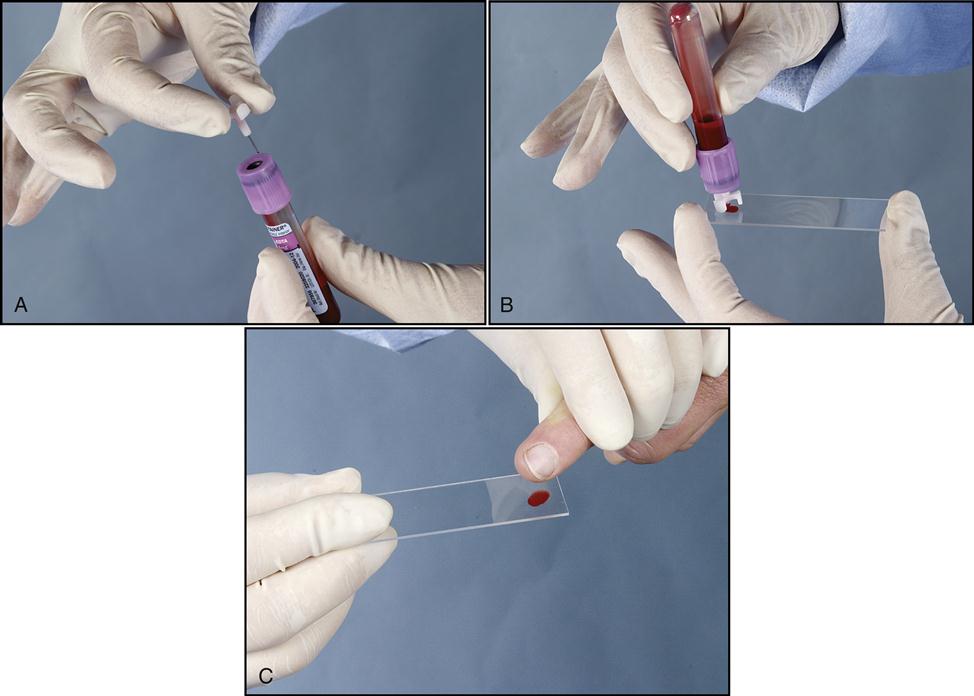
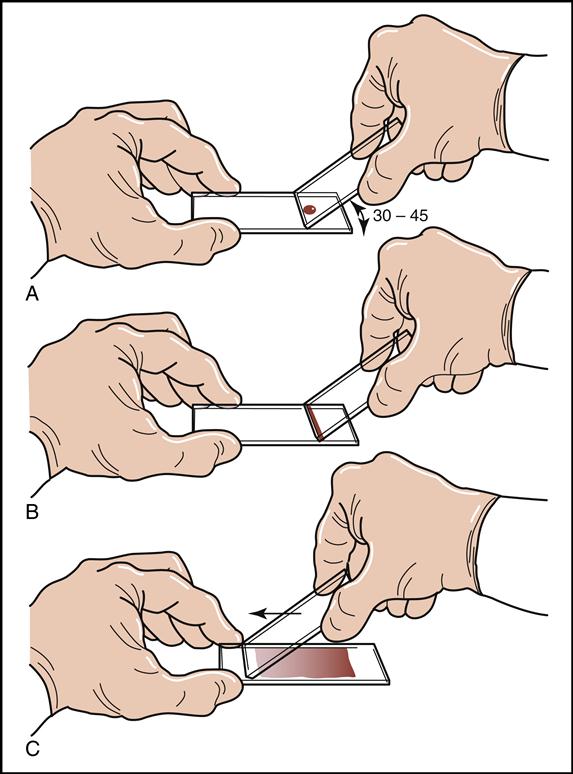
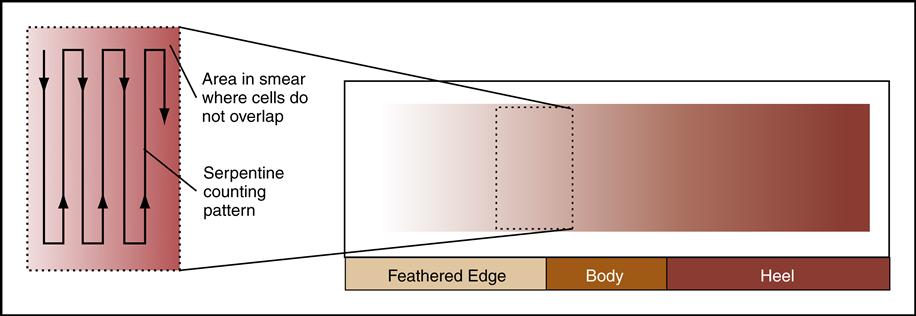
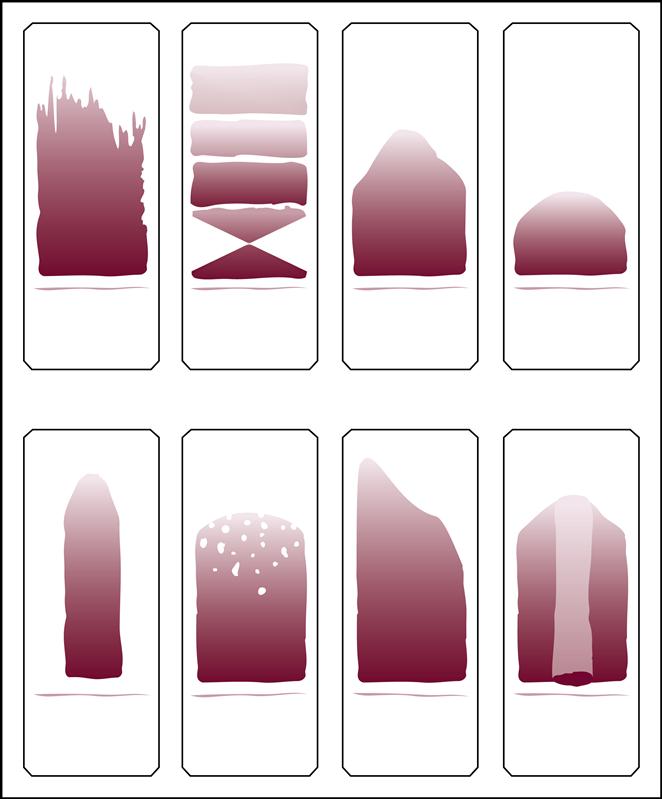
A blood smear can be made by using fresh blood from a gently mixed lavender-topped EDTA Vacutainer tube (Becton Dickinson, Franklin Lakes, N.J.) or from a fresh capillary puncture. A drop of blood is placed on the end of a clean slide with a Diff safety device (Becton Dickinson) inserted into the lavender EDTA tube. This device is a convenient and safe way to obtain a drop of blood from the Vacutainer tube without removing the sealed top of the tube. Push the white device into the top of the EDTA lavender tube, then invert the tube and press the device against the slide (Fig. 5-15). The pressure on the slide causes a drop of blood to flow onto the slide. A drop of blood can also be obtained from a finger capillary puncture. Next, the drop of blood is spread across the slide by bringing a clean “pusher” slide back into the drop at a 30- to 35-degree angle. As soon as the blood spreads approximately three fourths along the edge, quickly move the pusher slide forward (Fig. 5-16). Allow the slide to air dry. A properly performed smear will look like the one in Fig. 5-17. Improper, unacceptable smears resemble those in Fig. 5-18.
Evaluate the smear to see if the following are present:
2. A well-distributed “body” with margins on each side
3. Smooth, thick-to-thin spreading of the smear with no ridges or tails
The smeared blood slide is then stained with either Wright’s stain or a Quick Diff (Becton Dickinson) stain. Both of these staining methods use three ingredients: a methanol fixative, a red acid dye, and a blue alkaline dye. The Wright’s stain also uses a buffer solution to change the pH. Refer to the material safety data sheets in the workbook and the labels on the bottles when working with stains, and take appropriate precautions. The simple Quick Diff staining procedure used in ambulatory settings is provided in the workbook and is demonstrated in Procedure 5-1 at the end of this section.
Stained blood smears must be observed by the physician, a trained hematologist, or a laboratory technician.
White Blood Cell Identification and Differential
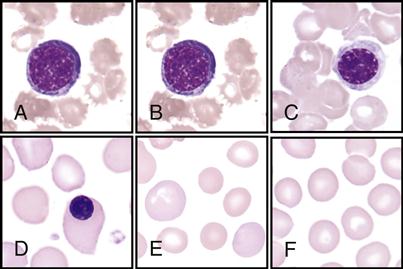
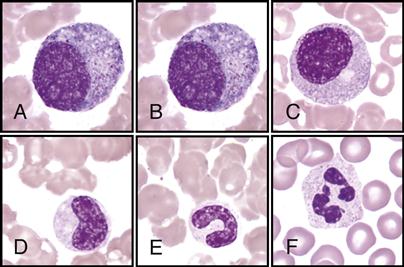
The hematologist scans stained blood smears to identify the distribution of the five types of leukocytes on the basis of their staining characteristics, shapes, and sizes. This procedure is called a differential count. See the WBC atlas on pp. 140-141, which lists and shows the distinguishing characteristics that are observed for each of the leukocytes.
Note the following facts found in the atlas for each of the following:
• Immature banded neutrophil; nucleus has a banded shape (B)
• Monocytes—largest of the leukocytes; has large, lacy nucleus (D)
• Eosinophils—has large red granules (E)
• Basophils—rarest; has large, dark-blue or black granules (F)
Each time a leukocyte is found, the hematologist identifies it and presses the corresponding key on a differential counter (Fig. 5-19). After identifying 100 leukocytes, the counter sounds a bell. Each of the five leukocytes is then displayed as a percentage of the total 100 cells identified.
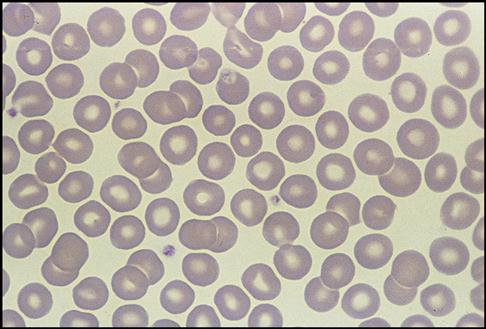
Red Blood Cell Identification and Description
Next, the hematologist observes and reports the appearance of the RBCs. Normal RBC appearance and distribution are shown in the Fig. 5-20). In a diseased state, the RBCs may become altered in appearance. The three columns in flow chart (A) in the RBC Atlas show how RBCs can vary in size (anisocytosis), shape (poikilocytosis), and color (polychromia, hypochromia, and hyperchromia). The hematologist reports the degree of these variances by using +1, +2, and so on. The following abnormal RBC sizes, colors, and shapes are shown in the RBC Atlas on pp. 142-143:
• Microcyte and hypochromic RBCs (small cell and faded color cells) (C)
• Polychromatic RBCs (multicolored cells) (D)
• Dacryocytes (tear-shaped cells) (E)
• Ovalocytes (elliptical-shaped cells) (F)
• Spherocytes (ball-shaped round cells) (G)
Platelet Description
Platelets (thrombocytes) are also assessed while scanning the slide (Fig. 5-21). Their quantity is approximated by the hematologist while observing various fields under oil immersion.
Theory of Hemostasis
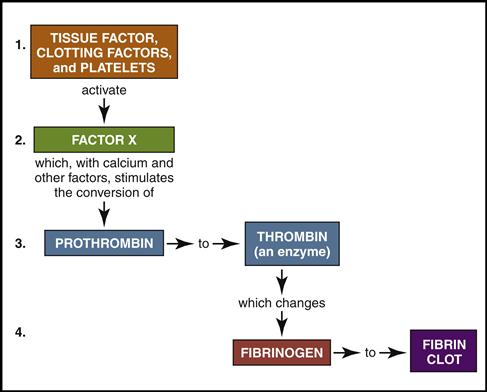
The cardiovascular system is equipped with a remarkable mechanism that swiftly stops blood from escaping out of a damaged blood vessel. First the blood vessel constricts, slowing down the blood flow. Then platelets concentrate around the damaged site and initiate a clot made of a sticky fibrin mesh (see Figs. 5-13 and 5-14). The fibrin strands are the result of a complex chain reaction of 13 clotting factors, including the two plasma proteins, fibrinogen (factor I), and prothrombin (factor II) (Table 5-1). The formation of the sticky fibrin can be simplified into four steps, as illustrated in Fig. 5-22.
TABLE 5-1
| Factor | Name |
| I | Fibrinogen |
| II | Prothrombin |
| III | Tissue factor; thromboplastin |
| IV | Calcium |
| V | Labile factor; proaccelerin |
| VII | Serum prothrombin conversion accelerator; proconvertin |
| VIII | Antihemophilic factor |
| IX | Plasma thromboplastin component |
| X | Stuart–Prower factor |
| XI | Plasma thromboplastin antecedent |
| XII | Hageman factor |
| XIII | Fibrin stabilizing factor |
| Platelet factor | Cephalin |
From Stepp CA, Woods M: Laboratory procedures for medical office personnel, Philadelphia, 1998, Saunders.
Once the clot is formed and has fulfilled its purpose, the body stops the reaction to prevent excessive clotting. The body produces a natural anticoagulant, heparin, to keep the clotting mechanism in balance. The anticoagulant will prevent the blood from forming an unwanted stationary clot, or thrombus, or releasing a traveling clot, or embolus.
Coagulation and Testing
Most coagulation tests are performed at the hospital or reference laboratory to help determine why a patient is bleeding, bruising, or forming clots abnormally. Table 5-2 lists reference values for selected coagulation tests.
TABLE 5-2
Reference Values for Selected Coagulated Tests
| Test | Reference Values |
| Bleeding time (Ivy) | 1-8.5 min (average, 3-6 min) |
| Platelet count | 150,000-400,000/mm3 (average, 250,000/mm3) |
| Platelet aggregation | Aggregation visible within <5 min |
| Capillary fragility | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|