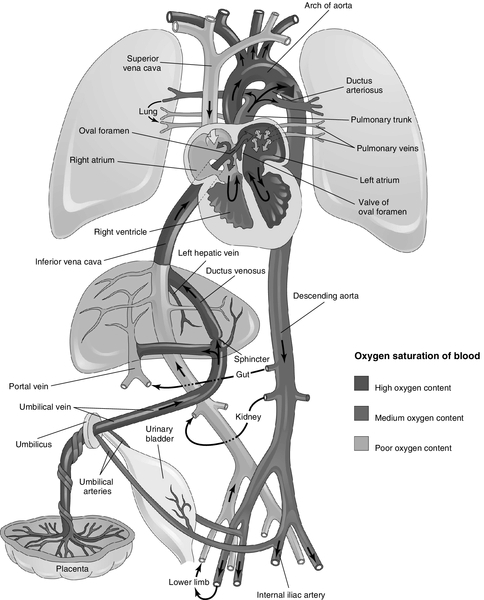
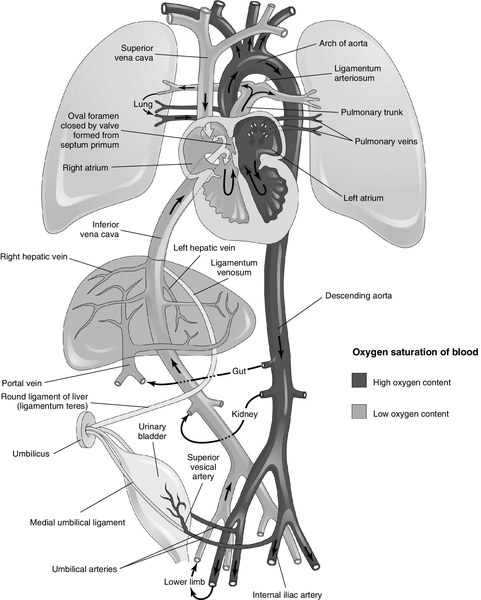
CHAPTER 4 1. Identify primary features of fetal circulation. 2. Identify physiologic changes that occur during transition to extrauterine life. 3. Identify routine care considerations for a newborn infant during the transition period. 4. Identify signs and symptoms of common problems in the transition period. 5. Define the methods and intervention times for parental teaching. The transition period is considered to be the first 6 to 10 hours of life, but more than a period of time, it is a process of physiologic change in the newborn infant that begins in utero as the child prepares for the transition from intrauterine placental support to extrauterine self- maintenance. The fetus prepares for transition during the course of gestation in such ways as storing glycogen, producing catecholamines, and depositing brown fat. The neonate’s ability to accomplish the transition to extrauterine life will depend on gestational age and the quality of placental support during gestation as well as any physical defects or anomalies that may affect major organ systems. 2. High rate of metabolism. The placenta uses one third of all the oxygen and glucose supplied to it by the maternal circulation for its own metabolic needs. 3. Low-resistance circuit. The placenta receives approximately 50% of fetal cardiac output. 4. Characteristics of uterine venous blood as it enters the intervillous space: PCO2 of 38 mm Hg, PO2 of 40 to 50 mm Hg, and pH of 7.36. B. Fetal shunts/blood flow (Fig. 4-1) (Moore et al., 2013). 1. Umbilical vein (PO2 32 to 35 mm Hg). It carries oxygenated blood from the placenta to the fetus. 2. Ductus venosus. Forty percent to 60% of the umbilical venous blood bypasses the liver through the ductus venosus to the inferior vena cava (IVC). It is a low-resistance channel that allows a significant portion of relatively well-oxygenated blood to enter the heart directly; the other half passes through the liver and enters the IVC via the hepatic veins. This mixing of blood slightly lowers the PO2. 3. IVC blood and the blood from the coronary sinuses (PO2 = 25 to 28 mm Hg). This blood is largely deflected across the right atrium, through the foramen ovale, and into the left atrium. In contrast, most of the blood from the superior vena cava (SVC), also returning to the right atrium, is deflected to the right ventricle (see item 6, below). The crista dividens (lower edge of the septum secundum) separates the flow of blood from the IVC into two streams, with 50% to 60% of the blood from the IVC being diverted across the foramen ovale into the left atrium (Blackburn, 2013) and the remainder of blood from the IVC remaining in the right atrium and mixing with poorly oxygenated blood from the SVC and coronary sinus. 4. Left atrium. Blood is received from the right atrium via the foramen ovale and mixes with a small amount of blood returning from the lungs via the pulmonary veins. 5. Left ventricular blood (PO2 = 25 to 28 mm Hg). Virtually all this blood is from the IVC by way of the right atrium–foramen ovale–left atrium pathway. Left ventricular blood is pumped out through the aorta to the brain from the upper part of the aortic arch. Approximately 90% of the blood from the ascending aorta feeds the coronary, carotid, and subclavian arteries and thus the brain and upper extremities. 6. The SVC. Unoxygenated blood returning from the brain and upper extremities is received by the SVC. Ninety-seven percent enters the right atrium and flows to the right ventricle through the tricuspid valve; only 3% flows to the left atrium via the foramen ovale. 7. Right atrium. Some mixing occurs here between the unoxygenated SVC blood and the oxygenated IVC blood not shunted directly into the left atrium via the foramen ovale. 8. Right ventricle. The dominant ventricle (PO2 = 19 to 22 mm Hg) ejects about 66% of the total cardiac output. Most of the blood is shunted across the ductus arteriosus, away from the lungs, and into the descending aorta to supply the kidneys and intestines. It then divides into two arteries, which subsequently return back to the placenta. 9. Ductus arteriosus. Equal in size to the aorta, it connects the pulmonary artery to the descending aorta. The blood flows right to left (pulmonary artery to aorta) across the ductus arteriosus because of high pulmonary vascular resistance and low placental resistance. Patency is maintained by the low oxygen tension in utero and by the vasodilating effect of prostaglandin E2. 10. Low pulmonary blood flow (only 10% to 12% of the right ventricular output). This results from high pulmonary vascular resistance (Blackburn, 2013). 11. Descending aorta. It supplies the kidneys and intestines, divides into two arteries, and returns blood to the placenta for oxygenation. B. Pulmonary arteries. The small pulmonary arteries of the fetus have a thick, muscular medial layer; they are very reactive and are actively constricted by the low PO2 normally present during fetal life. Pulmonary vascular resistance increases throughout fetal life. C. Lung fluid secretion. Fetal lungs actively secrete fluid; secretion of fluid is decreased near term. At term, the lungs contain 30 mL of plasma ultrafiltrate per kilogram of body weight. This is comparable to a postnatal thoracic gas volume of 25 mL/kg. An adequate fluid volume is necessary for lung development. Fluid moves into and out of the lungs through the trachea. D. Fetal breathing. In-utero fetal breathing movements have been detected as early as 11 weeks of gestation. They contribute to lung development. E. Surfactant. Surfactant is secreted into the amniotic fluid by the fetal lung before 20 weeks of gestation. The absolute quantity of surfactant increases throughout gestation in both the lung and amniotic fluid and can support extrauterine respiration at approximately weeks 34 of gestation. B. Glycogen. Large glycogen stores (2 to 10 times that of an adult) provide large energy reserves to sustain the newborn infant through the transition period. C. Hemoglobin. Fetal hemoglobin has an increased affinity for oxygen. Fetal hemoglobin is progressively replaced by adult hemoglobin from weeks 32 to 36 of gestation and is approximately 80% of the total hemoglobin at term. A. Placenta. Maternal placental perfusion ceases with uterine contractions. B. Stress hormones. High concentrations of stress hormones (predominantly norepinephrine) are released as a direct effect of the resultant hypoxia on the adrenal medulla. A. Cardiovascular adaptation (Fig. 4-2) (Hillman et al., 2012; Moore et al., 2013). a. Placenta is separated from the circulation, and the umbilical arteries and veins constrict. b. As the low-resistance placental circuit is removed, there is a resultant increase in systemic blood pressure; the systemic vascular resistance then exceeds the pulmonary vascular resistance. 2. The three major fetal shunts (ductus venosus, foramen ovale, and ductus arteriosus) functionally close during transition (Leone and Finer, 2013). b. Foramen ovale. The fall in pulmonary vascular resistance results in a drop in right ventricular and right atrial pressure, and the increased systemic vascular resistance results in an increase in left atrial and left ventricular pressures, causing the foramen ovale to close against the atrial septum. (2) Until the foramen ovale is anatomically sealed, anything that produces a significant increase in right atrial pressure can reopen the foramen ovale and allow a right-to-left shunt. c. Ductus venosus. Absent umbilical venous return leads to closure of the ductus venosus. It functionally closes within 2 to 3 days and becomes the ligamentum venosum. 3. Postnatal circulation (see Fig. 4-2) (Hillman et al., 2012; Moore et al., 2013). a. Systemic venous blood enters the right atrium from the SVC and the IVC. b. Poorly oxygenated blood enters the right ventricle and passes through the pulmonary artery into the pulmonary circulation for oxygenation. c. The oxygenated blood returns to the left atrium through the pulmonary veins. d. This blood passes through the left ventricle and into the aorta to supply the systemic circulation with oxygenated blood. B. Pulmonary adaptation (Blackburn, 2013; Hillman et al., 2012). 2. Stimuli for initiating respiration. The mild hypercapnia, hypoxia, and acidosis that result from normal labor are due partially to the intermittent cessation of maternal–placental perfusion with contractions. The decreased pH stimulates the respiratory center directly; the low PO2 and high PCO2 stimulate the respiratory center by means of central and peripheral chemoreceptors (Gauda and Martin, 2013). Other stimuli include cold, light, noise, and touch. 3. Entry of air into lungs with the first breath. b. The pulmonary vessels respond to the increase in PO2 with vasodilation. Pulmonary vascular resistance progressively decreases until adult levels are reached by 6 to 8 weeks of age (Blackburn, 2013). 4. Inspiration of air and expansion of lungs. After the thoracic squeeze (during labor and vaginal delivery, this empties the lungs of approximately one third of the fetal lung fluid), the subsequent recoil of the chest wall causes inspiration of air and expansion of the lungs. Negative intrathoracic pressures generated with the first breath may be as high as 20 to 70 cm H2O because of the mechanical advantage created by the high resting level of the diaphragm in the nonaerated lung (Niermeyer and Clarke, 2011). Subsequent breaths in the normal newborn infant require 15 to 20 cm H2O pressure. 5. Respiratory augmentation. Head’s paradoxic reflex is a vagally mediated hyperinflation triggered by distention of stretch receptors in the large airways. 6. Work of inspiration. This is predominately related to overcoming the surface tension of the walls of the terminal lung units at the gas–tissue interface. On expiration, the ability to retain air depends on surfactant (Hillman et al., 2012). b. Surfactant has the ability to lower the surface tension at an air–liquid interface. c. As the surfactant lowers the surface tension in the alveolus at end-expiration, it stabilizes the alveoli and prevents collapse. 7. There is an increase in the functional residual capacity with each breath. Less inspiratory pressure is thus required for subsequent breaths. 8. Lung compliance. Improves in the hours after delivery as a result of circulating catecholamines. The increased levels of catecholamines (especially of epinephrine) also clear the lungs by decreasing the secretion of the lungs’ fluids and increasing their absorption through the lymphatic system. A. Assessment/observation. Within 2 hours of birth, the neonate’s health status should be evaluated and the neonate assessed for risks that may complicate transition to extrauterine life (American Academy of Pediatrics [AAP] and American College of Obstetricians and Gynecologists [ACOG], 2012). 1. Body measurements. Head circumference, length, and weight are recorded. 2. Vital sign assessment. Vital signs recorded on admission will include heart rate, respiratory rate, and axillary temperature. Universal blood pressure screening in the well newborn infant is not warranted (AAP, 1993). 3. Gestational age assessment. Weight, head circumference, and length are graphed against the assessed gestational age and medical record (refer to Chapter 7). 4. Clinical changes. During the first hours of life, vital signs stabilize and the newborn proceeds through sleep–wake cycles associated with readiness-to-feed behaviors (Verklan, 2002). The time sequence of changes is altered in infants with low Apgar scores, immaturity, maternal medications, and intrinsic disease (refer to Chapter 5). 5. Head-to-toe physical examination (refer to Chapter 7). The following findings seen during transition are within normal limits as the infant progresses through the physiologic changes described under cardiopulmonary adaptation, above. (2) Petechiae of the face and facial bruising. A vertex presentation, a rapid second stage of labor, or a tight nuchal cord may result in these skin changes. (With severe facial bruising, central color may be assessed by looking at the mucous membranes in the mouth.) b. Head. (2) Caput succedaneum (edema of the presenting part of the scalp, caused by pressure that restricts the return of venous and lymph flow during vaginal delivery) may be present. c. Respirations/breath sounds. (2) Prolonged expiratory phase. (3) Respiratory rate of 30 to 60 breaths per minute. (4) Grunting and retracting (intercostal and substernal). These findings may be present during the first hours of life as lung fluid is cleared. d. Heart sounds. (2) Soft grade 2/6 systolic murmur may be present; represents a left-to-right shunt across the ductus arteriosus before its closure. e. Heart. (2) Consistently high or low heart rate suggests a pathologic condition. f. Intestines. (1) Blood flow is reduced initially. (2) As the bowel begins to fill with air, normal motility and bowel sounds are present within 15 minutes. (3) The normal term neonate passes meconium within the first 24 hours after birth. If a term neonate has not passed meconium by 48 hours after birth, the lower gastrointestinal (GI) tract may be obstructed. g. Urinary function. (1) Urine is normally passed within the first 24 hours after birth. (2) Failure to void within the first 24 hours may indicate genitourinary obstruction or abnormality. h. Extremities. Findings may include deformities resulting from the intrauterine position. B. Thermoregulation considerations in the nursery (Brown and Landers, 2011). The admission assessment and observation should be done in a controlled environment, such as a radiant warmer or incubator, that both provides warmth and prevents heat loss so that the infant maintains a normal temperature without increasing oxygen consumption, using glucose stores, or exceeding brown fat stores in the process of nonshivering thermogenesis (mediated by norepinephrine released by cold stress). Normal axillary ranges are 36.5° to 37.5° C (97.7° to 99.5° F) for the term neonate and 36.3° to 36.9° C (97.3° to 98.6° F) for the preterm neonate. Hypothermia and hyperthermia occur when the infant’s attempts to maintain a normal temperature fail, which has serious metabolic consequences for the newborn infant (refer to Chapter 6). 2. First bath. Delay bath until body temperature has stabilized and is within normal limits. Check temperature 30 minutes after the bath and 1 hour after transfer to open crib. 3. Temperature. Check temperature at least every 4 hours until the infant’s condition is stable, and then every 8 hours until discharge. Temperatures may need to be monitored every 30 minutes until stable. 4. Environmental temperature (incubator or warmer). Record environmental temperature with temperature checks of the infant to monitor his or her environmental requirements. NOTE: These requirements can be checked against the normal ranges of neutral thermal environmental temperature needs as a tool in evaluating an infant’s condition. C. Transition nursery medications (AAP and ACOG, 2012; Taylor et al., 2013). b. Procedure. Instill medication into conjunctival sac within 1 hour of birth; however, this may be delayed until after the first breastfeeding. The medication should not be flushed from the eye after application. A new tube is used for each infant. 2. Vitamin K1 (phytonadione). Administer vitamin K within 1 hour of birth. b. Risk of deficiency. Maternal dietary inadequacy of vitamin K, hepatic immaturity, reduced liver stores, and absence of intestinal flora predispose the child to deficiency of vitamin K; gut bacteria are a substantial source. Vitamin K is needed to promote the hepatic biosynthesis of vitamin K–dependent clotting factors, including prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). Deficiency results in hemorrhagic disease of the newborn (HDN). (2) Early HDN. Maternal exposure to drugs, including warfarin, anticonvulsants, and antituberculosis drugs, may affect coagulation. Severe or life-threatening hemorrhage may occur during delivery or in the first day of life. Intracranial hemorrhage is a common complication. Early HDN is the only type that cannot be prevented by vitamin K prophylaxis. (3) Late HDN. The late form of HDN may occur between 1 and 3 months of life. Acute intracranial hemorrhage is the most common initial finding and is often fatal or neurologically devastating. Other findings include GI or mucous membrane bleeding. Late HDN can be prevented by vitamin K prophylaxis. 3. Hepatitis vaccine and hepatitis B immunoglobulin (HBIG). a. Rate of hepatitis B virus (HBV) infection. If the mother is hepatitis B surface antigen (HBsAg) and hepatitis B “e” antigen (HBeAg) positive, the baby has a 90% risk of HBV infection in the first 12 months if treatment is not received. There is a 20% risk of HBV infection if the mother is positive for HBsAg only (Venkatesh et al., 2011). b. Chronicity. In the absence of treatment, the neonate will likely become a carrier, and eventually develop primary hepatocellular carcinoma (Venkatesh et al., 2011). c. Maternal HBsAg-positive treatment recommendations: (1) Child is bathed as soon as temperature is stable. (2) Hepatitis B vaccine (Engerix-B, 10 mcg, or Recombivax-HB, 5 mcg) and HBIG, 0.5 mL intramuscularly (prepared from plasma to contain a high titer of antibody against HBsAg) should be administered before 12 hours of age; may be given concurrently at different sites; 85% to 95% effective in preventing both HBV infection and the chronic carrier state. (3) Remaining doses of hepatitis B vaccine are given at 1 and 6 months of age. d. Maternal HBsAg-negative treatment recommendations: (2) Hepatitis B vaccine (Engerix-B, 10 mcg, or Recombivax-HB, 5 mcg) is administered prior to discharge from the hospital. The second dose is administered at 1 to 2 months of age and the third dose at 6 to 18 months of age. e. Unknown maternal HBsAg status at delivery. Follow schedule for maternal HBsAg-positive status. Additional HBIG administration will depend on the results of maternal serologic screening done within 12 hours after delivery. f. Premature infants with birth weight less than 2 kg born to HBsAg-negative mothers should receive the vaccine just before hospital discharge if the infant weighs more than 2 kg, or the dose is delayed until 2 months of age when other routine immunizations are given. All premature infants born to HBsAg-positive mothers should receive immunoprophylaxis (HBIG) and vaccine beginning as soon as possible after birth, followed by appropriate postvaccination testing (Venkatesh et al., 2011). D. Glucose needs/first feeding (Anderson et al., 2011; Blackburn, 2013; McGowan et al., 2011). 2. Hepatic glycogen is mobilized immediately after birth in response to the increased catecholamines to provide a continuing source of glucose to the brain in the absence of placental supply; in a healthy term infant, up to 90% of the hepatic glycogen stores may be consumed by 3 hours of life. 3. Blood glucose concentration at birth is about 85% of the maternal level. The last maternal meal, the duration of labor, the mode of delivery, the type and amount of intravenous (IV) fluids administered to the mother, and medications given to the mother all influence the actual concentration of glucose. The glucose level then falls for 1 to 2 hours, followed by an increase and stabilization at mean levels of approximately 70 mg/dL by the age of 3 hours in healthy, nonstressed infants (Jain et al., 2013). 4. A screening blood glucose test (on capillary whole blood) should be performed in infants with risk factors at 30 minutes to 1 hour of age. A glucose level less than 40 mg/dL at any time in any newborn infant is an indication for evaluation and treatment. The goal is to maintain the glucose value at greater than 40 mg/dL in the first day and greater than 40 to 50 mg/dL thereafter. NOTE: The whole-blood glucose level on the screening test is usually 10% to 15% lower than the corresponding serum or plasma level because the erythrocytes will continue to metabolize the glucose in the sample. Risk factors include the following: b. Reduced stores of glucose in premature and small-for-gestational-age infants. c. Hyperinsulinemia in infants of mothers with diabetes or gestational diabetes and in large-for-gestational-age infants, resulting in rapid removal of glucose from the circulation. d. Any symptoms of a low blood glucose level. An infant may have jitteriness, irritability, seizures, hypothermia, temperature instability, lethargy, poor feeding, emesis, apnea, pallor, cyanosis, and weak or high-pitched cry (see also Common Problems and Clinical Presentation, item F: Metabolic Problems). NOTE: Capillary samples from an unwarmed heel may lead to a falsely low glucose value because of stasis of blood and ongoing transfer of glucose to the cells. 5. Early, frequent feedings should be given on demand; frequency is not to exceed 4 hours between feedings (maximum of 3 hours between feedings for infants weighing < 2.5 kg). Allow the infant to begin feeding when he or she is demanding nutrition and when evaluation findings are within normal limits; nursing or formula feeding can be used (type of formula is by family’s or physician’s choice). (2) Contraindications to nippling the feeding or to breastfeeding (consider gavage feeding for these infants). (b) Respiratory rate greater than 60 breaths per minute without other signs of respiratory distress. (c) Weak suck. (d) Absent coordination of suck and swallow. (3) Contraindications to any enteral feedings. (b) Severe birth asphyxia. (c) Shock. (d) Increased work of breathing and oxygen requirement. (e) Suspicion of GI obstruction. b. Sterile-water “test” feeding. It is difficult to evaluate an infant’s ability to suck and swallow with sterile water because most infants do not like the taste and on occasion will refuse to suck or swallow; therefore, it is advisable to forgo “sips of water” in favor of a thorough evaluation before feeding. c. Dextrose 5%. Use of dextrose 5% is not indicated as a “test” feeding because studies show that it is more irritating to the lungs after aspiration than is formula. Moreover, it is not indicated after a feeding. d. Guidelines for feeding in transition nursery for transient asymptomatic hypoglycemia: (2) Check glucose level 1 hour after feeding is given. e. Indications for IV glucose infusion (see Initial Stabilization of the Sick Newborn Infant): (1) Hypoglycemia is persistent and symptomatic. (2) Enteral feedings are contraindicated. (3) Oral feedings do not maintain normal glucose levels. (4) Initial glucose screening level is less than 40 mg/dL. E. Ongoing teaching during transition. 2. Demonstrate or point out infant’s response to stimuli (tactile, visual, auditory): self-consolability, body movements, gaze, head turning. 3. Discuss physical findings. a. Transient: head molding, acrocyanosis, birth trauma, positional deformities. b. Permanent: congenital anomalies, birthmarks. 4. A stable infant may stay with the family from birth through recovery to postpartum period with appropriate observation and teaching provided by all staff members in contact with the family unit. F. If newborn transitioned in a nursery, he or she may be transferred when the following are stable. 1. Temperature, heart rate, respiratory rate. 2. Glucose level. 3. Normal physical assessment findings, or abnormal findings that do not require continuous observation or immediate intervention or treatment. B. Medications or history of substance abuse: alcohol, nicotine, cocaine, opiates, marijuana, amphetamines, tocolytics, anticonvulsants, anticoagulants, and analgesics/anesthetics. C. Maternal illnesses: pregnancy-induced hypertension, diabetes, intrapartum fever/infection (e.g., with group B streptococcus, genital herpes simplex virus, human immunodeficiency virus, and varicella), HBsAg positive, thyroid disease, inherited disorders, and cardiac disease. D. Perinatal fetal distress, delivery complications: abnormal fetal heart rate pattern, meconium staining of the amniotic fluid, rapid delivery, difficult delivery, rupture of membranes more than 18 hours before delivery. E. Cesarean delivery and indications: breech presentation, fetal distress, placenta previa, abruptio placentae, cephalopelvic disproportion, failure to progress in labor. 2. Pale. 3. Mottled. 4. Cool to touch. 5. Poor perfusion. B. Respiratory system. 2. Tachypnea. 3. Decreased air entry. 4. Increased work of breathing: grunting, flaring, and retracting. 5. Apnea. 6. Unequal breath sounds. 7. Oxygen requirement. C. Cardiovascular system. 1. Abnormal heart sounds such as murmur. 2. Weak, absent, or unequal pulses. 3. Hepatosplenomegaly. D. Central nervous system. 2. Jitteriness, tremors. 3. Lethargy. 4. Bulging fontanelle (record baseline head circumference). 5. Seizures. 6. Irritability, high-pitched cry. E. Morphologic features. 1. Congenital anomalies (e.g., abdominal wall defects, imperforate anus). 2. Severe birth trauma. 3. Absent or decreased limb movement. 4. Asymmetry. F. GI tract. 1. Abdominal distention (measure baseline abdominal girth). 2. Increased gastric contents on aspiration. 3. Inability to pass an orogastric tube. 4. Excessive mucus. 5. Emesis soon after birth or after first feeding. A. Pulse oximetry (peripheral monitoring of oxygen saturation). 2. A baseline PaO2 value should be obtained to confirm the infant’s oxygen level. B. Transcutaneous oxygen tension. 2. Site must have adequate perfusion as the sensor heats the skin to dilate the local blood vessels and promote diffusion of oxygen to the skin surface. 3. Sensor needs to be regularly calibrated. C. Arterial blood gas determinations. If oxygen requirement persists, pulse oximetry saturations in room air are decreased and cyanosis is present. D. Chest x-ray examination. Anteroposterior and lateral views are needed if respiratory distress is present or cardiac disease is suspected. E. Transillumination. Use a high-intensity light placed over the side of the chest in question if pneumothorax or pneumomediastinum is suspected. F. Whole-blood glucose screening test or serum glucose determination if indicated by history or assessment results: see Routine Care Considerations During Transition, item D: Glucose Needs/First Feeding. G. Hematocrit determination. 2. Plethoric or pale infant. 3. Twins (to rule out twin-to-twin transfusion). 4. Heel-stick (capillary) samples tend to have higher results by approximately 10%. 5. Hematocrit variations. Highest hematocrit is at 2 to 4 hours of age and then progressively falls as a result of the beginning of red blood cell breakdown and the cessation of erythropoiesis in response to a comparatively oxygen-enriched environment. H. Complete blood cell count with differential examination of the white blood cells. 1. As part of a sepsis diagnostic evaluation. 2. To screen for normal and abnormal hematologic indices. I. Blood culture as part of a diagnostic evaluation for sepsis. J. Urine sample collection. 2. Screening test for drugs of abuse. K. Lumbar puncture: Performed at the discretion of the physician/advanced practice nurse as part of a diagnostic evaluation for sepsis. L. Ultrasonography, computed tomography, and magnetic resonance imaging. 1. Cranial evaluation for abnormal central nervous system (CNS) findings. 2. Abdominal examination if history of two-vessel cord to rule out renal anomalies. M. Echocardiography and electrocardiography: As part of a diagnostic study for a congenital cardiac defect. N. Passage of orogastric tube. 1. To check patency of esophagus in infants with excessive pooling of mucus in the oropharynx. 2. To decompress a distended abdomen. 3. To measure and assess gastric contents (> 25 mL and/or significant bile in the stomach indicates obstruction). A. Birth trauma (refer to Chapter 2). B. Perinatal hypoxia–ischemia (Blackburn, 2013; Scher, 2013; Volpe, 2008). 2. Fetal distress is indicated by an abnormal fetal heart rate pattern, meconium staining of the amniotic fluid, scalp pH less than 7.20, and Apgar scores less than 5 at 1 minute of age and less than 7 at 5 minutes of age. 3. Pathophysiologic sequelae include: b. Respiratory acidosis from elevated levels of carbon dioxide. c. Metabolic acidosis. (1) Results in high pulmonary vascular resistance. (2) Leads to decreased surfactant release. d. Hypoxic–ischemic damage to less vital organs such as kidney and gut after redistribution of blood to vital organs. e. The myocardium depends on its stored reserves of glycogen for energy as its supply of oxygen falls. Eventually this reserve is consumed and the myocardium is simultaneously exposed to progressively lower PO2 and pH levels. The combined effects lead to reduced myocardial function with decreased blood flow to vital organs. 4. All newborn infants have some degree of respiratory acidosis and hypoxia during labor and vaginal delivery; a healthy term infant has increased tolerance and reserves. The asphyxiated newborn infant has more prolonged hypoxia and respiratory acidosis and may have additional metabolic acidosis, hypothermia, and hypoglycemia. 5. Clinical findings. a. Mild to moderate perinatal hypoxia–ischemia. (1) Extended awake, alert state (45 minutes to 1 hour). (2) Dilated pupils. (3) Normal muscle tone. (4) Active suck. (5) Regular or slightly increased respiratory rate. (6) Normal or slightly increased heart rate. b. Moderate to severe perinatal hypoxia–ischemia. (2) Hypoglycemia. (3) Pupils constricted. (4) Respiratory distress manifested by grunting, flaring, retracting, tachypnea, and oxygen requirement. (5) Seizures (subtle and multifocal clonic; 12 to 24 hours of age). (6) Acute tubular necrosis following reduced blood flow to the kidneys. (7) Hypotonia initially, lethargy. (8) Bradycardia. c. Severe perinatal hypoxia–ischemia requires constant monitoring in a level III/IV (intensive care) nursery. (1) Pale skin, poor perfusion, hypotension. (2) Cerebral edema. (3) Seizures. (4) Apnea. (5) Intracranial hemorrhage. C. Pulmonary problems (Gardner et al., 2011; Martin and Crowley, 2013). a. Tachypnea, unequal breath sounds, shift of heart tones, and distant heart tones. b. Transillumination of chest is positive for free air. 2. Retained lung fluid, respiratory distress syndrome (because of prematurity or birth asphyxia), and pneumonia. a. Decreased air entry with respiratory distress syndrome and pneumonia. b. Increased work of breathing: grunting, flaring, and retracting. c. Tachypnea, apnea. d. Decreased saturations (SaO2), cyanosis, continued oxygen requirement. 3. Aspiration syndromes (meconium, blood). b. Tachypnea. c. Barrel chest. 4. Upper airway obstruction (e.g., choanal atresia or micrognathia). 5. Extrapulmonary (e.g., phrenic nerve injury with resultant diaphragmatic paralysis or eventration of the diaphragm). D. Cardiovascular problems (Phelps et al., 2013; Scholz and Reinking, 2013). (1) Patent ductus arteriosus with a left-to-right shunt. (2) Ventricular septal defect. (3) Atrial septal defect. (4) Endocardial cushion defect or atrioventricular canal defects. b. Obstructive lesions. (2) Coarctation of the aorta. (3) Pulmonary valve stenosis or atresia. (4) Hypoplastic left heart syndrome. c. Admixture of lesions. (1) Normal or increased pulmonary blood flow. (a) Complete transposition of the great vessels. (b) Truncus arteriosus. (c) Anomalous venous connections of the pulmonary veins. (2) Decreased pulmonary blood flow. (b) Tricuspid valve atresia. 2. Persistent fetal shunts. a. Patent ductus arteriosus with right-to-left shunt. b. Persistent pulmonary hypertension. 3. Clinical findings. b. Unequal or absent pulses, bounding pulses, decreased blood pressure in the lower extremities, and decreased perfusion. c. Increased precordial activity, shift of point of maximal impulse of heart tones to right, murmur. d. Congestive heart failure, indicated by tachypnea, moist breath sounds, tachycardia, peripheral edema, cardiomegaly, and hepatomegaly. E. Hemodynamics. a. Internal hemorrhage resulting from birth trauma; intracranial hemorrhage. b. External hemorrhage resulting from placenta previa or abruptio placentae; cord accident; fetal–maternal or twin-to-twin transfusion. c. Respiratory distress, pallor, poor perfusion, hypotension, weak or absent pulses, anemia. 2. Polycythemia. a. Plethoric, cyanotic, or excessively flushed with crying. b. Hypoglycemia. c. CNS symptoms, including jitteriness, hypotonia, lethargy, and seizures. 3. Anemia. a. Acute or chronic blood loss. b. Hemolysis from sepsis or ABO/Rh blood group incompatibilities. c. Reduced red blood cell production, manifested by severe asphyxia, sepsis, and aplastic anemia. d. Pale skin, murmur, tachypnea, normal arterial blood pressure, signs of congestive heart failure, including hepatosplenomegaly and increased vascular markings on x-ray film. F. Metabolic problems. 1. Hypoglycemia (Jain et al., 2013; McGowan et al., 2011). b. Clinical findings: (1) Jitteriness, irritability. (2) Seizures. (3) Hypothermia, temperature instability. (4) Lethargy. (5) Poor feeding, emesis. (6) Apnea. (7) Cardiorespiratory distress, cyanosis, oxygen requirement. (8) Pallor. (9) Tachycardia. (10) Weak or high-pitched cry. 2. Adverse effects of maternal medications; maternal use of illicit drugs. b. Tocolytics. Infants may present with hypoglycemia. c. Narcotics. Infants present with apnea, respiratory depression, and periodic breathing. d. Cocaine. Infants may present with apnea, poor muscle tone initially and then irritability and agitation, tremors, and feeding difficulties. e. Marijuana or methadone. Infants present with hyperthermia, agitation, and diarrhea. f. Alcohol. Infants have fetal alcohol syndrome with dysmorphic and behavioral abnormalities. G. Infection (see also Chapter 32). 1. Generalized bacterial or viral disease; acquired in utero or nosocomial. 2. Clinical findings. NOTE: Nearly 90% of neonates with early-onset group B streptococcus have signs of infection within 12 hours of birth (median age is 8 hours) (Ferrieri and Wallen, 2013). b. Tachypnea, apnea. c. Respiratory distress, cyanosis. d. Tachycardia. e. Cool, mottled skin; weak pulses; capillary refill lasting longer than 2 seconds; and hypotension. f. Disseminated intravascular coagulation. g. Hepatosplenomegaly. h. Unexplained jaundice. i. Purpura, petechiae. j. Hypoglycemia or hyperglycemia. k. Poor feeding, emesis, and abdominal distention. l. Lethargy, poor muscle tone. 3. In-utero viral infection. Infant may be small for gestational age with microcephaly. H. Congenital anomalies (frequently obvious on gross examination). a. Immediate onset, at birth, of significant respiratory distress. b. Shift in heart tones, decreased or unequal breath sounds, bowel tones heard in chest, scaphoid abdomen, and cyanosis. 2. Esophageal atresia with or without tracheoesophageal fistula. b. Increased pooling of secretions in the oropharynx, respiratory distress, unable to place orogastric tube. 3. Abdominal wall defects: omphalocele and gastroschisis. 4. Limb anomalies: amniotic banding, talipes equinovarus, polydactyly, and syndactyly. 5. Neural tube defects. 6. Intestinal obstructions. 7. Chromosomal abnormalities such as trisomy 21 or trisomy 18. 8. Urogenital abnormalities: exstrophy of bladder, hypospadias, epispadias, and ambiguous genitalia. 2. Monitor and record trends (i.e., improvement of respiratory rate toward normal, improved perfusion, and normal glucose screens). B. Avoid excessive handling. 2. Use pulse oximeter or cardiorespiratory monitor to reduce hands-on determination of vital signs. 3. Reduce background stimulation such as loud noises or bright lights. 4. Use nonnutritive sucking to lower activity levels and reduce energy needs. a. Infant may be more comfortable in a prone position. b. Crying can be stressful and is similar to a Valsalva maneuver, with prolonged exhalation, obstructed venous return, quick inspiratory gasp, and right-to-left shunting at the foramen ovale. c. Crying depletes energy reserves and increases oxygen consumption. C. Provide a neutral thermal environment (refer to Chapter 6). 1. Observe infant for apnea and hypotension during warming. 2. Avoid hyperthermia. D. Supply glucose. a. Give at least 0.5 to 1 ounce of formula by nipple or gavage if there are no contraindications to enteral feedings and the infant is free of symptoms (see Routine Care Considerations During Transition). If condition is stable, infant may be allowed to nurse 5 to 10 minutes on each breast. b. Begin maintenance formula at 50 to 70 kcal/kg/day or breastfeed on demand every 2 to 3 hours. c. Check blood glucose 30 minutes to 1 hour after feeding. d. Consider giving a formula designed for premature infants when treating hypoglycemia orally. (1) These formulas provide 50% of carbohydrate in the form of glucose polymers that are easily absorbed; salivary amylase retains its activity in the infant’s stomach because of increased gastric pH and is effective in the digestion of glucose polymers (Blackburn, 2013). (2) Approximately 50% of the fats are provided as medium-chain triglycerides (MCTs). As MCTs are absorbed from the stomach, they may also increase the level of plasma ketones, which can be used as an alternative substrate to glucose for brain metabolism (Blackburn, 2013). (3) The process of absorbing fat (fatty acid oxidation and ketogenesis) spares glucose for brain energy needs; free fatty acids and ketones promote glucose production by providing essential gluconeogenic cofactors. (4) Healthy newborn infants respond to a protein meal by preferentially increasing glucagon, which elicits a glycemic response. 2. Intravenous administration of glucose for hypoglycemia (see Routine Care Considerations During Transition, item D: Glucose Needs/First Feeding) is as follows: b. Monitor therapy with frequent glucose checks and titrate the infusion rate and concentration to meet the infant’s needs. NOTE: Do not administer an IV bolus of glucose greater than D10W because of reactive hypoglycemia and hypertonicity of the solution. Always follow a glucose bolus with a continuous infusion of glucose. E. Supply oxygen: Assess needs with a pulse oximeter or with arterial blood gas determinations and close observation. 2. Provide warmed, humidified oxygen by oxygen hood, continuous positive airway pressure by nasal prongs, or assisted ventilation by endotracheal tube according to the infant’s needs (refer to Chapter 26). 3. Monitor oxygen provided with an oxygen analyzer. Oxygen should be provided via a blender to provide the least FiO2 required. The percentage and liters per minute should be recorded. Record blow-by oxygen in liters per minute and as distance from the infant’s face. F. Supply volume expanders, including blood and normal saline solution. 1. For hypotension and blood loss. 2. Requires IV line placement and transfer to level II or III nursery for continued management and observation, including cardiorespiratory monitoring and blood pressure checks to adjust therapy as necessary. G. Naloxone hydrochloride (Narcan). 2. Do not use naloxone if the mother has a history of opioid dependency: may precipitate acute withdrawal symptoms. H. Antibiotics. 1. As indicated by history, current status of the infant, and initial results of sepsis evaluation. 2. Administer via peripheral IV or heparin-lock IV line. A. History. Review obstetric history; anticipate needs of the infant at delivery. B. Complications. If there are expected complications (preterm delivery, congenital anomalies) and time permits, discuss the anticipated plan of care with the family. 2. Allow the parents to tour the NICU if possible. C. Parental support. Encourage parents to express their feelings, fears, and misgivings; involve support people. B. After delivery room assessment, return the infant to the family if the infant’s condition is stable. C. Answer questions regarding acrocyanosis, Apgar scores, and morphologic findings. D. Allow parents time to visit, breastfeed, and see extended family. B. Encourage the support person to touch and talk to the infant. C. Discuss physical findings such as caput succedaneum, head molding, positional deformities, and birthmarks. D. Discuss the infant’s sensory capabilities, including sight, hearing, and smell. E. Listen to the parents. Allow them to express their reactions as they compare their “dream” infant with the real infant they now have (too tiny, not the right sex, deformed, or premature). F. After completion of admission procedures, the infant is returned to the family for feeding and visiting. G. Allow the family to participate in the infant’s care as permitted by the neonate’s condition. B. Short hospital stays and family instruction. With shorter hospital stays, there is less time available for teaching and an increased importance of teaching. This may be the only information many families receive on care of a newborn infant. 1. Classes; videotaped lectures. a. Cardiopulmonary resuscitation; safety. b. Breastfeeding. c. Developmental milestones. 2. Follow-up visits by the nurse to the home and phone calls from postpartum nurses. Encourage families to call the nursery if they have questions about their newborn’s care. 3. Follow-up visit with the primary care provider within 48 to 72 hours. Encourage the family to select a primary care provider and assist in making an appointment for the first visit. 4. Return visit for newborn screening if needed. A. Provide prenatal teaching—if possible, with visits to NICU. B. Provide information booklets, with location, phone numbers, visiting regulations, parent-to-parent groups, and necessary support personnel. C. Bring the mother to the infant’s bedside if the infant is unable to return to the mother after delivery. Allow family members to be near the infant as much as possible and encourage them to see past the equipment to the infant and his or her special needs (gentle touch, stroking, soft voice, a familiar person). D. When the infant is stable, allow family members to visit in the privacy of their postpartum room or a parent room if condition warrants; for example, an infant with a heparin lock for antibiotics can be taken to the mother’s room to nurse. E. Provide a picture and footprints of the infant for the family. This is especially important if transfer to a level II or III nursery will be to another facility. F. Facilitate the family in keeping in contact with the transfer facility, and be available to explain information given to the family.
Adaptation to Extrauterine Life
ANATOMY AND PHYSIOLOGY
Characteristics of Placental–Fetal Circulation
Fetal Lung Characteristics
Fetal Metabolism and Hematology
Labor
Cardiopulmonary Adaptation at Birth
ROUTINE CARE CONSIDERATIONS DURING TRANSITION (GARDNER AND HERNANDEZ, 2011; ALDEN, 2012A; ALDEN 2012B; TAYLOR, WRIGHT, AND WOODRUM, 2013)
RECOGNITION OF THE SICK NEWBORN INFANT
Review of Perinatal History (Rezaee et al., 2013)
Physical Assessment
Diagnostic Tools (Bradshaw and Tanaka, 2011)
Common Problems and Clinical Presentation
Initial Stabilization of the Sick Newborn Infant
PARENT TEACHING
Before Delivery
At Delivery
During Transition
Postpartum Period (Early Discharge)
Transfer to Level II or III Setting
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


