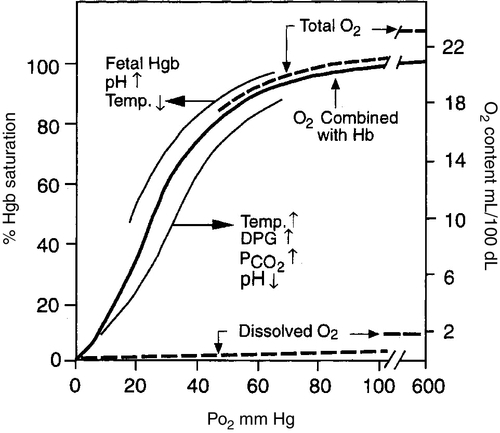
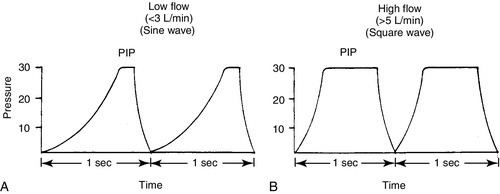
CHAPTER 26 Debbie Fraser; William Diehl-Jones 2. Describe the concepts of elastic recoil, compliance, resistance, and gas trapping and their importance in ventilating the lungs of the newborn infant. 3. Explain the relationship of fetal hemoglobin, pH, and temperature to the oxyhemoglobin dissociation curve. 4. List potential causes of respiratory and metabolic acid–base disturbance in the newborn infant. Identify ranges of pH, Pao2, Paco2, HCO3−, and base excess/deficit in various respiratory disease states in the newborn infant. 5. Identify treatment modalities for neonates in respiratory distress. 6. Describe various types of mechanical ventilation devices available for the neonate. 7. List nursing interventions required to care for ventilated infants, based on the theories of mechanical ventilation. 8. Differentiate between high-frequency jet ventilation and high-frequency oscillatory ventilation. 9. Identify the nursing interventions required for high-frequency ventilation that differ from those required for conventional ventilation. 10. Identify changes in patient status that indicate potential complications with assisted ventilation. 11. Describe various medications used to enhance lung status in the ventilated patient. Caring for an infant requiring assisted ventilation is a challenge. It is necessary for the nurse to understand the normal pulmonary physiology as well as the pathophysiology of pulmonary diseases in the neonate. An understanding of the basic mechanical principles of various ventilators is important to providing optimal care for a neonate. New ventilation techniques are being developed rapidly, and the choices for ventilating the neonate are greater now than ever before. The focus of this chapter is to provide the basic knowledge needed to care for the infant requiring oxygen therapy or mechanical ventilation. A. Definitions (Blackburn, 2013). 2. Vital capacity (VC): the volume of air maximally inspired and maximally expired (40 mL/kg). 3. Functional residual capacity (FRC): the volume of gas that remains in the lungs after a normal expiration (30 mL/kg). 4. Total lung capacity (TLC): the amount of air contained in the lung after a maximal inspiration (63 mL/kg). 5. Physiologic dead space: anatomic plus alveolar dead space. b. Alveolar dead space: the volume of inspired gas that reaches the alveoli but does not participate in gas exchange because of inadequate perfusion to those alveoli. 6. Mechanical dead space: gas that fills the ventilator circuit for availability in inspiration, as well as exhaled gas. Minimal dead space is desirable. Excessive dead space can cause increased retention of carbon dioxide. B. Concepts (Keszler and Abubakar, 2011). 2. Lung compliance: the change in volume that occurs with a change in pressure (elasticity of the lung). It also refers to the relationship between a given change in volume and the pressure required to produce that change. An infant with severe hyaline membrane disease will have decreased compliance (because of lack of surfactant) requiring increased pressure to overcome the resistance generated by the surface tension in the alveoli. The major force contributing to elastic recoil of the lung is surface tension at the air–liquid interface in distal bronchioles and alveoli. The amount of distal airway pressure needed to counteract the tendency of the alveoli and bronchioles to collapse is demonstrated by the Laplace relationship: the relationship between pressure, surface tension, and the radius of a structure. The pressure needed to stabilize an alveolus is directly proportional to twice the surface tension and inversely proportional to the radius of that alveolus. 3. Lung resistance: the result of friction between moving parts. Airway resistance is determined by the flow rate, viscosity, and density of the respiratory gases as well as the length and diameter of the airways (Keszler and Abubakar, 2011). An increase in airway resistance increases the time needed for air to reach the alveoli. High rates of airflow increase airway resistance by creating turbulence. Resistance to gas in a 2.5-mm endotracheal tube (ETT) is higher than in a 3.5-mm ETT because of the narrow lumen of the smaller tube. It takes greater pressure to force air through a small tube. Anatomic sources of resistance in the newborn infant include nasal passages, the glottis, the trachea, and the main bronchi. During intubation, the ETT is also a source of resistance. 4. Gas trapping: more gas entering the lung than leaving the lung. A partially occluded ETT can cause gas trapping. Debris from meconium can allow gas into the lung but may occlude the airway during exhalation (known as a ball–valve effect). 5. Inadvertent positive end-expiratory pressure (PEEP): a result of gas trapping in which volume and pressure increase in the distal airways through end-expiration. Providing oxygen by nasal cannula can result in inadvertent PEEP in the small premature infant. 6. Ventilation–perfusion ratio ( a. Too little ventilation with normal blood flow. b. Too little blood flow with normal ventilation. c. A combination of the above. 7. Mean airway pressure (MAP): mean or average pressure transmitted to the airways throughout an entire respiratory cycle (Gardner et al., 2011). MAP is dependent on the ventilator rate, gas flow through the ventilator circuit, peak inspiratory pressure (PIP), PEEP, and inspiratory time. Increasing MAP can greatly influence the management of respiratory distress in decreasing atelectasis and true intrapulmonary shunting and is a useful tool in determining oxygenation. 8. Permissive hypercapnia: ventilation strategy that allows carbon dioxide levels to remain elevated. This strategy is designed to minimize barotrauma by avoiding the use of mechanical ventilation or by using minimal ventilatory rates and pressures. C. Oxygen transport. 2. Oxygen is transported to tissue cells bound reversibly to hemoglobin and dissolved in plasma O2. The amount of O2 that is dissolved in the plasma is small (0.3 mL of O2 dissolved in 100 mL of plasma per 100 mm Hg of O2) compared with the amount that is bound to hemoglobin (1.34 mL of O2 per gram of 100% saturated hemoglobin). 3. The amount of oxygen carried in the blood by hemoglobin depends on the hemoglobin concentration and the percent saturation of the hemoglobin. Adequate saturation is affected by the amount of hemoglobin available. Hemoglobin is almost fully saturated at a PO2 of 80 to 100 mm Hg. 4. The binding of oxygen to hemoglobin varies with the PaO2. The relationship is nonlinear and gives rise to an S-shaped curve—the oxyhemoglobin dissociation curve. The amount of oxygen that combines with hemoglobin at a given PO2 depends on the position of the hemoglobin–oxygen dissociation curve (Fig. 26-2). Factors that determine the position of the dissociation curve are: b. Temperature. c. PCO2. d. pH. 5. With decreased affinity (shift to the right), hemoglobin releases O2 more easily to the tissues. 6. With increased affinity (shift to the left), oxygen is unloaded less rapidly and efficiently in the peripheral tissues. D. Control of breathing (Gardner et al., 2011). Control of ventilation (Box 26-1) is affected by both neurologic and chemical factors. The neurologic factors include central nervous system (CNS) maturity, sleep state, and reflexes. The chemoreflexes include responses to hypoxemia, hyperoxia, and hypercapnia. E. Hypoxia. Delivery of O2 to tissues is inadequate. Causes include the following: 2. Anemia. Hemoglobin available to transport oxygen is reduced, although completely saturated. PaO2 levels are usually normal. 3. Abnormal hemoglobin. O2 is not released to the tissues. b. Bart hemoglobin. 4. Cardiogenic or hypovolemic shock. F. Hypoxemia. O2 content of arterial blood is low because of extrapulmonary or intrapulmonary shunts. The blood has bypassed adequately ventilated alveoli. b. Can occur whenever alveoli are inadequately ventilated (hypoventilation). 2. Extrapulmonary shunt. b. Pulmonary artery hypertension. Blood shunts from the right side of the heart to the left side via a patent foramen ovale or ductus arteriosus, or both, causing blood to bypass the lungs. (2) In the presence of shunting, preductal blood obtained from the right radial artery has a greater than 5% difference in saturation from postductal blood obtained from the umbilical artery or posterior tibial artery. B. Nasal cannula. Humidified O2 is delivered at a set flow rate via a cannula, with the flow directed into the nares. The exact concentration of oxygen delivered by nasal cannula cannot be measured. 2. High-flow nasal cannula (HFNC). Flow is greater than 1.5 to 2 L/min, with oxygen blended to a known concentration. High flow rates result in the delivery of various levels of continuous positive airway pressure (CPAP) depending on the type of cannula, size of the infant, whether the infant’s mouth is open or closed, and the flow rate used (Mosca et al., 2012). Adequate humidification is essential with the use of high flow to prevent drying and damage to the nasal passages. HFNC may be comparable to nasal CPAP in level of respiratory support supplied (Miller and Dowd, 2010). 3. A blender should be used to adjust the concentration of oxygen being delivered by cannula, although the amount of oxygen entering the infant’s lungs cannot be accurately determined because of the entrainment of room air around the cannula. 4. Indications. A nasal cannula is used when there is a need for prolonged oxygen therapy, as in chronic lung disease, transfer or transport, and increased mobility of the infant for feedings or for other developmental activities. 5. Complications. Pressure-related tissue damage may occur because of improper or infrequent changing, O2 concentration may vary, hypoxemia may result from a displaced cannula, and the cannula may be occluded by nasal secretions (may cause significant respiratory distress). Flow through the cannula may result in drying of the nasal mucosa, predisposing the infant to tissue damage and thickened nasal secretions. C. CPAP. a. Indications: atelectasis, apnea, respiratory distress, and pulmonary edema. b. Advantages: short-term use to assist with alveolar expansion and to inhibit alveolar collapse (atelectasis); intubation not required. May be useful in infants whose nares are too small to accommodate CPAP prongs. Alternating between nasal prong CPAP and nasal mask CPAP may help to reduce skin breakdown around the nares in low birth weight (LBW) infants (Wisewell and Courtney, 2011). c. Complications: carbon dioxide retention in the dead space of the mask, pulmonary hyperexpansion potentially leading to air leaks (i.e., pneumothorax, pneumomediastinum), aspiration of stomach contents, and gastric distention. 2. Nasal CPAP: generally started at 5 to 6 cm H2O pressure and titrated up to 8 cm H2O pressure (Bingham and Fraser, 2012) delivered by prongs that fit into the nares, in addition to a measured concentration of oxygen. a. Indications: atelectasis, apnea, mild to moderate respiratory distress, and pulmonary edema. b. Advantage: intubation not required. c. Complications: ineffective ventilation, pneumothorax, variable pressure delivery when infant’s mouth is open, molding of the head from securing straps, erosion of the septum from poorly fitting prongs, nasal obstruction as a result of increased secretions, agitation, dislodging of prongs by an active infant, and gastric distention and perforation. 3. Nasopharyngeal CPAP: delivered by ETT or long nasal prongs, passed through the nares and positioned with the tip of the tube in the oropharynx. b. Advantage: stable placement of tube, which an infant is less likely to dislodge than with nasal CPAP. c. Disadvantages: need for a skilled provider to place the tube, possible damage to the nasal septum and oropharynx, more invasive than other forms of CPAP, variable pressure delivery when infant’s mouth is open, and gastric distention. Research has demonstrated that short nasal prongs are more effective in preventing reintubation than nasopharyngeal tubes (De Paoli et al., 2008). 4. Bilevel CPAP: provides continuous positive pressure at two separate CPAP levels. The background or baseline CPAP is set at 4 to 7 cm H2O with sighs or brief periods of increased pressure set at 2 to 4 cm of H2O higher than baseline. a. Indications: atelectasis, apnea, moderate respiratory distress, and pulmonary edema. May facilitate extubation in very low birth weight infants (Lista et al., 2010). b. Advantage: intubation not required. Higher MAPs may stabilize airways and assist in the recruitment of alveoli (Bingham and Fraser, 2012). c. Complications: ineffective ventilation, pneumothorax, variable pressure delivery when infant’s mouth is open, erosion of the septum from poorly fitting prongs, agitation, dislodging of prongs by an active infant, feeding intolerance, and gastric distention. 5. Nasal intermittent positive pressure ventilation (NIPPV): combines nasal CPAP with ventilator breaths delivered at a set peak pressure are delivered through nasal CPAP prongs or nasal CPAP mask. a. Indications: apnea, atelectasis, moderate respiratory distress. b. Advantages: Compared to nasal CPAP, NIPPV has been shown to reduce work of breathing and decrease the need for mechanical ventilation in the first 72 hours of life (Chang et al., 2011; Meneses et al., 2012). c. Complications: ineffective ventilation, pneumothorax, variable pressure delivery when infant’s mouth is open, erosion of the septum from poorly fitting prongs, agitation, dislodging of prongs by an active infant, feeding intolerance, and gastric distention. 6. Mechanical ventilation: respiratory support of infant using mechanical assistance. a. Gas-exchange mechanisms in spontaneous and conventional mechanical ventilation. (2) Molecular diffusion occurs in terminal airways and alveoli. This is the exchange of gases in adjacent spaces. (3) The status of alveolar ventilation is determined as follows: Alveolar ventilation = respiratory rate × (tidal volume delivered − anatomic dead-space volume). b. Indications: respiratory failure (hypoxemia, hypercapnia, and/or acidemia), pulmonary insufficiency, need for surfactant administration, severe apnea and bradycardia episodes, cardiovascular support, CNS disease, and surgery. c. Advantages: consistent delivery of assisted ventilation and oxygen therapy, decreases the work of breathing, and stabilizes the airway. d. Disadvantages: intubation by skilled provider, x-ray examination to confirm placement, possible intermittent x-ray examinations to verify placement or lung status, continuous monitoring of vital signs and oxygen saturation. Exposes the neonate to potential volutrauma/barotrauma of the lung tissue and increases the risk of chronic lung disease. e. Complications: tube malposition or dislodgment, underventilation or overventilation, tracheobronchial injury, pulmonary air leaks, infection, intracranial hemorrhage, bronchopulmonary dysplasia (BPD), and retinopathy of prematurity. 2. T-Piece resuscitator. Levels of positive pressure and PEEP are preset. Breaths are delivered by occluding the PEEP cap on the T-piece. A gas source is required to operate the T-piece resuscitator. Has the advantage of controlling the amount of pressure applied with each breath, thus eliminating variable pressure delivery inherent in bag-and-mask ventilation. A meta-analysis comparing the T-piece resuscitator to other self-inflating bags found no difference in morbidity or mortality (Hawkes et al., 2012). 3. Pressure-cycled ventilator. Inspiratory phase ends when a preset pressure is reached within the ventilator circuit, regardless of the volume of gas delivered during inspiration. 4. Time-cycled, pressure-limited, continuous-flow ventilator. A predetermined pressure of gas is administered; the duration of inspiration and expiration can be adjusted. Ventilator also allows for the infant’s spontaneous respiratory efforts, facilitating a gradual reduction of support. The operator determines the rate, PIP, PEEP, inspiratory time, and flow. 5. Volume-cycled ventilator. Inspiration ends when a preset volume of gas is delivered, regardless of the pressure reached within the ventilator circuit. The pressure used to deliver the breath will vary inversely with the infant’s lung compliance and respiratory effort. An increase in ventilation is achieved by increasing VT or rate; oxygenation is improved by increasing PEEP, FiO2, or VT (Harris and Fraser, 2012). 6. Pressure support ventilator. PSV supports breaths initiated by the infant by delivering a mechanical breath to a preset volume, and it uses a variable inspiratory time to allow the infant greater control and synchrony with the ventilator. PSV is flow cycled such that, when inspiratory flow decreases by a certain percentage, inspiration ends. PSV ventilators usually have a maximum inspiratory time that is preset. PSV is often used as a weaning mode of ventilation (Keszler, 2012). B. Patient-triggered ventilation. Mechanical breaths are delivered in response to a signal derived from the patient and detected as a spontaneous respiratory effort. The signal may be derived from a sensor that detects airflow, airway pressure, chest wall movement, or esophageal pressure (Keszler, 2012). The goal of patient-triggered ventilation is to avoid asynchrony of breathing by the patient and breaths given by the ventilator. Asynchrony may lead to air trapping, air leaks, CNS dysfunction, and irregularity of blood pressure and cerebral blood flow. Patient-triggered ventilation has been shown to improve gas exchange, decrease the need for and duration of ventilation, reduce the incidence of air leaks, and provide ventilation that better matches the infant’s own efforts (Brown and DiBlasi, 2011; Keszler, 2012). 1. Synchronized intermittent mandatory ventilation. b. Unassisted breaths occur between ventilator breaths, with continuous flow of gas from the ventilatory circuit. c. Partial asynchrony may occur if the patient attempts to terminate the inspiratory effort while the ventilator is in the inspiratory phase. 2. Assist/control (A/C) mode of ventilation. b. A detection system signals the start of inspiratory effort, which allows for synchronous initiation of inspiration. c. Asynchronous expiratory-phase breaths may still occur. 3. PSV. a. PSV is pressure limited and flow cycled. The ventilator supports each breath and terminates the breath when the inspiratory flow drops below a preset threshold. This termination mechanism avoids prolonged inspiration and maximizes synchrony (Keszler, 2012). 4. Neurally assisted ventilation (neurally adjusted ventilatory assist [NAVA]). b. Each breath is supported in proportion to the intensity of the infant’s inspiratory effort in an attempt to optimize synchrony (Biban et al., 2010). 5. Volume-targeted ventilation. b. A VT is set by the operator based on the infant’s weight and disease condition. c. The benefits of volume-targeted ventilation include reduced risk of death or chronic lung disease, reduced risk of air leaks, and a reduced duration of mechanical ventilation when compared to pressure-limited ventilation (Wheeler et al., 2010). A. Care of O2 delivery devices. 2. Nasal cannula. Remove and clean secretions every 4 to 6 hours as needed. Inspect surrounding tissue for pressure-related injury. If sudden onset of respiratory distress occurs, inspect cannula for secretions and suction nasopharynx for mucus. Change cannula according to unit protocol. Some infants receiving oxygen by nasal cannula benefit from the administration of saline drops to moisten the mucosa. These should be administered according to unit protocol. In addition, oxygen/air flow should be warmed and humidified. 3. Nasal prong CPAP. Ensure that CPAP device is of the correct size to decrease the incidence of pressure necrosis of the nares. Nasal CPAP units come in a variety of sizes and should be short and wide, with thin walls to allow for maximal airflow. They should be soft and flexible and should be easy to secure and maintain. Humidification of 90% to 100% should be provided in the CPAP system to prevent drying out of the mucous membranes and subsequent formation of thick secretions. Evaluate the need for suctioning every 2 to 4 hours. Inspect surrounding tissue for pressure-related injury. Secure the device to a stockinette cap or with soft straps provided by some manufacturers. Lightweight tubing is helpful for ease in securing the device and in keeping the unit in the nose. The infant can be positioned supine, on either side, or prone, generally with the head of the bed at approximately a 30-degree angle. Observe for abdominal distention resulting from excessive air entering the stomach from the CPAP device. Consider aspirating every few hours from an orogastric tube or leaving it open to continuously vent gas from the stomach. Feeding is not contraindicated during delivery of CPAP. The clinical condition must be evaluated before institution of feedings. Change prongs according to unit protocol, generally every 2 or 3 days. 4. ETT. After correct placement has been determined, note the depth of the tube at the gum or lip and post at the bedside. This is important for future reference in case the tube slips, if reintubation is needed, or to determine suction catheter length. Secure the ETT with tape or other method. Each institution generally develops a method that works well for the staff and patients. Observe for evidence of slipping or tape loosening and secure again when necessary to prevent accidental extubation. Position the infant supine, on either side, or in the prone position, with the head in a neutral position. Be aware that the tube moves with the chin and can move several centimeters with flexion or extension of the head. Signs of extubation include sudden deterioration in clinical status, abdominal distention, crying, decreased chest wall movement, breath sounds in the abdomen, agitation, cyanosis, and bradycardia. Notify the physician or neonatal nurse practitioner if extubation is a concern and prepare for reintubation as soon as possible. Intubation equipment should be readily available. A bag and mask with pressure manometer or a T-piece resuscitator should also be available at each bedside and should be tested during each shift. Suction the ETT when necessary. Complications of an ETT include palatal grooves (consider a palate protector, which can be made by a pediatric dentist or is commercially available), nasal erosion, subglottic stenosis, tracheoesophageal perforation during insertion of the tube, aspiration, infection, and tracheal granuloma. 5. Tracheostomy tube. Daily changing of the dressing and weekly changing of the tube are usually adequate. Inspect the site for signs of tissue pressure and/or necrosis. Suctioning is necessary to keep the airway clear of secretions. Family members need to be included in this procedure, thus facilitating discharge. B. Suctioning the airway. b. Suctioning can coincide with the cleaning or changing of the tubes or with routine caregiving. 2. Endotracheal tubes. a. The amount of secretions will be disease related. Infants with resolving respiratory distress syndrome, patent ductus arteriosus (PDA), BPD, and pneumonia are more likely to require suctioning because of an increased production of mucus. Patients with early-stage respiratory distress syndrome and those with most types of congenital heart disease will not have much mucus and will require less suctioning. Suctioning is done on an as-needed basis, never on a routine schedule (Gardner et al., 2011). Criteria for suctioning include evidence of secretions (audible or visible), changes in vital signs, agitation or restlessness, and changes in oxygenation or ventilation. b. Protocols for suctioning vary from one institution to another. Administering manual breaths with the ventilator before and after suctioning may be necessary to maintain lung volumes during the procedure. Hyperoxygenation prior to suctioning is generally not appropriate. c. In-line suction devices allow suctioning while ventilation continues and are associated with a decrease in episodes of hypoxia and smaller changes in heart rate; however, differences in long-term outcomes have not been demonstrated (Taylor et al., 2011). d. Do not advance the suction catheter farther than the distance of the ETT, and do not suction too vigorously. e. Vacuum pressure range should be 60 to 100 mm Hg (Gardner et al., 2011). A 5 F or 6 F suction catheter for a 2.5- to 3.5-mm ETT, or an 8 F suction catheter for a 4- to 4.5-mm ETT, is usually appropriate. f. Complications of suctioning include hypoxemia, bradycardia, barotrauma, changes in blood pressure, alterations in cerebral blood flow, intraventricular hemorrhage, tracheal damage, atelectasis, infection, and pneumothorax. C. Initiating mechanical ventilation. The goal of mechanical ventilation is to assist in providing adequate tissue oxygenation and eliminating CO2. 1. Establish an airway. Endotracheal intubation should be performed by a skilled provider (see Chapter 15). 2. Ventilator selection. The ventilator selected for use is based on the patient’s condition and disease process, the patient’s response to previous ventilatory support, and staff experience and comfort with the device. Patient-triggered or synchronized ventilation is now the preferred method of mechanically ventilating neonates in most NICUs. There are a number of devices and ventilator modalities that allow the operator to control the PIP or volume delivered to the neonate, as well as the rate, PEEP, flow, and inspiratory time. 3. Parameters to be set and/or monitored during mechanical ventilation. b. PIP. PIP is the primary factor used for determining VT and affecting PaO2 (Harris and Fraser, 2012). Determining the appropriate PIP requires careful and skilled assessment. The complications of excessive PIP include air leaks, decreased venous return, intraventricular hemorrhage, and decreased cardiac output. Factors such as weight, gestational age, disease process, lung compliance, and airway resistance must be considered. Auscultation of breath sounds to assess aeration and compliance is necessary. Visual inspection of chest wall movement should guide your assessment. A beginning PIP of 20 cm H2O is appropriate for most preterm infants. The lowest PIP that will provide adequate ventilation is ideal, with the goals of preventing barotrauma and volutrauma and decreasing the incidence of air leaks and chronic lung disease (Dargaville and Tingay, 2012). Certain conditions may warrant use of high PIP, including poor compliance, atelectasis, or pulmonary hypertension. Before connecting the patient to the ventilator, ensure that the inspiratory pressure is correct. Recheck after the connection has been made, and adjust as necessary. c. VT. VT is the primary factor affecting both oxygenation and ventilation. When using volume-targeted ventilation, the operator determines the desired volume to be delivered with each breath rather than setting the PIP. Based on averaging a series of breaths, the ventilator determines the PIP needed to deliver the desired volume of gas. An upper limit for the PIP should be set by the operator to prevent the delivery of excessive pressure.An increase in ventilation is achieved by increasing the volume of gas to be delivered (VT) or the rate. Increased oxygenation is achieved by increasing the FiO2, the PEEP, or the VT (Harris and Fraser, 2012). Determination of VT is based on the infant’s weight, with a beginning VT of 4 to 5 mL/kg being most common. d. PEEP. This measure aids in maintaining FRC, stabilizing and recruiting atelectatic areas for gas exchange, improving compliance, and improving ventilation–perfusion matching in the lung (Gardner et al., 2011). PEEP is important in assisted ventilation for infants with surfactant deficiency because of the likelihood of alveolar collapse. Physiologic PEEP is estimated at 2 cm H2O. Levels lower than 2 cm H2O are not generally recommended. In most instances, medium levels, about 4 to 7 cm H2O, are recommended. Levels greater than 8 cm H2O are associated with pulmonary air leaks and reduction of cardiac output. e. Inspiratory/expiratory (I/E) ratio: ratio of time spent in inspiration to time spent in expiration. Determining this time should be based on the underlying reason for ventilation. A physiologic I/E ratio in a nondisease state is equal to 1:2 or 1:3, meaning a short inspiratory time with a longer expiratory time. Prolonged expiratory time is useful during weaning, when oxygenation is not as problematic. The I/E ratio will affect the PaO2 and PaCO2. Changes affect MAP and oxygenation. f. Flow rate: flow of gas (measured in liters per minute) through the patient’s circuit. The flow rate determines the ability of the ventilator to deliver the desired amount of PIP, waveform, I/E ratio, and respiratory rate. A flow rate of at least twice the infant’s minute ventilation ensures that the ventilator can reach the desired pressure. Flow rates of 4 to 10 L/min are usually adequate (Harris and Fraser, 2012). With low flow rates (< 3 L/min), inspiratory pressure gradually builds to a peak just before expiration, closely resembling a sine waveform (normal breaths are shaped like a sine waveform). There may be less barotrauma to the airways with a sine waveform. High flows of 4 to 10 L/min or higher are necessary with square waveform ventilation or when high rates are used. A square waveform pattern moves the ventilator breath rapidly from the resting or expiratory pressure level to the PIP. Because the PIP is reached sooner than with a sine waveform, the PIP is held for a longer period (Fig. 26-3). This may be advantageous with atelectatic areas of the lung. It may also contribute to barotrauma.
Assisted Ventilation
PHYSIOLOGY
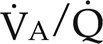 ) (Fig. 26-1). Matching pulmonary ventilation and perfusion is necessary for efficient gas exchange. The relationship between ventilation and perfusion is expressed as a ratio and describes the relationship between alveolar ventilation and capillary perfusion of the lungs. A 1:1 ratio indicates that the alveoli are in perfect contact with the pulmonary capillaries, allowing exchange of O2 and CO2. A
) (Fig. 26-1). Matching pulmonary ventilation and perfusion is necessary for efficient gas exchange. The relationship between ventilation and perfusion is expressed as a ratio and describes the relationship between alveolar ventilation and capillary perfusion of the lungs. A 1:1 ratio indicates that the alveoli are in perfect contact with the pulmonary capillaries, allowing exchange of O2 and CO2. A 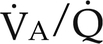 ratio of zero indicates a shunt whereby no ventilation occurs during passage of blood through the lungs. Abnormalities of the
ratio of zero indicates a shunt whereby no ventilation occurs during passage of blood through the lungs. Abnormalities of the 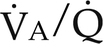 ratio may be due to:
ratio may be due to:
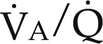 ) ratios on blood gas tensions. A, Direct venoarterial shunting (
) ratios on blood gas tensions. A, Direct venoarterial shunting (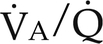 = 0). Venous gas tensions are unaltered, and arterial blood has the same tension as venous blood. B, Alveolus with a low
= 0). Venous gas tensions are unaltered, and arterial blood has the same tension as venous blood. B, Alveolus with a low 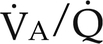 ratio. Only partial oxygenation and CO2 removal take place in this alveolus because of underventilation in relation to perfusion. C, Normal alveolus. D, Underperfused alveolus with high
ratio. Only partial oxygenation and CO2 removal take place in this alveolus because of underventilation in relation to perfusion. C, Normal alveolus. D, Underperfused alveolus with high 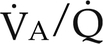 ratio. Note that although the oxygen tension is 32 mm Hg greater than in normal alveolus (C), this results in only a slightly higher saturation and O2 content. (From Thibeault, D.W. and Gregory, G.A.: Neonatal pulmonary care. Norwalk, Conn., 1986, Appleton-Century-Crofts.)
ratio. Note that although the oxygen tension is 32 mm Hg greater than in normal alveolus (C), this results in only a slightly higher saturation and O2 content. (From Thibeault, D.W. and Gregory, G.A.: Neonatal pulmonary care. Norwalk, Conn., 1986, Appleton-Century-Crofts.)
TREATMENT MODALITIES
Types of Assisted Ventilation
Ventilator Modes
NURSING CARE OF THE PATIENT REQUIRING RESPIRATORY SUPPORT OR CONVENTIONAL MECHANICAL VENTILATION

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


26: Assisted Ventilation
FIGURE 26-1 ■ Effects of various ventilation–perfusion (
FIGURE 26-2 ■ Hemoglobin–oxygen dissociation curve. Nonlinear or S-shaped oxyhemoglobin curve and the linear or straight-line dissolved O2 relationships between the O2 saturation (SaO2) and the PO2. Total blood O2 content is shown with division into a portion combined with hemoglobin and a portion physically dissolved at various levels of PO2. Also shown are the major factors that change the O2 affinity for hemoglobin and thus shift the oxyhemoglobin dissociation curve either to the left or to the right. DPG, 2,3-diphosphoglycerate. (Modified from West, J.B.: Respiratory physiology: The essentials [2nd ed.]. Baltimore, 1979, Williams & Wilkins, pp. 71, 73.)
FIGURE 26-3 ■ Comparison of ventilator waveforms. A, Relative sine wave. B, Relative square wave. (From Goldsmith, J.P. and Karotkin, E.H. [Eds.]: Assisted ventilation of the neonate [3rd ed.]. Philadelphia, 1996, W.B. Saunders, p. 169.)
