CHAPTER 37 Debbie Fraser; William Diehl-Jones 1. Describe the normal anatomy of the eye. 2. Identify the normal anatomy of the ear. 3. Identify the major function(s) of each structure. 4. Describe the components of a nursing assessment of the eyes and ears in the neonate. 5. Describe the nurse’s role in assisting the physician with neonatal eye examinations. 6. Discuss the factors to consider in universal hearing screening of newborns. 7. For each of seven types of eye disorders in the neonatal period—traumatic injuries to the eye, conjunctivitis, nasolacrimal duct obstruction, cataracts, retinoblastoma, infections (TORCH diseases), and retinopathy of prematurity—(1) provide an overview of the pathogenesis and (2) describe commonly used treatment modalities, outlining the specific nursing care measures designed to meet the needs of neonates with these disorders. 8. Outline the most common causes of hearing loss in the newborn. 9. Outline teaching points for the family of a newborn at risk for hearing or vision problems. An examination of the neonate’s eyes and ears is an important, though often neglected, portion of a physical assessment. There is a great deal of clinically significant information that the astute nurse can glean from a thorough evaluation of these systems. Evidence of intrauterine infection, birth trauma, congenital malformations, disease, and a variety of genetic abnormalities can be detected during the course of the nurse’s assessment of the neonate’s eyes and ears. This chapter provides the neonatal nurse with a review of normal anatomy of the eye and ear, together with the major function(s) of each structure; the essential components of an assessment of the newborn’s eyes and ears; an overview of the most common eye disorders in the neonate; and common treatment modalities and nursing measures used in the treatment of various ocular disorders in the newborn infant. The essential elements of a universal hearing screening program for newborns are addressed, as are the most common causes of hearing loss in neonates. B. Conjunctiva: mucous membrane lining the inner aspect of the eyelids (palpebral) and onto the eyeball to the periphery of the cornea (bulbar). C. Lacrimal system: manufactures and drains away tears; cleans, lubricates, and moistens the eyeball. D. Bony orbit or socket: surrounds and protects the eyeball. Most important opening within the orbit is the optic foramen, through which the optic nerve, ophthalmic artery, and ophthalmic vein from each eye pass en route to the brain. A. Outer layer (fibrous tunic). 1. Cornea: transparent; reflects light rays. 2. Sclera: the “white” of the eye; normal bluish appearance in newborn infants; gives shape to the eyeball and protects the inner parts. B. Middle layer (vascular tunic): the uveal tract. 2. Ciliary body: the anterior portion of the choroid. 3. Choroid: a vascular, pigmented membrane that lines most of the internal surface of the sclera, absorbs light rays, and nourishes the retina. C. Inner layer: the retina. 1. Extends from the ora serrata to the optic nerve. 2. Functions in image formation. a. Photoreceptors: rods and cones. b. Bipolar cells. c. Ganglion cells. 3. Optic disc: retinal blood vessels enter the eye, and optic nerve exits the eye. Blind spot in field of vision because optic disc has no photoreceptors. 4. Optic nerve: second cranial nerve. 5. Macula: exact center of the retina and location of sharpest vision. D. Anterior cavity (filled with aqueous humor). 1. Anterior chamber: behind the cornea, in front of the iris. 2. Posterior chamber: behind the iris, in front of the suspensory ligament and lens. E. Lens: a biconvex, transparent capsule that refracts light; the most important focusing mechanism of the eye. 1. Lens remains cloudy until 30 to 34 weeks of gestation. F. Posterior cavity (filled with vitreous humor): lies between the lens and the retina. Contributes to intraocular pressure, gives shape to the eyeball, and holds the retina in place. B. Innervation. The extraocular muscles are innervated by the oculomotor (third cranial) nerve, the abducens (sixth cranial) nerve, and the trochlear (fourth cranial) nerve. 1. Pupillary reflex is functional by 36 weeks. C. Function (Blackburn, 2013; Gardner and Goldson, 2011). 1. At birth, newborns are able to see an object best at a distance of 8 to 10 inches with a visual acuity of 20/400 (de Alba Campomanes et al., 2012). 2. A healthy term newborn is able to fix on an object and follow it up to 90 degrees in a horizontal arc. 3. Newborns prefer black-and-white patterns and the human face. 4. Color discrimination develops at 2 to 3 months of age (Graven, 2004). B. Birth history: gestational age, duration of labor, use of forceps. C. Family history: incidence of ocular disorders, especially retinoblastoma; systemic diseases. The examination is performed with the baby in a quiet, alert state. To facilitate the spontaneous eye-opening, use an auditory stimulus, change the infant’s position from supine to upright, or dim the lights (Johnson, 2009). Eye prophylaxis may make the examination more difficult. 2. Spontaneous eye movements: note range of motion and conjugation (the ability of the eyes to move together). Infants can track and follow objects with both eyes. Erratic or purposeless movements may be observed during the first few weeks of life. Median focal distance for the term neonate is about 8 inches (20 cm). B. Reaction to light or visual stimuli: strong blink reflex to bright light or stimulation of the lids, lashes, or cornea. A somewhat unsteady gaze can be observed shortly after birth, with ability to fixate on a stimulus for 4 to 10 seconds and refixate every 1 to 1.5 seconds. Ability to maintain fixation and to follow does not occur until 5 to 6 weeks of age. C. Pupils: shape should be round and reaction to light should be equal; constriction to both direct and contralateral stimulation should occur. The red reflex should be elicited bilaterally; normally appears as a homogeneous bright red-orange. Opacities or interruptions may indicate cataracts or retinoblastoma. D. Eyelids: note symmetry, epicanthal folds, bruising or edema, lacerations, ptosis, and presence of lacrimal puncta. E. Conjunctivae: should be pink and moist; redness or exudate is abnormal. F. Corneas: may be somewhat less than transparent or slightly hazy in the first few days of life in both premature and term infants. Sclerae may be bluish in premature or small babies as a result of thinness. G. Irises: should be similar in appearance; note pigmentation. A coloboma, or keyhole pupil, may be associated with congenital anomalies. Brushfield’s spots are silvery gray spots scattered around the circumference of the iris—strongly associated with Down syndrome. H. Lenses: should be clear and black with direct illumination. Examination of the anterior vascular capsule of the lens is a useful adjunct to determination of gestational age in preterm infants between 27 and 34 weeks. 1. Direct result of duration and difficulty of delivery. 2. Improperly applied forceps. 3. Compression of cranial nerves. B. Clinical presentation. 1. Petechiae, ecchymoses, edema, and/or lacerations of pinna, lids, conjunctiva, or globe. 2. Bright red patches on conjunctiva (subconjunctival hemorrhage): occurs in up to 13% of births (Isenberg, 2005). 3. Droopy eyelids. C. Complications: These injuries are generally mild and transient, often resolving spontaneously. Conjunctivitis is an inflammatory reaction resulting from invasion of the conjunctivae by pathologic organisms. A wide variety of infectious agents are capable of producing conjunctivitis in the newborn infant. The most common causes in North America include the following: A. Neisseria gonorrhoeae: peripartum transmission, onset 3 to 4 days. B. Chlamydia trachomatis: peripartum transmission, onset 5 to 7 days. C. Staphylococcus aureus: acquired during the neonatal period, onset 5 to 14 days. D. Herpes simplex: peripartum transmission, onset 6 to 14 days A. Incidence: One third of neonates born vaginally to infected women develop ophthalmic gonococcal infection (Embree, 2011). May be higher in areas with poor perinatal care or irregular antibiotic eye prophylaxis after birth. B. Onset of infection: onset of symptoms usually between days 3 and 4 of life. C. Clinical presentation. 2. Purulent discharge. 3. Redness/hyperemia of the conjunctivae. D. Diagnostic findings. a. Maternal history of sexually transmitted disease. b. Age at onset of infection. 2. Physical examination. a. Clinical signs of inflammation. b. Purulent discharge. 3. Laboratory. a. Gram stain shows gram-negative diplococci. b. Culture positive for gonococci from conjunctival surface or exudate. E. Nursing care. 1. Isolate infant in accordance with infection control guidelines. 2. Irrigate eyes with sterile normal saline solution hourly until discharge is eliminated. 3. Promptly administer appropriate systemic therapy. Topical antimicrobial therapy is not required. a. Penicillin-sensitive N. gonorrhoeae: aqueous crystalline penicillin G, intravenous (IV) or intramuscular (IM), for 10 days (Venkatesh et al., 2011). b. Penicillin-resistant N. gonorrhoeae: ceftriaxone, 25 to 50 mg/kg (maximum 125 mg) IV or IM in a single daily dose (de Alba Campomanes et al., 2012) or cefoxitin (Venkatesh et al., 2011). 4. Parents of infected infant should be referred for evaluation and treatment. F. Complications. 2. Systemic complications involving the blood, joints, or central nervous system may occur in a small number of infants. 1. The most common cause of conjunctivitis in the neonatal period, especially in areas with poor perinatal care or irregular administration of erythromycin eye prophylaxis after delivery. Chlamydial eye infections occur in up to 1% of births in developed countries (Isenberg, 2005). 2. About 20% to 50% of babies born vaginally to mothers with a C. trachomatis infection of the cervix will develop conjunctivitis; 10% to 20% develop pneumonia (Darville, 2011). 3. Prevention of infection in the newborn infant is dependent on prenatal detection and treatment of the mother or on the use of an effective form of eye prophylaxis at birth (e.g., erythromycin ointment). B. Onset: Symptoms are usually observed between 5 and 7 days of age. C. Clinical presentation: Symptoms vary from mild conjunctivitis to intense edema of the lids with purulent discharge. A pseudomembrane may be present over the conjunctiva. D. Diagnostic findings. 1. Identification of Chlamydia antigen. 2. Stains of conjunctival scrapings. 3. Culture of conjunctival scrapings. E. Patient management. 1. Therapy of choice is oral erythromycin (estolate preparation), for 14 days (Darville, 2011). 2. Topical therapy alone is inadequate to eradicate the organism from the upper respiratory tract. 3. Parents of infected infants should be referred for evaluation and therapy. F. Complications: Infection is spread via the nasolacrimal system to the nasopharynx, leading to Chlamydia-related pneumonia. 2. Term and preterm newborn infants have the capacity to secrete tears (reflex tearing to irritants) but usually do not secrete emotional tears until 2 to 3 months of age. 3. Congenital obstruction is usually caused by an imperforate membrane at the distal end of the nasolacrimal duct. 4. Congenital nasolacrimal obstruction is the most common abnormality of the neonate’s lacrimal apparatus. Incidence of this condition ranges between 5% and 10% of all newborn infants (de Alba Campomanes et al., 2012). B. Clinical presentation. 1. Usually within the first few weeks of life. 2. Persistent tearing (epiphora): need to rule out congenital glaucoma. 3. Crusting or matting of the eyelashes: “sticky eye.” 4. Spilling of tears over the lower lid and cheek: a “wet look” in the involved eye(s). 5. Absence of conjunctival infection. 6. Mucopurulent material refluxing from either punctum when gentle pressure is applied over the involved nasolacrimal sac. C. Nursing care. 2. Technique consists of placing the index finger over the common canaliculus to block the exit of material through the puncta, and stroking downward firmly. 3. Digital pressure increases hydrostatic pressure in the nasolacrimal sac, which may cause a rupture of the membranous obstruction. 4. If a mucopurulent discharge is present, antibiotic eyedrops (sodium sulfacetamide) or ointment (erythromycin) may be required. 5. Cleansing of eyes: eyes should be cleaned with moist compresses, with secretions mechanically removed. 6. Duration of conservative management: conservative management is advocated for the first year of life. 7. Resolution: the majority of nasolacrimal obstructions resolve spontaneously or with massage by 1 year of age. 8. Surgical treatment: unresolved obstructions can be successfully treated surgically; tear duct probing is done, with the infant under general anesthesia, after the first year of life. D. Complications. 1. Acute dacryocystitis: inflamed, swollen lacrimal sac. 2. Fistula formation. 3. Orbital or facial cellulitis. Congenital cataracts are the main treatable cause of visual impairment in infancy. To ensure optimal visual development, congenital cataracts should be surgically removed within 6 to 8 weeks of birth (de Alba Campomanes et al., 2012). 2. Cataract: a cataract is an opacity of any size or degree in the lens of the eye. 3. Path of light: normally the light from an object passes directly through the lens to a focal point on the retina, producing a sharp image. Cataracts result in a degraded image or no image at all. 4. Visual impairment: cataracts lead to varying degrees of visual impairment, from blurred vision to blindness, depending on the location and extent of the opacity. In neonates, cataracts may be transient, disappearing spontaneously within a few weeks. B. Etiology or precipitating factors. 1. Idiopathic (30%): developmental variation, not associated with other abnormalities. 2. Genetically determined (30%): most common mode of inheritance—autosomal dominant. 3. Congenital rubella: cataracts are present in 30% of newborn infants with congenital rubella syndrome (Plotkin et al., 2011). 4. Other congenital infections. b. Cytomegalovirus (CMV) infection. c. Herpes simplex. d. Varicella. 5. Metabolic disorders (e.g., galactosemia). 6. Chromosomal abnormalities (e.g., Down syndrome, trisomy 13, Turner’s syndrome). 7. Clinical syndromes (e.g., Crouzon’s disease, Pierre Robin syndrome). 8. Prematurity. C. Clinical presentation. 2. Searching nystagmus (at 1 to 2 months of age). The presence of nystagmus is a marker for poor visual prognosis. D. Diagnostic findings. a. Family history of ocular disease or systemic disorders. b. Pregnancy, especially first-trimester intrauterine infections. 2. Physical examination. b. Examine to detect a white pupil by shining a light into each eye, with the light source held to one side. c. If the opacity is small, it may be identified only when the pupils are dilated and with the use of an ophthalmoscope. d. Consider other diseases of the eye that may produce a white pupil (e.g., retinoblastoma). E. Nursing care. 2. Parental education: in collaboration with the physician, assist parents in understanding the nature, possible cause, and treatment of cataracts in the newborn infant, together with the prognosis for future vision. Surgery is indicated whenever the cataract is likely to interfere with vision. 3. Explore any feelings of guilt the parents may have in relation to the cause of the cataracts; provide appropriate support. 4. Encourage parent–infant attachment: neonate may not be able to see the parents but can learn to know their voices, smell, and touch. 5. Care for the patient postoperatively. b. Administer eyedrops or ointments as ordered postoperatively. c. Apply clean eye patches or protective shields to protect the eye from rubbing or bumping and to prevent irritation from light. d. Monitor for complications of cataract surgery. These are relatively infrequent but include infection within the eye, glaucoma, and retinal detachment. Note any increased redness or haziness of the eye, increased tearing, photophobia, or cloudiness of the cornea. Increased crying, irritability, disruption in sleeping patterns, or rubbing of the eye may indicate pain. e. Assist the parents in understanding the essential role of optical correction devices, such as glasses or contact lenses, in their infant’s vision and development. f. Promote appropriate visual stimulation and foster normal infant development by teaching parents about newborn visual preferences (e.g., black-and-white contrast or medium-intensity colors, the human face, geometric shapes, checkerboard designs). F. Complications. 1. Varying degrees of visual impairment, leading to developmental delay. 2. Presence and/or severity of associated ocular defects, such as microphthalmos and glaucoma. G. Outcome. Visual prognosis depends not only on the extent of cataracts, age at removal, surgical outcome, and rapid optical correction but also on the nature of other associated anomalies of the eye or syndromes. Retinoblastoma is the most common ocular malignancy in children, with an incidence of 1 in 15,000 to 20,000 live births. 2. Strabismus (19%). 3. Loss of visual acuity (4%). 4. Red eye (5%). B. History. 1. Family history of retinoblastoma. C. Physical examination. 1. Examine for presence of red reflex and to detect a white pupil. 2. Consider congenital cataracts as an alternative cause of leukokoria. D. Treatment. 2. Chemotherapy. 3. Focal destruction of the lesion. E. Complications. 1. Loss of vision in the affected eye. 2. Survival is greater than 90% with early recognition and treatment. 3. Long-term survival is 50% if spread outside the eye has occurred. The developing eyes are highly vulnerable to the damaging effects of prenatal infection (Mets and Chhabra, 2008), and ocular abnormalities may in fact be the predominant manifestation of the disease. A number of the congenitally acquired infections are associated with abnormal ocular conditions, including cataracts, chorioretinitis, corneal opacities, and glaucoma. The most common of these infections are toxoplasmosis, rubella, CMV, and herpes (see also Chapter 32). In the prevaccine era, rubella was a common childhood infection. Since the introduction of vaccinations, congenital rubella syndrome occurs infrequently. In fact, between 2001 and 2004, only 25 cases of congenital rubella syndrome were reported (Anderson and Gonik, 2011). 2. Infection in the first trimester of pregnancy presents the greatest hazard to organogenesis, including that of the eyes. B. Incidence. Ocular abnormalities are frequently seen in congenital rubella, with “salt-and-pepper” retinopathy being the most common ocular abnormality (Plotkin et al., 2011). C. Clinical presentation. 2. Ocular manifestations. a. Cataracts: in approximately 30% of patients. b. Pigmentary retinopathy. c. Microphthalmos. d. Glaucoma. 3. Other common manifestations include severe hearing loss, intrauterine growth restriction, hepatomegaly, thrombocytopenia, and cardiac anomalies (see also Chapter 32). D. Nursing care. 2. See E. Nursing Care, under Cataracts. E. Outcome. 1. Prognosis: depends on severity of symptoms and number of organ systems involved. 2. Mortality rate: in first year of life may approach 80% when multisystem involvement occurs. 3. Multiple disabilities: common in surviving infants. 4. Consequences of congenital rubella: may not be evident at birth but may become apparent in subsequent months. 1. CMV can cause a perinatal viral infection. 2. Congenital illness is most severe if infection occurs early in pregnancy, the period of greatest susceptibility of the developing fetus. Congenital infections can result from either a primary or recurrent maternal infection. B. Etiology. 1. Ubiquitous virus: CMV can cause infection in all age groups. 2. Route of transmission: Infection may be acquired transplacentally, during birth (via the cervix), or through breast milk. In seropositive mothers, the risk of transmission through breast milk is 30% to 59%. 3. Transfusion: An important possible cause of morbidity in premature infants is transfusion-acquired CMV. All premature infants should receive seronegative blood products. C. Incidence. 1. The most common congenital viral infection, affecting 1% to 2% of all newborns in the United States (Britt, 2011). 2. In the presence of primary acute maternal infection, 30% to 40% of fetuses are affected (Anderson and Gonik, 2011). D. Clinical presentation. 2. Laboratory diagnostic methods (e.g., isolation of the virus from the urine) must be used if this condition is suspected. 3. Chorioretinitis is present in 10% to 15% of infants with symptomatic CMV (Britt, 2011). 4. Other eye abnormalities include conjunctivitis, microphthalmos, strabismus, cataracts, and optic atrophy. 5. Other manifestations include intrauterine growth restriction, microcephaly, hepatosplenomegaly, jaundice, and bleeding disorders (see also Chapter 32). E. Nursing care. 1. Limited research suggests some benefit from IV ganciclovir or oral valganciclovir. 2. Use of gowns and good handwashing technique are essential to prevent the spread of infection. 3. Seronegative pregnant women should not care for infants with known or suspected infection. 4. These infants require long-term follow-up. F. Complications. 1. Cytomegalic inclusion disease. 2. Sensorineural hearing loss, the most important late sequela, and the most common cause of congenital hearing loss (Britt, 2011). 3. Chorioretinitis or optic atrophy. G. Outcomes. 1. Mortality rate: Overall mortality rate for symptomatic congenital infection is up to 30% (Freij and Sever, 2005). 2. In 10% to 15% of infants who are free of symptoms at birth, neurologic sequelae, such as microcephaly, neurodevelopmental delay, or sensorineural deafness, may develop in the first 2 years of life. B. Etiology. 2. Infection is acquired through contact with the excrement of infected cats and ingestion of improperly cooked meat. C. Incidence. 1. The incidence of maternal infection varies considerably according to geographic location. Estimates in the U.S. population suggest a seroconversion rate of 1 in 1000 during pregnancy (Anderson and Gonik, 2011). 2. The risk of transmission for an infection acquired during pregnancy is 20% to 50% (Anderson and Gonik, 2011). D. Clinical presentation. 2. Eighty percent to 90% of infected infants are symptomatic at birth (Anderson and Gonik, 2011). 3. Chorioretinitis is the most common manifestation. Toxoplasmosis infection is the most common cause of chorioretinitis in the United States (Remington et al., 2011). 4. Other manifestations include hydrocephalus, intracranial calcifications, hepatosplenomegaly, jaundice, and bleeding disorders (see also Chapter 32). E. Specific nursing care. 2. Give supportive care to the family, with sensitivity to feelings of guilt they might have. 3. Teach parents to recognize the signs of sequelae, including visual impairment in infancy (e.g., failure to fix and focus on objects or faces). F. Outcome. 2. Eighty percent of treated infants with moderate to severe disease have normal psychomotor development. 3. Recurrent eye lesions develop in 10% of treated infants with mild disease and in up to 30% of treated infants with moderate or severe disease. Formally referred to as retrolental fibroplasia, retinopathy of prematurity (ROP) is a vasoproliferative retinopathy that occurs primarily in low birth weight infants (Fierson, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists, 2013). 2. Nasal periphery is vascularized by about 32 weeks of gestation, but the process is not complete in the more distant temporal periphery until 40 to 44 weeks. Most cases of ROP begin at 31 to 32 weeks of gestation. 3. After premature birth, this process of normal vasculogenesis may be arrested as a result of injury from some noxious agent(s) or stressor(s). 4. Vasoproliferation: This arrest of normal vasculogenesis is later followed by a phase of rapid, excessive, irregular vascular growth and shunt formation (vasoproliferation), stimulated by vascular endothelial growth factor (VEGF) and interleukin growth factor. 5. Area of new growth generally forms an abrupt ridge between the vascular and avascular retina, particularly in the temporal periphery. 6. ROP may resolve if the vasculature in the area recovers and resumes advancing normally, allowing the retina to become completely vascularized. 7. If the new vasculature proceeds to develop abnormally, these capillaries may extend into the vitreous body and/or over the surface of the retina (where they do not belong). Leakage of fluid or hemorrhage from these weak, aberrant blood vessels may occur. 8. Blood and fluid leakage into various parts of the eye can result in scar formation and traction on the retina. 9. Traction may pull the macula out of its normal position, thus affecting visual acuity. If the macula is slightly out of position, vision will be mildly affected. 10. Tractional exudative retinal detachment results in blindness. B. Etiology. 1. Complex multifactorial disorder. 2. Possible risk factors (Gardner et al., 2011). a. Prematurity/low birth weight: most important clinical factor associated with ROP. b. Supplemental oxygen, hypoxia, and hyperoxia. c. Hyper-/hypocapnia. d. Growth hormone deficiency. e. Ventilator support. f. Surfactant therapy. g. Apnea/bradycardia. h. Asphyxia/acidosis/shock. i. Blood transfusions. j. Steroid exposure. k. Sepsis. l. Patent ductus arteriosus. m. Intraventricular hemorrhage/seizures. n. Nutritional deficiencies such as vitamin E and vitamin A. o. Hyperglycemia. p. Prenatal complications: maternal hypertension, diabetes, bleeding, smoking. q. Ethnicity—ROP is more common and more severe in white infants than in African American infants. r. Exposure to bright light. s. Elevated bilirubin and exposure to phototherapy. C. Incidence. 1. Incidence of ROP appears to increase significantly as birth weight and gestational age decrease. Up to 67% of neonates weighing less than 1251 g will develop ROP; up to 37% of these infants will progress to vision-threatening disease (Hartnett and Penn, 2012). D. Stages of retinopathy. 1. Standardized approach for describing ROP, developed by the International Committee for the Classification of Retinopathy of Prematurity (2005) according to five stages. b. Stage 2: ridge or elevation at the junction of the avascular and vascularized regions of the retina. c. Stage 3: ridge with extraretinal fibrovascular proliferation, either (1) Continuous with the posterior edge of the ridge, (2) Posterior but disconnected from the ridge, or (3) Into the vitreous. d. Stage 4: subtotal retinal detachment. (2) Involving the foveae. e. Stage 5: total retinal detachment. 2. Aggressive posterior ROP: significant dilation and tortuosity of posterior pole vessels, present in zone I or II. 3. “Plus” disease: an indicator of activity. Signs (in increasing order of severity) include the following: a. Engorgement and tortuosity of vessels of the posterior pole in two or more quadrants. b. Iris vessel engorgement. c. Pupil rigidity. d. Vitreous haze. 4. Pre–plus disease: abnormal vessels, dilation and tortuosity in two or more quadrants not yet sufficient for a diagnosis of plus disease. 5. Zones for classification of ROP (Fig. 37-2). a. Zone I: extends from the optic disc to twice the disc–foveal distance—a radius of 30 degrees. b. Zone II: extends from the periphery of the nasal retina (ora serrata) in a circle around the anatomic equator. c. Zone III: anterior to zone II; present temporally, inferiorly, and superiorly but not in the nasal retina.
Ophthalmologic and Auditory Disorders
ANATOMY OF THE EYE (FIG. 37-1)
Protective Structures
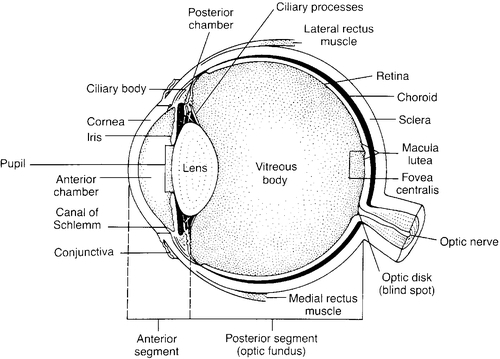
The Eyeball
Extraocular Muscles
PATIENT ASSESSMENT
History
Examination (de Alba Campomanes et al., 2012; Johnson, 2009)
PATHOLOGIC CONDITIONS AND MANAGEMENT
Birth Trauma
Conjunctivitis
Etiology
Neisseria gonorrhoeae
Chlamydia trachomatis
Nasolacrimal Duct Obstruction
Cataracts
Retinoblastoma (de Alba Campomanes et al., 2012)
Congenital Infections
Congenital Rubella Syndrome
Cytomegalovirus
Toxoplasmosis (Remington et al., 2011)
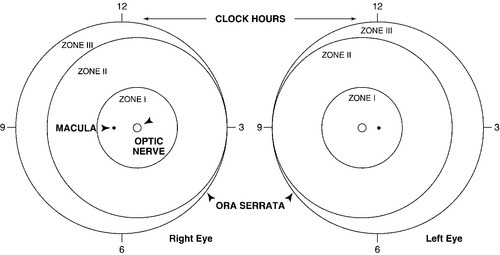
Retinopathy of Prematurity

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


37: Ophthalmologic and Auditory Disorders
FIGURE 37-1 ■ Cross-section of eyeball. (From Boyd-Monk, H.: The structure and function of the eye and its adnexa. Journal of Ophthalmic Nursing and Technology, 6[5]:176-183, 1987.)
FIGURE 37-2 ■ Zones in retinopathy of prematurity. (From George, D.S.: The latest on retinopathy of prematurity. MCN: American Journal of Maternal Child Nursing, 13[4]:254-258, 1988.)
