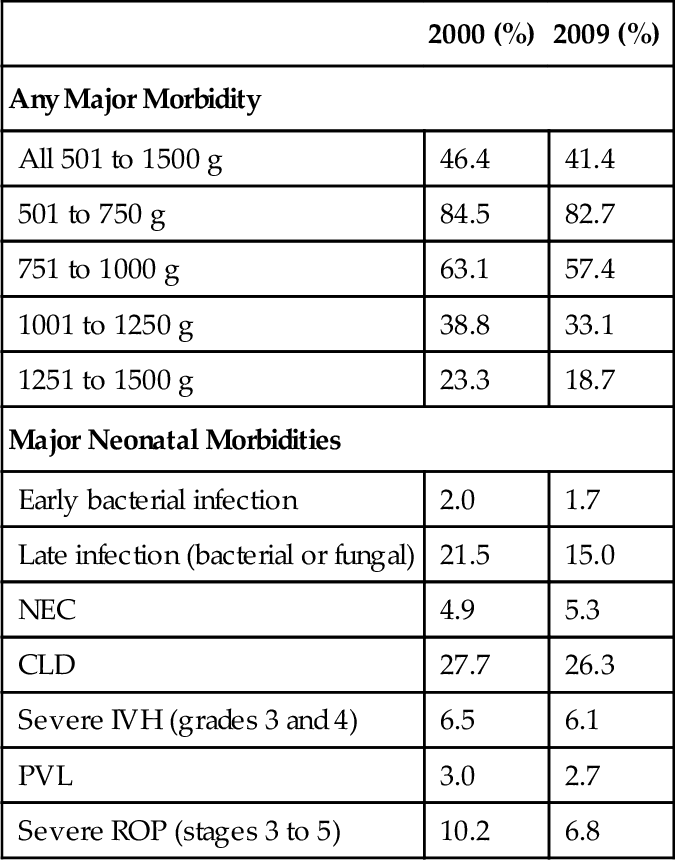
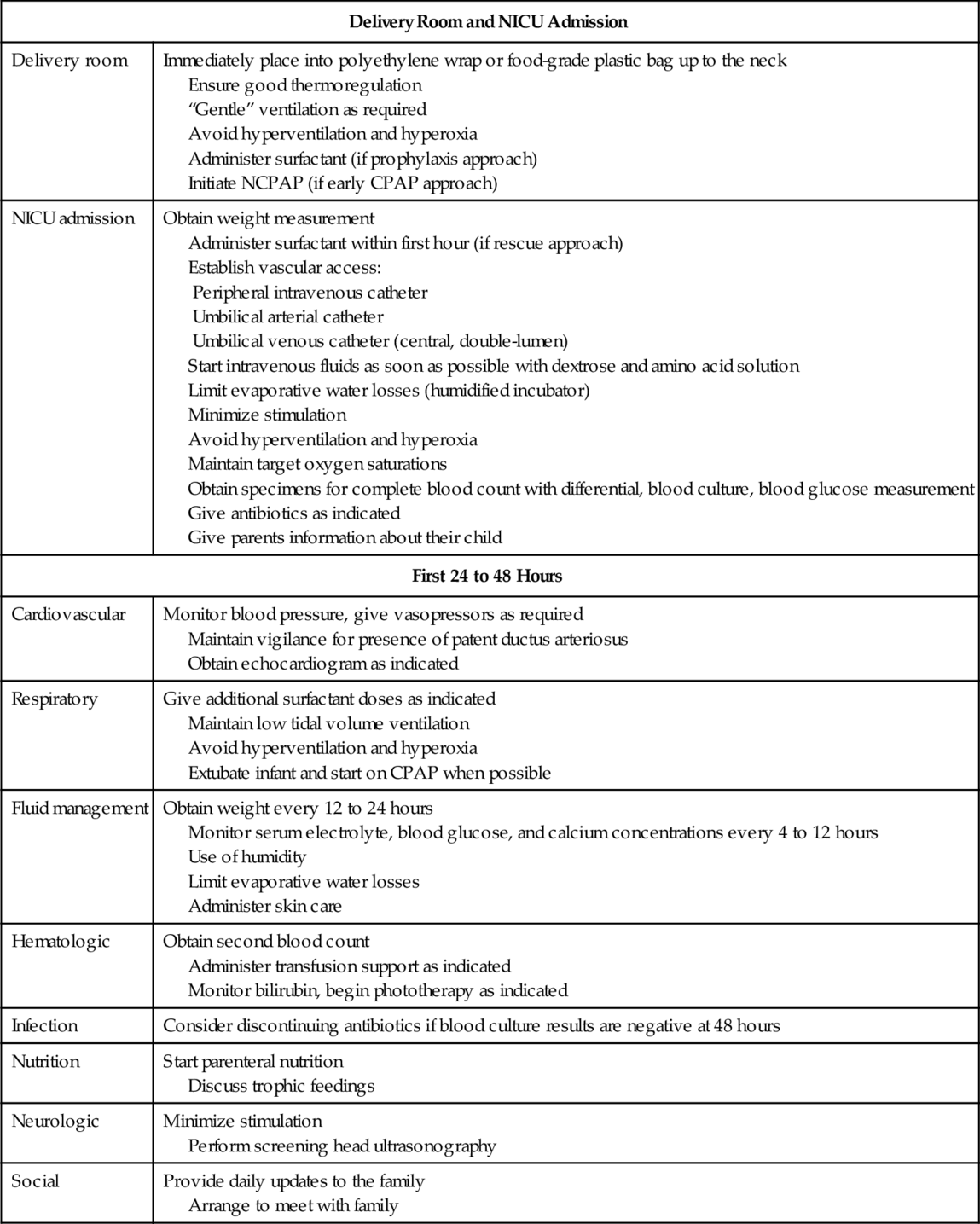
CHAPTER 22 1. Discuss the mortality and morbidity associated with extreme prematurity. 2. Identify principles of nursing care specific to the ELBW infant population and their families. Care of premature infants with birth weights between 1000 and 1500 g has become almost routine in most neonatal intensive care units (NICUs) in the United States. The most recent challenge in neonatology is the care of ELBW infants (birth weight < 1000 g). Although they represent a small percentage of overall births and NICU admissions, ELBW infants are often the most critically ill and at the highest risk for mortality and long-term morbidity of any NICU patient. They remain hospitalized for long periods of time, suffer the most acute and long-term complications of neonatal intensive care, and consume a disproportionate number of hospital days. The purpose of this chapter is to provide an overview of topics related to care of the ELBW infant. Detailed information on resuscitation, thermoregulation, ventilation, and fluids and electrolytes can be found in those specific chapters. B. Preterm–related causes of death together accounted for 35% of all infant deaths in 2008, more than any other single cause. Preterm birth is also a leading cause of long-term neurologic disabilities in children. C. Preterm birth costs the U.S. health care system more than $26 billion each year (CDC, 2013). Women who are at increased risk of having a premature baby include African American women, women younger than 17, and women older than 35, as well as women who have a low income. D. Births at the threshold of viability, early preterm birth, or birth of an ELBW infant—especially those weighing less than 750 g or less than 26 weeks of gestation, pose a variety of complex medical, social, and ethical considerations. E. The effect of such births on the infants, their families, the health care system, and society are profound. Although the prevalence of such births is less than 1%, they account for nearly one half of all cases of perinatal mortality (American Academy of Pediatrics [AAP] and American College of Obstetricians and Gynecologists [ACOG], 2012). B. In 2009, nearly half of all infants 501 to 1500 g and 89% of those weighing 501 to 750 g either died or survived after experiencing ≥ 1 major morbidity during their initial hospital stay, highlighting the continuing challenges facing these vulnerable patients, their families, and the health professionals who care for them. C. There has been a dramatic increase in the number of multiple births in the United States over the past two decades, largely due to artificial reproductive technology and fertility–inducing drugs. D. Perinatal risks that may be associated with assisted reproductive therapy include high–order multiple pregnancy, prematurity, low birth weight, small for gestational age, perinatal mortality, cesarean delivery, placenta previa, placental abruption, preeclampsia, and birth defects. E. Recent studies have shown that triplet or higher–order births are associated with an increased risk of death or neurodevelopmental impairment at 18 to 22 months‘ corrected age when compared with ELBW singleton infants (Wadhawan et al., 2011). Table 22-1 details the rates for mortality and morbidity for 2000 and 2009 (Horbar et al., 2012). B. Because ELBW and/or very preterm infants are at increased risk of predischarge mortality, they should be delivered at a level III facility unless this is precluded by the mother’s medical condition or geographic constraints (Committee on Fetus and Newborn, 2012). C. Antepartum transport avoids separation of mother and infant in the immediate postpartum period, allows mothers to communicate directly with NICU health care providers, and supports the goal of family–centered health care. B. Whenever possible, data specific to the age, weight, and gender of the fetus should be used to aid management decisions made by obstetricians and parents of fetuses at risk of preterm delivery before 26 completed weeks of gestation. This information may be developed by each institution and should indicate the population used in determining estimates of survivability. C. The Eunice Kennedy Shriver National Institute for Child Health and Development (NICHD) Neonatal Research Network‘s Extremely Preterm Birth Outcome Data website provides a calculator to estimate infant outcomes based on birth weight, gestational age, gender, and antenatal corticosteroid administration. The calculator is available at http://www.nichd.nih.gov/about/org/der/branches/ppb/programs/epbo/pages/epbo_case.aspx. D. If the physicians involved believe that there is no chance of survival, resuscitation is not indicated and should not be initiated. If the physicians consider a good outcome to be very unlikely, then parents should be given the choice of whether resuscitation should be initiated and physicians should respect their preference. E. When the physicians’ judgment is that a good outcome is reasonably likely, physicians should initiate resuscitation and, together with the parents, continually evaluate whether intensive care should be continued. Enhancement of fetal pulmonary function with the use of antenatal steroids lessens the prevalence and severity of neonatal respiratory distress syndrome. Current recommendations in the Guidelines for Perinatal Care (AAP and ACOG, 2012) include: B. A single course of antenatal corticosteroids should be administered to women with premature rupture of membranes (PROM) before 32 weeks of gestation to reduce the risks of respiratory distress syndrome, perinatal mortality, and other morbidities. C. The efficacy of corticosteroid use at 32 to 33 completed weeks of gestation for preterm PROM is unclear, but treatment may be beneficial, particularly if pulmonary immaturity is documented. Sparse data exist on the efficacy of corticosteroid use before fetal age of viability, and such use is not recommended. D. A single rescue course of antenatal corticosteroids may be considered if the antecedent treatment was given more than 2 weeks prior, the gestational age is less than 326/7 weeks, and the woman is judged by the physician to be likely to give birth within the next week. However, regularly scheduled repeat courses or multiple courses (more than two) are not recommended. B. The single most important clinical benefit for preterm infants is the possibility for a nearly 50% reduction of intraventricular hemorrhage (IVH). It is important to note that the timing of umbilical cord clamping should not be altered for the purpose of collecting umbilical cord blood for banking. A. Careful adherence to details in the delivery room and during the first few hours after birth is essential to help avoid immediate and long–term complications of the ELBW infant. NICUs should have a consistent approach to the initial care of the ELBW infant in the delivery room and upon admission to the NICU (Table 22-2). B. All neonatal resuscitations and delivery room management should follow the guidelines found in the Textbook of Neonatal Resuscitation, 6th edition (American Academy of Pediatrics and American Heart Association 2011). Also please see Chapter 5. B. The infant’s temperature must be monitored closely because overheating has been described when plastic wrap is used in combination with an exothermic mattress. The goal should be an axillary temperature of approximately 36.5° C (97.7° F). A. Administration of supplemental oxygen in the delivery room. 2. Because many babies born at less than 32 weeks of gestation will not reach target saturations when resuscitated with air, blended oxygen may be used. If blended oxygen is not available, resuscitation should be initiated with air. 3. The use of room air for resuscitation of these infants has been proposed to protect from hyperoxia and damage to the lungs by oxygen free radicals. Many NICUs have developed their own policies regarding starting values of blended oxygen in the delivery room (Eichenwald, 2012). B. Assisted ventilation in the delivery room. 1. If positive pressure ventilation is required, it should be provided with low inspiratory pressure to prevent overdistention of the lungs, which can result in air leak and other lung injury. Adequate positive end-expiratory pressure (PEEP) will help to maintain lung volume (Eichenwald, 2012). C. Surfactant administration in the delivery room. 1. There continues to be uncertainty as to whether to give surfactant in the first few minutes of life to an ELBW newborn, apply nasal continuous positive airway pressure (NCPAP) without surfactant, or intubate giving surfactant and then extubate to CPAP. The potential benefits and risks of these strategies are still under study (Goldsmith and Karotkin, 2011). 2. No matter the timing of the surfactant administration, it is only given after endotracheal tube position is confirmed (AAP and ACOG, 2012). 3. Novel methods of surfactant administration are currently being studied. Klebermass-Schrehof et al. (2013) have described a new mode of surfactant administration without intubation, known as less invasive surfactant administration. CPAP is administered by a nasopharyngeal tube after delivery. At 20 to 30 minutes after birth, a thin catheter (1.3 mm diameter) is inserted into the trachea. Surfactant is then administered over 2 to 5 minutes via this catheter while the infant is breathing spontaneously. About 65% of the extremely premature infants (23 to 25 weeks postmenstrual age) could be managed without mechanical ventilation (MV) during the first week of life, and 41% without any MV during the entire hospital stay. The authors found significantly higher survival rates (especially for the most immature infants), and less IVH, cystic periventricular leukomalacia (PVL), need for supplementary oxygen at day 28, and duration of MV compared to historical controls. 2. Because of the high transepidermal fluid losses in these infants, intravenous (IV) fluids containing 5% to 10% dextrose should be started as quickly as possible after admission and efforts should be made to reduce evaporative water losses by increasing the relative humidity surrounding the infant. B. Ambient humidity. 2. 70% or higher humidity in the first week of life is needed to affect a difference in TEWL. C. Oxygen saturation range. 1. The optimal range for oxygen saturation continues to be controversial. 2. The SUPPORT trial found that the risk of death during the initial hospitalization was increased among neonates randomly assigned to the lower-oxygen-saturation (85% to 89%) group as compared with those assigned to the higher-oxygen-saturation (91% to 95%) group. The authors state that lower oxygen saturation targets cannot be recommended in these extremely preterm infants (Vaucher et al., 2012). 3. In general, oxygen is given for saturations less than 85% and weaned for saturations greater than 95%. A. Umbilical arterial catheter (UAC). 1. Close monitoring of blood pressure, arterial blood gases, and serum chemistries during the first few days after birth is necessary for high risk ELBW infants. Insertion of a UAC allows for reliable arterial access in critically ill infants. For infants greater than 1200 g, a 5 F catheter should be used. Infants less than 1200 g require a 3.5 F catheter (MacDonald and Ramasethu, 2007). B. Umbilical venous catheter (UVC). C. Percutaneous central venous catheter. A. Gestational age is strongly linked to epidermal barrier function. Poor epidermal barrier function in the extremely preterm infant leads to disturbances in temperature regulation and water balance. The skin barrier of premature infants is injured easily and can serve as a portal of entry for agents, causing serious bacterial infections (Telofski et al., 2012). B. Because of these risks, preservation of skin integrity should be incorporated into the care of the extremely preterm infant. Objective tools such as the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) Neonatal Skin Condition Score Tool can facilitate assessment of neonatal skin conditions (Lund et al., 2013). C. Limited use of adhesives and extreme care upon their removal, frequent repositioning of the infant to avoid pressure points on the skin, and use of soft bedding or a water mattress are the minimum requirements (Eichenwald, 2012). D. Assessment of the overall skin condition involves evaluating all skin surfaces, head–to–toe, daily or more frequently. Risk factors for skin injury include less than 32 weeks of gestation, edema, and adhesives applied to the skin to secure tubes, lines, and monitoring equipment. E. Turning the infant a minimum of every 4 hours is necessary, along with careful inspection of skin surfaces. Even when turning side-to-side is not feasible, lifting the head, shoulders, and hips and supporting these areas with pressure-reducing surfaces is helpful (Harris et al., 2003; Lund, 2011; Telofski et al., 2012). A. Noninvasive respiratory support (NRS). 2. A variety of CDP devices available today allow breaths to be delivered above the baseline CPAP pressure and to be synchronized or nonsynchronized to the infant’s own breaths. The goal of such devices is to enhance CO2 removal and stimulate breathing. Various CDP devices provide an adjunct to weaning infants off MV after they have been extubated and also help manage apnea of prematurity (Wiswell and Courtney, 2011). 3. Marked bowel distention (“CPAP belly”) is frequently seen. The air from the CPAP easily passes into the esophagus and is swallowed. Increased abdominal girth and visibly dilated intestinal loops may be seen. An increased risk of gastric perforation has been reported. An orogastric tube should always be placed whenever CPAP is used (Wiswell and Courtney, 2011). B. Mechanical ventilation. 2. A variety of devices are now available for use: synchronized intermittent mandatory ventilation (SIMV), assist–control ventilation, volume-controlled ventilation, high-frequency jet ventilation (HFJV), and high-frequency oscillatory ventilation (HFOV). C. Methylxanthines. 2. The Caffeine for Apnea of Prematurity Trial Group (Schmidt et al., 2007) reported on the short- and long-term effects of caffeine at 18 to 21 months in infants with very low birth weight. The study found a decreased rate of BPD, decreased death, less cerebral palsy, and decreased incidence of severe ROP in the treatment group. B. All infants have a physiologic weight loss that is a reduction in extracellular water. In very premature infants, the loss of extracellular fluid can be 15%. C. Fluid requirements. 2. Maintenance fluids required during the first week of life are displayed in Table 22-3. Fluids should be adjusted based upon the infant’s clinical condition and any factors that may alter the fluid requirements. TABLE 22-3 Maintenance Fluid Requirements During the First Week of Life
Care of the Extremely Low Birth Weight (ELBW) Infant
OVERVIEW
EPIDEMIOLOGY (CENTERS FOR DISEASE CONTROL AND PREVENTION [CDC], 2013; MARCH OF DIMES, 2013)
MORTALITY AND MORBIDITY (AAP AND ACOG, 2012)
PERINATAL MANAGEMENT (AAP AND ACOG, 2012)
PERINATAL CONSULTATION (AAP AND ACOG, 2012)
ANTENATAL STEROIDS
TIMING OF UMBILICAL CORD CLAMPING AFTER BIRTH (AAP, 2013)
DELIVERY ROOM CARE SPECIFIC TO ELBW INFANTS
THERMOREGULATION (SEE CHAPTER 6) (AAP AND ACOG, 2012)
VENTILATORY PRACTICES IN THE DELIVERY ROOM (AAP AND ACOG, 2012)
ADMISSION TO THE NEONATAL INTENSIVE CARE UNIT
VASCULAR ACCESS (SEE CHAPTER 15)
SKIN CARE (SEE CHAPTER 36)
ASSISTED VENTILATION (SEE CHAPTER 26)
NUTRITIONAL MANAGEMENT (SEE CHAPTERS 8 AND 10) (DELL, 2011)
Birth Weight (g)
Dextrose (g/100 mL)
Day 1 to 2 (mL/kg/day)
Day 3 to 7 (mL/kg/day)
< 750
5 to 10
100 to 200
120 to 200
750 to 1000
10
80 to 150
100 to 150
1001 to 1500
10
60 to 100
80 to 150
> 1500
10
60 to 80
100 to 150 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nurse Key
Fastest Nurse Insight Engine
Get Clinical Tree app for offline access