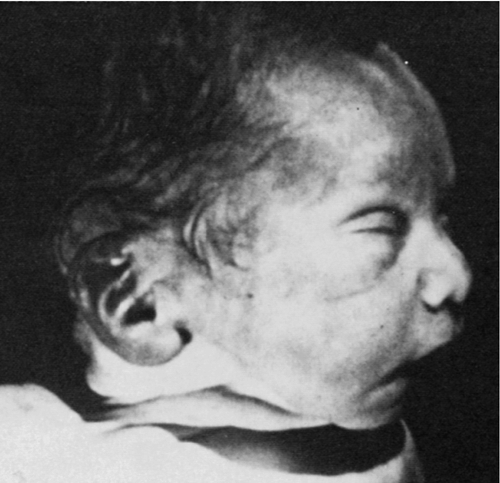
CHAPTER 33 1. Relate congenital renal/genitourinary disorders to embryologic development. 2. Apply knowledge of normal renal anatomy and physiology to renal pathophysiology that presents in the neonatal period. 3. Explain the etiology of selected neonatal renal/genitourinary disorders. 4. Describe clinical manifestations and complications that may be associated with selected neonatal renal/genitourinary disorders. 5. Determine the appropriate management of each disorder discussed. 6. Formulate an appropriate plan of care for each disorder discussed. Homeostasis of the newborn is dependent on a functional renal system. In utero, the placenta is the organ responsible for fluid and electrolyte homeostasis. After birth, the kidney must assume its role as the regulator. An understanding of basic principles in renal developmental physiology is essential for successful clinical management of sick preterm and term neonates. This chapter presents information on the anatomy and physiology of the kidney as a base from which to discuss selected renal/genitourinary disorders. Fetal renal function is closely linked to both the developmental stage of the kidney and the intrauterine or extrauterine environment of the fetus. Maternal exposure to a variety of drugs not only may alter function of the fetal kidney but also may cause permanent impairment of renal development and function. The Barker hypothesis, as well as the theory of the developmental origins of disease, contend that adverse events in utero induce compensatory responses in the fetus, such as altered kidney development. These changes in organ structure may persist permanently and have long-term consequences for renal functions, such as the future development of hypertension. These considerations underscore the lifetime importance of optimal prenatal and neonatal care. 2. Nephrogenesis is the formation of new nephron units. 3. Embryologic development of the urinary system begins within the first weeks after conception and progresses through three stages. 4. Both the urinary and the genital systems develop from the same germ layer of the embryo. B. Pronephros. 1. Pronephros is a primitive and nonfunctional kidney that appears early in the fourth week of gestation, then disappears by the fifth week (Moore et al., 2013). 2. Pronephroi degenerate but the ducts and tubes persist, which are then used by the mesonephroi (Moore et al., 2013). C. Mesonephros. 1. This intermediate kidney appears from 5 to 12 weeks’ gestation and consists of glomeruli and tubules (Moore et al., 2013). 2. The pronephric tube extends into the cell lining of the mesonephros to form the wolffian duct. An outbranch of the Wolffian duct called the ureteric bud aids in the development of the metanephros (Guignard and Sulyok, 2012). 3. The mesonephroi function as the intermediate kidney, then undergo involution through apoptosis (programmed cell death) in preparation for the next stage of development (Guignard and Sulyok, 2012). D. Metanephros. 1. Differentiation of the metanephros—a primitive form of the permanent kidney—starts around 5 weeks’ gestation, and the first nephrons are formed by week 8 (Moore et al., 2013). 2. This primitive kidney is functional at around the ninth week of gestation and begins the formation of urine (Moore et al., 2013). 3. Interactions between the ureteric bud and the metanephric mesenchyme result in the formation of both the collecting duct system and the nephrons of the permanent kidney. The stalk of the ureteric bud becomes the ureter and the cranial part of the bud branches to form the collecting tubules. This branching morphogenesis leads to the formation of the renal collecting system, with the first generations of the collecting tubules enlarging to form the major calices while subsequent generations form the minor calices (Guignard and Sulyok, 2012; Moore et al., 2013). 4. Nephrogenesis is complete at 34 weeks of gestation, when each kidney contains its definitive complement of approximately 800,000 to 1.2 million nephrons (Vogt and Dell, 2011). E. Positional changes of the fetal kidney (Moore et al., 2013). 2. Early in development, the hilum of the kidney (area where the veins, arteries, and ureters enter the kidney) faces ventrally (toward the abdomen). As the kidneys ascend upward, they also rotate medially, and by the ninth week of gestation the hilum is directed anteromedially. Eventually the kidneys are located on the posterior abdominal wall. 3. The kidneys become fixed once they come into contact with the suprarenal (adrenal) glands in the ninth week of gestation. F. Changes in blood supply of the fetal kidney (Moore et al., 2013). 2. The permanent renal arteries develop from branches of the abdominal aorta. The right renal artery is longer and often more superior. A. The bladder and urethra are formed during the second and third months of gestation. 2. Caudal portions of the ureters, which originate from the mesonephric ducts, enter the bladder. 3. During these processes, the ureteral orifices move cranially and the mesonephric ducts move closer together to enter the prostatic urethra, forming the trigone of the bladder. A. During intrauterine life, the kidneys play only a minor role in regulating fetal salt and water balance because this function is maintained primarily by the placenta. The most important functions of the prenatal kidneys are the formation and excretion of urine to maintain an adequate amount of amniotic fluid (Chevalier and Norwood, 2011). B. Renal blood flow is low during fetal life, only 2% to 4% of the total cardiac output. This proportion increases after birth, from a value of 5% in the first 12 hours of life to 10% at the end of the first week (Guignard and Sulyok, 2012). 2. Medulla: middle section of the kidney, which contains the renal pyramids, straight portions of tubules, loops of Henle, vasa recta, and terminal collecting ducts. 3. Renal sinus and pelvis: innermost portion of the kidney. The renal sinus contains the uppermost part of the renal pelvis and calyces, surrounded by some fat in which branches of the renal vessels and nerves are embedded. 4. Ureter: excretory duct of the kidney, which transports urine from the kidney to the bladder. B. Microscopic renal anatomy: the nephron (Hall, 2011). 2. Each nephron contains a tuft of glomerular capillaries called the glomerulus, through which large amounts of fluid are filtered from the blood, and a long tubule in which the filtered fluid is converted into urine on its way to the pelvis of the kidney. 3. The glomerulus contains a network of glomerular capillaries that is encased in Bowman’s capsule. Fluid filtered from the glomerular capillaries flows into Bowman’s capsule and then into the proximal tubule, which lies in the cortex of the kidney. 4. From the proximal tubule, fluid flows into the loop of Henle, which dips into the renal medulla. Each loop consists of a descending and an ascending limb. 5. Collecting ducts empty into the renal pelvis of the kidney. Urine flows from the collecting ducts into the renal calyces, then into the ureters. Peristaltic contractions force urine down the ureters into the bladder. B. Cardiac output: The proportion of cardiac output distributed to the kidneys is 4% to 6% in the first 12 hours of life and increases to 8% to 10% in the first week of life. In comparison, 25% of cardiac output is distributed to the kidneys in the normal adult. C. Systemic vascular resistance: Systemic vascular resistance increases markedly after birth, which may cause a redistribution of blood flow to organs other than the kidneys, which may immediately contribute to the low neonatal renal blood flow. D. Contribution of structural changes: New vascular channels from nephrogenesis, as well as the continued formation of new glomeruli and vascular remodeling, may influence renal hemodynamics postnatally. E. Vasoactive factors that regulate renal blood flow: Several vasoactive agents participate in the regulation of renal blood flow in the postnatal maturing kidney: 2. Angiotensin I rapidly converts to angiotensin II predominantly in the lungs, although other tissues such as the kidneys and blood vessels contain converting enzyme and can form angiotensin II. 3. Angiotensin II is an extremely powerful vasoconstrictor but is rapidly inactivated in the blood after 1 to 2 minutes. A second effect is to decrease excretion of sodium and water, which slowly increases the extracellular fluid volume (Hall, 2011). 4. Natriuretic peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). ANP and BNP are produced in the atria and ventricles, whereas CNP is found mainly in the brain, pituitary gland, vascular endothelium, and kidneys. The primary effects of ANP are: (1) vasodilation and decrease in mean blood pressure; (2) increase in renal blood flow, glomerular filtration rate (GFR), and filtration fraction; (3) inhibition of sodium and water reabsorption in both proximal and distal tubules; and (4) decreased concentrating ability. 5. Nitric oxide synthesized endogenously is an important regulator of renal hemodynamics in the immature kidney. Nitric oxide can function as a vasodilator to counterbalance vasoconstrictors such as angiotensin II (Solhaug et al., 1996). 6. Prostaglandins, which are endogenously produced, can cause vasodilation. Renal prostaglandin production is increased during the perinatal period, and the urinary excretion of prostaglandin E is 5 times that noted at term (Peruzzi et al., 1999). F. Glomerular filtration (Vogt and Dell, 2011). 2. GFR. a. Factors that may contribute to decreased GFR at birth include: (1) Small glomerular capillary area available for filtration. (2) Structural immaturity of glomerular capillary, which is associated with decreased water permeability. (3) Decreased blood pressure. (4) Increased hematocrit. (5) Renal vasoconstriction, which results in decreased glomerular plasma flow. b. Neonates born at less than 34 weeks’ gestation have low GFR (0.5 mL/min) until nephrogenesis is completed. 3. Three primary factors determine GFR: a. Glomerular capillary hydrostatic pressure. b. Hydrostatic pressure in Bowman’s capsule. c. Capillary colloid osmotic pressure. 4. Glomerular capillary hydrostatic pressure is the major controller of GFR. 5. Additional factors that affect GFR are: b. Permeability of capillary basement membrane. c. Rate of renal plasma flow. d. Changes in renal blood flow. e. Changes in blood pressure. f. Vasoactive changes in afferent or efferent arterioles. g. Ureteral obstruction. h. Edema of kidney. i. Changes in the concentration of plasma proteins: (2) Hypoproteinemia. j. Increased permeability of the glomerular filter. k. Decrease in total area of glomerular capillary bed. G. Tubular function (Vogt and Dell, 2011). 2. Tubules modify the glomerular ultrafiltrate, leading to production of urine, which is accomplished by the process of tubular reabsorption and secretion. b. Tubular secretion is the movement of substances into the tubular epithelium from the peritubular capillary plasma. Tubular secretion is necessary for regulation of fluid and electrolyte balance, along with other renal processes. 3. Regulation of fluids and electrolytes is an important tubular function. 4. Tubular function is altered in the neonate as a result of decreased renal blood flow and GFR. 5. Tubular portions of the neonatal nephron are smaller and less functionally mature, resulting in an altered ability to transport sodium, urea, chloride, and glucose, with decrease renal thresholds for many substances. 6. Rapid maturation of proximal tubular cells occurs between 32 and 35 weeks of gestation. H. Concentration and dilution mechanism (Vogt and Dell, 2011). 2. Sites of urinary concentration and dilution: b. Collecting duct. 3. Factors responsible for the limited ability of the neonatal kidney to concentrate urine: a. Anatomic immaturity of the renal medulla. b. Decreased medullary concentration of sodium chloride and urea. c. Diminished responsiveness of the collecting ducts to arginine vasopressin. 4. Normal range of neonatal specific gravity is 1.002 to 1.010. 5. Maximum concentrating ability: a. Term neonate: 700 mOsm of water/kg. b. Preterm neonate: 600 to 700 mOsm of water/kg. 6. Capacity for urine dilution: a. Thirty to 50 mOsm of water/kg. b. Ability of neonate to excrete a hypotonic load is limited, presumably because of the low GFR. I. Renal regulation of acid–base balance (Hall, 2011). 1. The kidneys are one of the most powerful acid–base regulatory systems. b. The term alkalosis refers to excess removal of H+ from the body fluids, in contrast to the excess addition of H+, which is referred to as acidosis. c. Excreting acidic urine reduces the amount of acid in extracellular fluid, while excreting alkaline urine removes base from the extracellular fluid. 2. The kidneys regulate extracellular H+ concentration through three processes: (1) secretion of H+, (2) reabsorption of filtered bicarbonate, and (3) production of new bicarbonate. a. Glucocorticoids can accelerate the renal processes of acidification (Guignard and Sulyok, 2012). J. Renal regulation of potassium (Benchimol and Satlin, 2011). 2. The kidney is the major excretory organ for potassium. 3. Acidosis is associated with intracellular potassium release resulting in an increase in plasma potassium. Alkalosis results in a shift of potassium into cells and a consequent decrease in plasma potassium. 4. Acute metabolic acidosis causes the urine pH and potassium excretion to decrease. Acute respiratory alkalosis and metabolic alkalosis result in increases in urine pH and potassium excretion. 5. Potassium uptake into cells is stimulated by insulin, β-adrenergic agonists (e.g., albuterol and terbutaline), and alkalosis. A. Renal anomalies may be diagnosed prenatally. The kidneys may be visualized at 10 weeks’ gestation, but accurate determination of renal anatomy is usually not possible until 16 weeks (Stamilio and Morgan, 1998). B. The antenatal history should be reviewed thoroughly, with particular attention devoted to medications, toxins, and unusual environmental exposures during the pregnancy. C. A review of the medical history of the family is important, including any prior fetal or neonatal deaths. While there is no established genetic basis for many congenital renal anomalies, certain disorders such as renal hypoplasia/dysplasia, multicystic dysplastic kidney, and vesicoureteral reflux may be familial. Certain diseases such as polycystic kidney disease and congenital nephrotic syndrome do have a clear genetic basis. A. Prenatal findings that suggest renal or urinary tract disease include hydronephrosis, renal cysts, hyperechoic kidneys, renal mass, oligohydramnios, and polyhydramnios. Hydronephrosis (dilation of the renal pelvis) is the most common identified renal anomaly (Dicke et al., 2006). B. Hydronephrosis may indicate upper urinary tract obstruction (ureteropelvic junction obstruction) and lower urinary tract obstruction (posterior urethral valves and prune-belly syndrome). C. Prenatal renal cysts can be seen with autosomal dominant polycystic kidney disease, multicystic dysplastic kidneys, and cystic renal dysplasia. D. Hyperechoic kidneys can result from tubular dilations (autosomal recessive polycystic kidney disease), dysplasia, or multiple microscopic cysts (Meckel–Gruber syndrome and nephrotic syndrome). E. Oligohydramnios is not limited to renal disorders. However, it can indicate renal agenesis, renal dysplasia, and lower urinary tract obstruction. F. Polyhydramnios is generally caused by gastrointestinal anomalies but may have a renal etiology in a small percentage of cases. Renal dysplasia, nephrotic syndrome, or inherited renal tubular defects may present with polyhydramnios. G. Congenital renal tumors are rare. The most common tumor is a mesoblastic nephroma, which appears as a unilateral, single, solid mass on prenatal ultrasound. B. Hypertension in the newborn period is suggestive of renal disease and should be evaluated. Hypotension usually results from hypovolemia, hemorrhage, or sepsis, which may lead to acute kidney injury. C. Delayed voiding greater than 12 hours may be seen after stressful deliveries but nearly all neonates will produce urine within 24 hours of birth. Prolonged anuria should be evaluated. D. Palpable abdominal masses, in particular flank masses, most often originate from the urinary tract and must be evaluated. E. Edema occurs when there is an imbalance between capillary hydrostatic and interstitial oncotic forces. The major renal causes include fluid overload secondary to a decrease in GFR from acute or chronic renal injury. Low intravascular oncotic pressure from urinary protein losses (congenital nephrotic syndrome) may also cause edema. F. Ascites can arise as an imbalance between hydrostatic and oncotic pressures, but it can also occur secondary to decreased lymphatic drainage. The most common renal cause is urinary ascites from perforation of the ureter, renal pelvis, or bladder from obstruction (e.g., posterior urethral valves). Congenital nephrotic syndrome and renal vein thrombosis may also cause ascites. G. Other anomalies such as abnormal ears, microcephaly, meningomyelocele, pectus excavatum, abnormal genitalia, cryptorchidism, imperforate anus, and limb deformities may be associated with underlying renal defects. H. Oligohydramnios (Potter) sequence may be seen in infants with bilateral renal agenesis. The absence of fetal renal function results in anhydramnios, which causes fetal deformation from compression by the uterine wall. Features generally include wide-set eyes, depressed nasal bridge, beaked nose, receding chin, and posteriorly rotated, low-set ears. Other anomalies include a small, compressed chest wall and arthrogryposis. The condition is uniformly fatal. “Potter-like” features may be seen in infants with significant renal impairment and oligohydramnios. Such patients often have pulmonary hypoplasia and require careful respiratory management (Vogt and Dell, 2011) (Fig. 33-1).
Renal and Genitourinary Disorders
OVERVIEW
FETAL DEVELOPMENT OF THE KIDNEY
DEVELOPMENT OF THE BLADDER AND URETHRA (VOGT AND DELL, 2011)
RENAL FUNCTION
RENAL ANATOMY
REGULATION OF POSTNATAL RENAL HEMODYNAMICS (SOLHAUG AND JOSE, 2011)
CLINICAL EVALUATION OF RENAL AND URINARY TRACT DISEASE (VOGT AND DELL [2011])
Prenatal Diagnosis of Renal Disorders (Bates and Schwaderer, 2012)
Postnatal Evaluation of Renal and Urinary Tract Disease (Bates and Schwaderer, 2012)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


33: Renal and Genitourinary Disorders
FIGURE 33-1 ■ Potter facies. Note epicanthal folds, hypertelorism, low-set ears, crease below lower lip, and receding chin. (From Martin, R.J., Fanaroff, A.A., and Walsh, M.C.: Fanaroff and Martin’s neonatal-perinatal medicine: Diseases of the fetus and infant [9th ed.]. St. Louis, 2011, Elsevier Mosby.)
