CHAPTER 32 Diana J. Wilson; Carol Ingals Tyner 1. Differentiate the methods of acquisition of neonatal infection. 2. Identify risk factors for infection associated with term and preterm infants. 3. Describe clinical signs and symptoms of infection and septic shock. 4. Calculate the absolute neutrophil count and immature/total neutrophil ratio from a complete blood cell count and differential cell count. 5. Identify the common gram-positive and gram-negative organisms responsible for bacterial infections in the neonatal period. 6. Name common broad-spectrum antimicrobial agents used to treat neonatal sepsis and discuss indications for and risks of their use. 7. Differentiate between mucocutaneous, systemic, and cutaneous candidiasis. 8. Identify clinical symptoms and therapies associated with ToRCHES CLAP spectrum infections. 9. List prevention strategies for hospital-acquired infections. The immature immune system of a neonate is characterized by immature activation and function of immune responses that make newborns more susceptible to infections with a frequency and an intensity greater than at any other period of life. In the first month of life, the risk of sepsis is the highest and remains the major cause of death. Up to 40% of the survivors will have some neurologic sequelae. Greater than 1 million newborns globally die each year from sepsis. In the United States, as many as 1 to 5 infants per 1000 live births will have early-onset sepsis (EOS) and as many as 250 infants per 1000 live births will acquire late-onset sepsis (LOS) (Weston et al., 2011; Wynn and Wong, 2010). The incidence of septicemia increases substantially in very low birth weight (VLBW) and extremely low birth weight (ELBW) premature infants and results in 50% to 61% mortality. Neonatal sepsis is a general term used to define actual or potential infection in the neonatal and newborn period and is defined on a continuum. The diagnosis of sepsis remains one of the most difficult diagnostic tasks. Early detection and implementation of therapy are critical. Failure to identify early signs of sepsis contributes to morbidity, mortality, and increased health care costs. This chapter provides a comprehensive review of common neonatal infections, treatment modalities, and the latest recommendations for reducing hospital-acquired infections. A. Vertical transmission: mother to infant. 2. Intrapartum acquisition after rupture of membranes: organism may ascend through genital tract with prolonged rupture of membranes. Infection may also result from aspiration of infected amniotic fluid or occur by colonization during passage of the fetus through the birth canal. Infection has also been demonstrated to occur through skin abrasions such as scalp electrodes during labor. 3. Postpartum acquisition: transmission through maternal breast milk to infant of several viruses, including HIV, hepatitis B virus, and cytomegalovirus. B. Horizontal transmission: transmission to the infant from nursery personnel, family members and visitors, contaminated hospital equipment, invasive procedures and devices, and blood products; also known as a nosocomial infection or hospital-acquired infection (HAI). 2. Intrapartum risk factors include: prolonged rupture of membranes (> 12 to 18 hours), vaginal GBS colonization, chorioamnionitis, uterine tenderness, purulent amniotic fluid, foul-smelling amniotic fluid, maternal fever greater than 38 ° C/101 ° F, prolonged or difficult labor, premature labor, maternal UTI, invasive intrapartum procedures (i.e., internal fetal monitoring), elevated maternal heart rate (> 100 beats per minute [bpm]), and elevated fetal heart rate (> 180 bpm). B. Neonatal. C. Environmental. B. Specific manifestations of sepsis by systems: 1. Thermoregulatory instability: temperature instability, fever, and hypothermia. 2. Neurologic: lethargy, jitteriness, irritability, seizures, hypotonia or hypertonia, bulging fontanelles, high-pitched or abnormal cry. 3. Respiratory distress (most common clinical sign): tachypnea, grunting, nasal flaring, retractions, cyanosis, apnea, respiratory acidosis, respiratory insufficiency, and radiologic evidence of pneumonia or pleural effusion. 4. Cardiovascular: tachycardia or bradycardia; arrhythmias; hypotension or hypertension; cold, clammy, or mottled skin; decreased peripheral perfusion or vasoconstriction; poor peripheral pulses; delayed capillary refill (> 3 seconds); cardiomegaly; and poor cardiac function. 5. Gastrointestinal: poor feeding, vomiting, diarrhea, abdominal distention, feeding intolerance, hypoactive bowel sounds, and radiologic evidence of ileus, portal venous gas, pneumatosis, or free air. 6. Integumentary: rash, pustules or vesicles, jaundice, pallor, and petechiae. 7. Internal organ: hepatomegaly and splenomegaly. 8. Metabolic: hyperglycemia, hypoglycemia, or glucose instability, and metabolic acidosis. C. Septic/distributive shock: profound sepsis resulting in insufficient perfusion, oxygenation, and delivery of nutrients to satisfy tissue requirements, resulting in cellular dysfunction and ultimately cell destruction. Rapid identification and treatment is paramount to preventing impending death (Agrawal, 2012) (see also Chapter 28). 1. Clinical presentation of septic shock: a. Tachycardia or bradycardia. b. Increased work of breathing, tachypnea, retractions, apnea, progressing to respiratory insufficiency and failure. c. Persistent pulmonary hypertension with resulting decreased oxygenation. d. Poor perfusion with pallor, mottling, delayed capillary refill (> 3 seconds), weak pulses, cool extremities, wide pulse pressures. e. Hypotension, decreased urinary output, organ dysfunction, ileus. f. Metabolic acidosis and other electrolyte disorders. g. Coagulopathy, oozing or bleeding, may progress to disseminated intravascular coagulation. h. Edema from capillary leak. i. Myocardial dysfunction, arrhythmias, impending cardiac arrest. A. Complete blood cell count (CBC). 1. White blood cell (WBC) count: interpretation is often difficult because of the wide range of normal values in the neonate (5000 to 30,000 cells/mm3) (Oski and Naiman, 1966). WBC count is an unreliable indicator of infection as leukocyte counts are normal in greater than 30% of infants with proven bacteremia, and leukopenia (< 5000 cells/mm3) or leukocytosis (> 20,000 cells/mm3) may be present when greater than 50% of culture results are negative (Weinberg and D’Angio, 2011). This suggests leukocytosis can be a normal finding in the newborn infant. Leukopenia less than 1750 cells/mm3 is generally considered an abnormal finding and may be due to sepsis, but may also be associated with maternal hypertension, asphyxia, or hemolytic disease. 2. Differential cell count (Fig. 32-1). (1) Absolute neutrophil count (ANC) is calculated as: (a) Manroe and colleagues (1979) developed a reference range for the ANC in term infants (Fig. 32-2). (2) Neutropenia: less than 1800 cells/mm3 (Manroe et al., 1979). Affects 6% to 8% of all NICU patients, or as many as 48,000 infants in the United States annually (Maheshwari and Black, 2012). Incidence is highest in premature infants and LBW infants, with 6% to 58% occurrence. (a) Relationship between neutropenia and risk of infection is not well established in neonates, and infants with greater than 1000 cells/mm3 may not be at increased risk (Maheshwari and Black, 2012). (b) Decision to treat should not be based upon neutropenia alone. (3) Neutrophilia. (a) Inconsistent response to infection, often normal with serious infection. (b) May be elevated at birth (as high as 26,000 cells/mm3) because of birth stress, maternal fever, ≥ 6 hours of oxytocin, asphyxia, meconium aspiration, pneumothorax with uncomplicated hyaline membrane disease, seizures, prolonged crying (≥ 4 minutes), hypoglycemia less than 30 mg/dL, hemolytic disease, surgery, and high altitude (Weinberg and D’Angio, 2011). b. Immature/total neutrophil (I/T) ratio. (2) Not a consistent correlation with presence of serious infection. Low immature band counts may be due to depleted bone marrow reserves producing misleading low I/T ratios. (3) Best used for negative predictor value. If I/T ratio is normal, likelihood infection is absent with 99% predictive value (Weinberg and D’Angio, 2011). (4) I/T ratio greater than 0.2 to 0.25 is suggestive of infection (Fig. 32-3). (5) Calculation of I/T ratio: 3. Platelet count. b. Thrombocytopenia (< 100,000/mm3): possible association with bacterial sepsis or viral infection, but usual onset does not occur until 1 to 3 days after infection onset (late indicator). May also occur with maternal HELLP syndrome (hemolysis, elevated liver function test results, and low platelet count), pregnancy-induced hypertension, and intrauterine growth restriction, as well as some syndromes such as trisomies 13, 18, and 21, Turner’s syndrome, and hemolytic disease. c. Severe and early-onset thrombocytopenia (< 50,000/mm3) is often an indicator of bacterial infection (Saxonhouse and Sola-Visner, 2012). With a clinically well infant without proven infection, neonatal alloimmune thrombocytopenia must be considered. B. C-Reactive protein (CRP). 2. May act as a carrier protein facilitating removal of foreign or altered materials from invading microorganisms or damaged tissues. Role remains unclear in activation of immune system pathways (Weinberg and D’Angio, 2011). 3. Elevated cord blood CRP levels are associated with chorioamnionitis with prolonged rupture of membranes. 4. Elevated CRP is also associated with noninfectious conditions that cause tissue damage or inflammation, including: asphyxia, respiratory distress syndrome, intracerebral hemorrhage, surgery, gastroschisis, meconium aspiration, and recent immunizations. This reduces the positive predictive value of the CRP level and its usefulness as a diagnostic tool. 5. Serial CRP levels are helpful in exclusion of serious infection. CRP levels increase rapidly and are elevated within 1 day of bacterial infection, peak at 2 to 3 days, and remain elevated until infection and associated inflammatory process are resolved. CRP levels then return to normal within 5 to 10 days in most infants. For infants undergoing sepsis evaluation, normal CRP levels 1 to 3 days after initiation of evaluation may have a negative predictive value of 99%, allowing the discontinuation of antibiotic therapy in light of negative cultures. 1. A minimum of 1 mL of blood should be obtained to improve chances for detection of bacteremia. Collection of only 0.5 mL has been shown to be unreliable in detection of pathogens (Nizet and Klein, 2011). Many clinicians suggest cultures from two sites are preferable in order to substantiate a positive culture. Adequate blood sample volume is associated with 90% sensitivity for bacterial detection and is twice as likely to yield a noncontaminant positive result (Piantino et al., 2013). b. Bacterial growth is evident within 48 hours for most cultures. Culture results are followed at 24-hour intervals, with a final report at 5 to 7 days. c. About 92% of positive blood cultures will be positive by 24 hours (Byington et al., 2003). d. False-positives can occur with contaminated specimens. Differentiating true sepsis from contamination can be challenging. Indicators of possible contamination include: increased time (> 2 to 3 days) until culture became positive, only a single culture is positive, infant is clinically well, organism is part of normal skin flora, multiple organisms grow in one culture bottle, or different organisms grow in separate cultures. If the infant has clinical deterioration, bacteremia must be presumed rather than a contaminant. e. In light of positive cultures, appropriate therapy should be continued and daily cultures drawn until a minimum of two consecutive cultures are negative. B. Cerebrospinal fluid (CSF) culture and polymerase chain reaction (PCR). 1. Routine use of lumbar puncture remains controversial in early sepsis evaluation. Up to 15% of infants with sepsis develop meningitis, and may not have any accompanying clinical symptoms to indicate meningitis. Bacterial meningitis occurs in less than 1 case per 1000 infants, but risk increases with LBW and prematurity (Nizet and Klein, 2011). 2. Lumbar puncture may be reserved for infants with clinical symptoms of central nervous system (CNS) involvement or proven bacteremia, or delayed for several days in an unstable infant until stabilization is achieved. The CSF culture results may be sterile after several days on antibiotic therapy, but abnormal CSF assays will still identify the presence of pleocytosis and an inflammatory reaction. 3. Interpreting CSF findings. b. Polymorphonuclear leukocytes are often present in newborns. c. Elevated protein concentration occurs in newborns and is higher in premature and VLBW than term infants. By the third month of life, protein levels reach the normal values for older infants in term babies (< 40 mg/dL), but may take longer in premature infants. d. CSF glucose concentrations are lower in neonates than in older infants, and may be as low as 30 mg/dL in term infants or 20 mg/dL in premature infants (Nizet and Klein, 2011). e. Gram stain smear of CSF can detect organisms in up to 78% of gram-negative meningitis, and up to 83% of group beta streptococci (GBS) meningitis. Gram-positive bacteria can be detected for up to 36 hours of antimicrobial therapy, and some gram-negative organisms can be seen for several days (Nizet and Klein, 2011). f. CSF culture. (1) Positive culture may reflect bacteremia from a bloody tap. (2) Repeat the CSF tap every 24 to 36 hours until culture is sterile. (3) Duration of antibiotic therapy is based on when the first negative culture is documented. (4) There is a direct correlation between adverse neonatal outcomes and persistence of bacteria in the CSF. C. Urine culture. 1. Incidence of UTI is low in EOS (1% to 2%), yield is significantly higher in LOS (7% to 8%) (Nizet and Klein, 2011). 2. Contamination with urine obtained by an external collection bag is high. Urine sample should be obtained by sterile catheterization to avoid contamination and false-positive results. D. Repeat studies. 2. Persistent bacteremia may be caused by resistance to antibiotics, incorrect administration of antibiotics, an occult site of infection that may require surgical intervention (e.g., abscess), or presence of invasive devices left in place during treatment for bacteremia. B. Definitions of and guidelines for SIRS have been modified from adult literature to fit the neonatal population by the International Pediatric Sepsis Consensus Conference, and published in 2005, (Goldstein, et al., 2005) but have limitations due to significant variations in normal vital sign values in premature and term infants alike (Piantino et al., 2013). Based on the guidelines, a neonate must have the presence of two of the following criteria, one of which must be an abnormal temperature or leukocyte count: 1. Core temperature of greater than 38.5 ° C/101.3 ° F or less than 36 ° C/96.8 ° F. 2. Tachycardia, defined as a mean heart rate greater than 2 standard deviations above normal for age in the absence of external stimulus, painful stimulus, or chronic medications. Otherwise, persistent unexplained elevation over a half-hour to 4-hour time frame. 3. Bradycardia with heart rate less than 10th percentile for age in the absence of external vagal stimulus, β-blocker medications, or heart disease. Otherwise, persistent unexplained bradycardia over a half-hour to 4-hour time frame. 4. Mean respiratory rate greater than 2 standard deviations above normal for age, mechanical ventilation related to acute deterioration, not due to neuromuscular disease or general anesthesia. 5. Elevated or depressed leukocyte count for gestation, or greater than 10% immature neutrophils. 6. Proven or suspected sepsis caused by any pathogen, or a clinical syndrome associated with high likelihood of infection. Positive evidence of infection, including derangements in examination, imaging, or laboratory tests, such as WBCs in normally sterile body fluid, perforated viscus, chest x-ray with pneumonia, petechial or purpuric rash, or purpura fulminans. 7. SIRS in the presence of or as a result of proven or suspected infection. C. Practitioners have the difficult task of deciding to discontinue empirical antibiotics with negative cultures. 1. Differential etiologies for SIRS with negative cultures include: viral infections, cardiopulmonary diseases (structural heart lesions, patent ductus arteriosus, pulmonary hypertension, pulmonary hypoplasia, surfactant protein deficiency, and bronchopulmonary dysplasia), intraventricular hemorrhage, seizures, subgaleal and intracranial hemorrhage, opiate withdrawal, NEC, malrotation, bowel obstruction (meconium plug syndrome, meconium ileus, Hirschsprung’s disease), metabolic disorders, and inborn errors (galactosemia, urea cycle disorders, organic acidemias, adrenal hyperplasia, hypoglycemia, autoinflammatory diseases) (Piantino et al., 2013). b. Treatment for suspected LOS must include coverage for prevalent HAIs such as coagulase-negative staphylococci (CoNS) and Staphylococcus aureus, or organisms with increased incidences specific to the individual nursery. 2. Ampicillin is commonly used in combination with an aminoglycoside such as gentamicin or tobramycin for synergy for initial broad-spectrum treatment of suspected or confirmed bacterial EOS. 3. If meningitis is suspected with EOS, ampicillin and cefotaxime are the antibiotics of choice until a specific organism has been identified. b. Third-generation cephalosporins may lead to rapid emergence of drug-resistant bacteria when used extensively for presumptive therapy and therefore should be limited to infants with evidence of meningitis or gram-negative sepsis. 4. Vancomycin and an aminoglycoside for synergy are used for initial treatment of suspected bacterial LOS due to penicillin G and ampicillin resistance of the majority of S. aureus organisms. a. Currently all staphylococcal strains in neonates have been susceptible to vancomycin, but vancomycin-resistant S. aureus has been identified in older populations (Nizet and Klein, 2011). Judicious use of vancomycin may help prevent further development of resistance in neonates. 5. Duration of antibiotic therapy is a minimum of 10 to 14 days for proven sepsis with minimal or absent focal infection, and a minimum of 21 days for meningitis. 6. If culture results are negative, antimicrobial agents may be discontinued after 48 to 72 hours, though antibiotics are often continued for 5 to 7 days of treatment in symptomatic infants or infants with deteriorating clinical condition despite negative culture results. 7. If the mother was treated before delivery, the antimicrobial course may be extended in the face of negative culture results, or if multiple courses of antibiotics have presumably induced low bacterial load (i.e., recent course within 4 days of new-onset infection). A. Treatment goals of septic shock includes timely management of airway, breathing, and circulation to rapidly restore adequate tissue perfusion and improve patient outcomes (Adcock, 2012; Agrawal, 2012). 1. Provide adequate ventilatory support and secure appropriate airway. 2. Treat hypovolemia with isotonic crystalloid (normal saline, lactated Ringer’s solution) for acute volume expansion. 3. Transfuse with packed red blood cells (RBCs), platelets, fresh frozen plasma, or cryoprecipitate for anemia, blood loss for disseminated intravascular coagulation. 4. Correct metabolic and electrolyte derangements, provide adequate nutrition, and avoid hypoglycemia due to increased energy demands and to avoid catabolism. 5. Treat hypotension with inotropic agents and consider cortisol. b. Epinephrine—potent inotropic and chronotropic effects, frequently used in infants nonresponsive to dopamine. c. Hydrocortisone—cortisol therapy in premature neonates is often used as a third-line treatment of refractory hypotension due to suspected adrenal insufficiency and may result in improved myocardial contractility, stroke volume, and cardiac output. It has been shown to increase blood pressure, decrease heart rate, and reduce the need for other vasoactive medications in neonates (Wang et al., 2010). 6. Treat underlying infection. 7. Evaluate cardiac function and arrhythmias with echocardiogram and electrocardiogram. 8. Extracorporeal membrane oxygenation for infants greater than 34 weeks of gestation if nonresponsive to other interventions. 2. Spirochete infections—syphilis. 3. Parasitic infections—congenital malaria, toxoplasmosis. 4. Fungal infection—candidiasis. 5. Other bacterial infections include UTIs, osteomyelitis or septic arthritis, pneumonia, and tuberculosis. 6. Other diagnoses that may present with similar nonspecific findings include neonatal hypoxia, inborn errors of metabolism, cyanotic congenital heart disease, and neonatal respiratory distress. 1. The overall incidence of neonatal sepsis in the United States in the last 10 years is between 1 and 5 per 1000 live births. Term infants have a reported rate of 1 to 2 per 1000 live births. The late preterm rate was 4 to 6 per 1000 live births (Cohen-Wolkowiez et al., 2009). 2. Defined by the age at which onset occurs. b. LOS is infection presenting at greater than 72 hours or ≥ 7 days of age and is attributed to pathogens acquired postnatally. c. Late-late-onset sepsis is defined as occurring in infants born at ≤ 28 weeks and infection presenting after 3 months of age. 3. Recent database results from the NICHD Neonatal Research Network in infants born from 2006 to 2009 estimated the overall incidence of EOS to be 0.98/1000 live births, with increasing rates in premature infants. Mortality was 7% (CDC, Active Bacterial Core Surveillance, Emerging Infections Program Network, 2011; Stoll et al., 2011). b. Rates of LOS are most common in preterm LBW infants, with rates from 1.87% to 5.42%, with decreasing rates as birth weight increases. c. African American preterm neonates account for 5.14 cases per 1000 births, with a case fatality rate of 24.4%. B. Responsible Organisms. 2. In VLBW infants with birth weights of less than 2500 g, rates of GBS were 2.1 in 1000 births, E. coli infection rate was 5.1 in 1000 births. 3. Cases with LOS are most commonly associated with CoNS (rate of 48%), S. aureus (rate of 8%), Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter spp., Citrobacter spp., and Candida. Gram-negative LOS is less common but has the greater mortality of 19% to 36%. Fungal infections account for roughly 12% of LOS in VLBW infants, although the incidence among centers varies widely (Edwards, 2012; Stoll et al., 2011). Following are brief descriptions of common focal sites and pathogens that contribute to the overall septicemia seen in neonates. 1. Incidence of meningitis has remained stable since the 1970s. Depending upon the inclusion criteria, the incidence is between 0.25 and 0.32 per 1000 live births. Occurs in as many as 15% of neonates with bacteremia. In a study done between 2006 and 2009 in the United States, 72% of infants who had sepsis or meningitis and who presented within 72 hours of birth had infection caused by GBS or E. coli (Stoll, et al., 2011). 2. EOS infections acquired in the first 2 to 6 days after birth reflect vertical transmission from maternal genital tract flora. Those infections acquired after the first week of life suggest vertical transmission with neonatal colonization from maternal flora or horizontal transmission through contact with colonized household or community caregivers. 3. Of neonates who present with early-onset GBS bacteremia, 5% to 10% will develop meningitis. Of those with late-onset GBS infection, 25% will have meningitis (Heath et al., 2003, Stoll et al., 2011). 4. Gram-negative bacillus is responsible for 3.6% of cases in which neonates present with meningitis. It is the fifth most common cause of meningitis in infants. Gram-negative bacilli seed the CSF secondary to bacteremia, trauma, and surgery. Subsequent meningitis can develop as a result of bacterial multiplication and induction of inflammation in the subarachnoid and ventricular space; this is followed by a progression of inflammation and development of neuronal damage. 5. Chronic complications of neonatal meningitis include (Libster et al., 2012): (1) Developmental delay in approximately 25%. (2) Late-onset seizures in approximately 25%. (3) Cerebral palsy in approximately 20%. (4) Hearing loss in approximately 10%. (5) Cortical blindness in less than 10%. b. Term infants, after GBS meningitis, had mild to moderate neurologic impairment in 25% of cases, and 19% had severe neurologic impairment (Libster, et al., 2012). 6. The clinical presentation of neonatal meningitis typically is indistinguishable from that of neonatal sepsis without meningitis. The most commonly reported clinical signs are: b. Neurologic signs of neonatal meningitis (Nizet and Klein, 2011; Pong and Bradley, 1999): (1) Irritability is present in up to 60% of infants. (2) Seizures have been reported as a presenting feature in 20% to 50%. Meningitis resulting from gram-negative pathogens presents with seizures, usually focal rather than generalized, more often than gram-positive pathogens. (3) Bulging fontanelle is present in approximately 25% of infants, and nuchal rigidity in approximately 15%. Findings of a full, but not bulging, fontanelle and normal neck flexion at the time of initial presentation are more the rule than the exception. c. Other findings reported are: (1) Poor feeding/vomiting in approximately 50%. (2) Respiratory distress in 33% to 50%. (3) Apnea in 10% to 30%. (4) Diarrhea in 20%. 7. Diagnostic features that are characteristic of neonatal bacterial meningitis include: (2) Neonates in whom a traumatic lumbar puncture occurred should be treated for presumed sepsis and meningitis, pending results of CSF culture. b. Increased white blood cell counts in CSF of greater than 1000 WBCs/mcl with predominately neutrophils. c. Decreased CSF glucose of less than 30 mg/dL in term and less than 20 mg/dL in preterm infants. d. Elevated CSF protein of greater than 100 mg/dL in term and greater than 150 mg/dL in preterm infants. e. Neuroimaging is indicated to assist in defining the potential complications of neonatal meningitis. It is suggested that a neuroimaging study be done 48 to 72 hours before the anticipated end of therapy. (2) Computed tomography or magnetic resonance imaging can demonstrate the degree of cerebral edema, obstruction of CSF flow, infarction, abscess, and subdural fluid collections. 8. Antibiotic therapy. Prompt initiation is crucial for optimal outcome, and antibiotics may be administered before results of CSF specimen are obtained. b. When meningitis resulting from a gram-negative organism is strongly suspected or CSF Gram stain reveals gram-negative bacilli, the empirical regimen of ampicillin and an aminoglycoside should be expanded to include cefotaxime for CSF penetration. c. Antibiotic choice in the neonate who presents with LOS bacterial meningitis depends on the preliminary CSF findings. (2) Once the causative pathogen and susceptibility pattern are known, the antimicrobial therapy should be altered accordingly. d. GBS is uniformly susceptible to penicillin and ampicillin, and a combination of either with an aminoglycoside is recommended. The aminoglycoside is to improve synergy. e. Gram-negative enteric bacteria: E. coli—ampicillin is the antimicrobial of choice. f. Ampicillin-resistant E. coli and other gram-negative organisms usually are treated with cefotaxime in combination with gentamicin. (1) Once sterility of the CSF is documented, the combination is continued for 7 to 14 days. g. Coagulase-negative staphylococci—vancomycin is the antibiotic of choice. 9. Management. b. Gram-positive bacteria usually clear from the CSF within 24 to 48 hours after initiation of appropriate therapy. Gram-negative pathogens may persist for several days in severe cases. c. Failure to respond to clinically and bacteriologically appropriate antimicrobial therapy can lead to significant neurologic sequelae such as hydrocephalus, multicystic encephalomalacia, porencephaly, and cerebral cortical and/or white matter atrophy. d. Infants with suspected bacterial meningitis need to be in the NICU setting and monitored for signs of increased intracranial pressure, such as bradycardia, hypertension, respiratory distress, bulging fontanelle, accelerated head growth, separation of the cranial sutures, hemiparesis, and focal seizures and/or new-onset seizures. (2) Evaluation and management with infectious disease experts to ensure adequate duration of antimicrobial therapy. e. Long-term follow-up for survivors of neonatal meningitis includes monitoring of hearing, visual acuity, and development status. (1) Hearing should be evaluated by evoked-response audiometry within 4 to 6 weeks of completion of therapy (Estripeaut and Saez-Llorens, 2009). f. Survivors are also at risk for developmental delay and may be eligible to receive early intervention services in the United States (eligibility criteria vary by state). g. Appropriate referrals should be made as indicated, and developmental surveillance should continue throughout childhood. B. Pneumonia b. Intrauterine aspiration of contaminated amniotic fluid. c. Aspiration of infected amniotic fluid during or after birth. The neonate can aspirate vaginal organisms that are colonizing the maternal genital area, leading to respiratory colonization. 2. Late-onset pneumonia usually occurs after 3 days of life, and generally arises from colonized organisms present in the hospitalized newborn. Horizontal transmission, or nosocomial infection, is acquired from infected caregivers, family members, or contaminated equipment. Microorganisms can invade through injured tracheal or bronchial mucosa or through the bloodstream. 3. The pathologic changes vary with the type of organism, either bacterial or viral. b. Viruses typically cause an interstitial pneumonia. The extensive inflammation occasionally occurs with hyaline membrane formation, followed by varying degrees of interstitial fibrosis and scarring. c. In the United States, GBS is the most common early-onset pathogen. Injury from GBS in the lungs increases the permeability that contributes to the development of alveolar edema and hemorrhage and possibly the mechanism that is responsible for bloodstream extension. 4. Pathogens responsible for EOS with pneumonia include: E. coli > GBS > Klebsiella > S. aureus > S. pneumoniae (Duke, 2005). b. Viral—Of the viral infections, HSV is the most common viral agent to cause early-onset pneumonia. HSV pneumonia occurs in 33% to 54% of disseminated HSV infections and usually is fatal in spite of treatment (Speer, 2012). c. Fungal—Approximately 25% of VLBW infants are colonized by Candida in the gastrointestinal and respiratory tracts, presumably during labor and delivery. (1) Pneumonia occurs in 70% of infants with systemic candidiasis (Baley et al., 1986). 5. Late-onset pneumonia occurs usually in those infants who have prolonged hospitalization that results in deviations to normal flora. There is a predominance of gram-positive organisms, including S. pyogenes, S. aureus, and S. pneumoniae. a. S. aureus and K. pneumoniae cause extensive tissue damage, abscess formation, and empyema. b. E. coli, Serratia marcescens, Enterobacter cloacae, S. pneumoniae, and P. aeruginosa may cause pneumatoceles. c. Citrobacter diversus is frequently associated with brain abscess and can also cause lung abscess. d. Chlamydia trachomatis has a long incubation period and typically is associated with pneumonia occurring between 2 and 4 weeks of age. Occurs where untreated sexually transmitted disease is present. 6. Risks: Patients who require assisted ventilation are at highest risk for late-onset pneumonia. 7. Clinical manifestations: b. Some infants develop pulmonary hypertension. c. Ventilatory-dependent infants may have increased oxygen and ventilator requirements or purulent tracheal secretions. 8. Diagnosis: Because signs of pneumonia are nonspecific, any newborn infant with sudden onset of respiratory distress or other signs of illness should be evaluated for pneumonia and/or sepsis. a. Cultures of blood and CSF should be obtained. b. If viral or other nonbacterial infection is suspected, specific studies should be obtained, including PCR. c. Gram stain and culture of tracheal aspirates may identify the causative organism. d. Chest x-ray examination. (b) Pleural effusions occur in up to 67% of infants with pneumonia, but are rarely found in RDS. However, pleural effusions can also be seen in infants with transient tachypnea of the newborn, congenital heart disease, and hydrops fetalis. 9. Therapy: The choice of empirical regimens is based upon whether the infection is early- or late-onset. (1) Third-generation cephalosporins should not be used for suspected sepsis or pneumonia. (2) Gram-negative bacilli can rapidly develop resistance to cephalosporins. b. For late-onset pneumonia, antibiotic choice depends on the prevalence sensitivity of bacteria in both the community and the hospital. The choice for term infants is vancomycin plus gentamicin. c. The usual treatment course for uncomplicated pneumonia is 10 to 14 days. C. Urinary tract infections. UTIs in newborns frequently are associated with bacteremia and may result in long-term complications. Newborns with UTI should be evaluated for associated systemic infection and anatomic or functional abnormalities of the urinary tract. 2. E. coli is the most common organism found, accounting for 80% of cases responsible for UTIs. a. Other organisms found were Klebsiella > Enterobacter > Citrobacter. b. Most common gram-positive organisms were Staphylococcus and Enterococcus species, but they occur less frequently than do gram-negative pathogens. 3. Most UTIs in newborns represent upper tract infection (pyelonephritis). Pathogenesis includes either hematogenous spread secondary to bacteremia or ascending infections, especially in infants with urinary tract abnormalities. Approximately 30% to 50% of term newborns with a UTI have urinary tract abnormalities, of which vesicoureteric reflux is the most common. Other lesions found in infants with UTI include obstructive abnormalities such as posterior urethral valves, malformations such as ectopic ureter, and renal conditions such as polycystic diseases. 4. Clinical manifestations: General signs are often nonspecific and may include temperature instability, poor weight gain, poor feeding, cyanosis, abdominal distention, hyperglycemia, hematuria, and proteinuria. Localized signs consist of a weak urinary stream and/or bladder distention. 5. Diagnosis: A urine specimen should be obtained by sterile catheterization. a. Diagnosis of UTI is based on culture of an organism from the urine. b. Because of the associated risk of bacteremia, blood cultures should be obtained. 6. Therapy: antimicrobial agents. Treatment with parenteral IV broad-spectrum antibiotics as soon as cultures of urine, blood, and CSF (if indicated) have been obtained. 7. Management: Repeat urine culture should be sterile within 36 to 48 hours after initiation of antimicrobial therapy. A. Streptococcus agalactiae is a gram-positive diplococcus encapsulated bacterium that has been the principal GBS pathogen in the neonate for over 50 years. GBS accounts for more than 95% of EOS and more than 90% of LOS in neonates in the United States today. The reported fatality rate among preterm neonates with birth weights greater than 1500 g was 20%, nearly an eight-fold increase compared to term infants (Phares et al., 2008). 2. GBS infection in neonates is classified by age at onset into early-onset GBS, occurring within 24 hours of birth up to day 7 of life; late-onset GBS, which has a broader range from 7 to 89 days; and late, late-onset GBS, which occurs in infants older than 3 months of age. 3. Risk factors include: a. Delivery at less than 37 weeks of gestation. b. Premature rupture of membranes. c. Rupture of membranes ≥ 18 hours before delivery. d. Clinical signs of chorioamnionitis, maternal temperature ≥ 100.4 ° F or (38 ° C) during labor. e. Maternal GBS bacteriuria during the current pregnancy. f. Prior delivery of an infant with GBS disease. g. Heavy maternal colonization inoculum (> 105 colony-forming units/mL) vaginally. 4. Since the implementation of universal screening of pregnant women at 35 to 37 weeks of gestation and recommendations for providing intrauterine antibiotic prophylaxis (IAP) in 2002, surveillance data by the CDC from 2010 demonstrate a decline from 1.7 cases per 1000 live births (1993) to 0.28 cases per 1000 live births (2008) in the United States. b. Despite highly effective prevention efforts, an estimated 1100 infants still become infected each year in the United States. Early-onset GBS accounts for 80% to 85% of cases. Late-onset GBS accounts for 50% of cases that present usually at 5 to 6 weeks after birth (American Academy of Pediatrics, Committee on Infectious Diseases, 2012; CDC, 2010; Remington et al., 2011). c. Late-onset GBS usually occurs at 4 or 5 weeks of age. Up to 50% of LOS is thought to be attributable to vertical transmission at birth and horizontally in household and community settings. d. Late, late-onset GBS occurs in infants older than 3 months of age. The infants who most commonly develop late, late-onset GBS are infants who were born before 28 weeks of gestation or those with a history of immunodeficiency. 5. Early-onset GBS infection most commonly manifests as: b. Pneumonia. c. Meningitis. d. Infants whose mothers receive IAP are less likely to have sepsis, need assisted ventilation, or have documented GBS bacteremia (Feng-Ying, et al., 2011). 6. Sepsis without a focus of infections occurs in 80% to 85% of cases of early-onset GBS. The signs and symptoms are nonspecific. Late-onset GBS disease most often presents as bacteremia without a focus in about 65% of cases. Meningitis remains the most common presentation of late-onset GBS and occurs in approximately 25% to 30% of cases. b. Penicillin remains the preferred agent, with ampicillin an acceptable alternative. 7. Other less common (2% to 3%) but well-described late-onset GBS focal infections are: b. Osteoarthritis. 8. Guidance with empirical therapy for early- or late-onset GBS can be reviewed online at http://www.cdc.gov/groupbstrep/guidelines/index.html. New in the 2010 guidelines: b. There is a revised colony count threshold for laboratories to report GBS in the urine of pregnant women. c. There are revised algorithms for GBS screening and use of IAP for women with threatened preterm delivery, including one algorithm for preterm labor and one for preterm premature rupture of membranes. d. Recommendations for IAP agents are presented in an algorithm format in an effort to promote use of the most appropriate antibiotic for penicillin-allergic women. e. A minor change has been made to penicillin dosing to facilitate implementation in facilities with different prepackaged penicillin products. f. The neonatal management algorithm’s scope was expanded to apply to all newborns. g. It provides management recommendations that depend upon clinical appearance of the neonate and other risk factors such as maternal chorioamnionitis, adequacy of IAP if indicated for the mother, gestational age, and duration of membrane rupture. h. Changes were made to the algorithm to reduce unnecessary evaluations in well-appearing newborns at relatively low risk for early-onset GBS disease. A. Candidiasis is caused by Candida, budding yeasts that form long chains called pseudohyphae. 1. Species causing most infections is Candida albicans, though Candida parapsilosis and Candida glabrata are also responsible for candidiasis in increasing numbers over the last 10 years in VLBW neonates (Remington et al., 2011). 2. Candida species are found on the skin and in the mouth, intestinal tract, and vagina. Transmission to neonate can occur in utero, intrapartum, or postnatally through breastfeeding. Risk factors include infants less than 1500 g, intubation, abdominal or cardiac surgery, NEC, spontaneous intestinal perforation, neutropenia, use of corticosteroids and histamine2 (H2) blockers, long-term use of broad-spectrum antibiotics, use of total parenteral nutrition and lipids, hyperglycemia, and invasive central catheters. Of VLBW infants less than 1000 grams, 5% to 20% develop disseminated or invasive candidiasis and approximately 75% have prolonged hospitalization, neurodevelopmental impairment (AAP, Committee on Infectious Diseases, 2012), and up to 30% mortality even with appropriate therapy (Remington et al., 2011). 3. Mucocutaneous candidiasis results in oral–pharyngeal (thrush) or vaginal and cervical candidiasis (lesions of gluteal folds, buttocks, groin, neck, and axillae). a. Most common form of candidiasis in the newborn infant. b. Mucosal lesions appear as white plaques on oral mucosa. c. Diaper dermatitis presents with intense erythema and satellite lesions. 4. Congenital candidiasis presents with widespread erythematous maculopapular rash, and preterm infants may present with pneumonia. 5. Disseminated candidiasis may involve any organ or anatomic site and may present with acute respiratory deterioration, acidosis, feeding intolerance, hypotension, skin abscesses, temperature instability, and/or erythematous rash. a. Common sites of lesions include the brain, kidneys, liver, spleen, lungs, and retinas but lesions typically do not appear until late in the course of the disease (AAP, Committee on Infectious Diseases, 2012). 6. Mucocutaneous candidiasis is usually diagnosed clinically with the visualization of lesions. Disseminated candidiasis is confirmed through isolation of the organism from blood, CSF, or urine cultures. Focal lesions are diagnosed with ophthalmologic examination, ultrasonography, computed tomography, and magnetic resonance imaging. 7. Treatment: Guidelines for management were updated in 2009 by the Infectious Diseases Society of America and contain recommendations for neonates (available at: http://cid.oxfordjournals.org/content/48/5/503.1.full#sec-6) (Pappas, et al., 2009). b. Invasive disease is treated by prompt removal of infected invasive catheters, and IV amphotericin B remains the drug of first choice for neonates. (2) Echinocandins are reserved for drug-resistant species and should be used with caution as safety and dosing have not been established in neonates. (3) Most Candida species are susceptible to amphotericin B, though lipid preparations of amphotericin B may not penetrate into the kidney parenchyma. c. Fungal prophylaxis of VLBW infants less than 1000 or less than 1500 g has demonstrated significant reduction in Candida colonization, invasive disease, and related mortality in four prospective randomized controlled trials and 10 retrospective cohort studies. Based on these studies, fluconazole has been shown to be safe and effective as prophylaxis in this population and is recommended for NICUs with moderate (5% to 10%) or high (≥ 10%) incidence of invasive disease (AAP, Committee on Infectious Diseases, 2012; Remington et al., 2011). Routine prophylaxis is not recommended for all NICUs due to the potential development of drug resistance, which has been documented in adult populations. 8. Isolation procedures: Standard Precautions. A. Classification of ToRCHES CLAP organisms. 1. Common congenital infections were traditionally classified as TORCH infections for ease of identification and management. Some professionals have expanded the “O” (other infections) category beyond the original syphilis to include other well-described pathogens (Johnson et al., 2013). A new acronym—ToRCHES CLAP—has been suggested to be more inclusive of other well-described pathogens acquired congenitally and in the neonatal period (Remington et al., 2011) and is utilized here with the addition of respiratory syncytial virus (RSV) under the “R” and hepatitis B virus (HBV) under the “H” due to their prevalence as causes of neonatal infection. a. To—Toxoplasmosis (Toxoplasma gondii) b. R—Rubella virus and Respiratory syncytial virus c. C—Cytomegalovirus d. H—Herpes simplex virus and Hepatitis B virus e. E—Enteroviruses f. S—Syphilis (Treponema pallidum) g. C—Chickenpox (varicella-zoster virus) h. L—Lyme disease (Borrelia burgdorferi) i. A—Acquired immunodeficiency syndrome/human immunodeficiency virus j. P—Parvovirus B19 B. ToRCHES CLAP organisms. a. Caused by intracellular protozoan parasite, Toxoplasma gondii. b. Maternally acquired from consumption of poorly cooked meat or by exposure to infected cat feces. Highest risk to fetus is primary acute maternal infection during pregnancy, but can occur with subsequent infections in immunocompromised women. Risk of transmission increases with increasing gestational age, but severity of the disease decreases. Estimated incidence of congenital toxoplasmosis ranges from 1 in 1000 live births in some areas of Latin America to 0.1 in 1000 live births in the United States (Guerina et al., 2013a). c. Most infants are asymptomatic and without apparent abnormalities at birth. Some may present with fever, maculopapular rash, petechiae, purpura, hepatosplenomegaly, jaundice, pneumonitis, microcephaly, meningoencephalitis, hydrocephalus, intracranial calcifications, seizures, thrombocytopenia, lymphadenopathy, myocarditis, chorioretinitis, cataracts, optic atrophy, and bone lesions (Johnson et al., 2013; Remington et al., 2011). (1) Classic triad of associated morbidities includes chorioretinitis, hydrocephalus, and intracranial calcifications, but triad occurs in less than 10% of cases (Guerina et al., 2013a). (2) The most common late finding is chorioretinitis, which can result in vision loss, retinal detachment, cataracts, glaucoma, and blindness. Additional sequelae include mental retardation, seizures, deafness, spasticity, and learning disabilities. d. Diagnosis may be made by a number of methods: isolation or histologic demonstration of the organism by PCR of amniotic fluid, CSF, urine, blood, or cord blood; observation of parasites (detection of Toxoplasma cysts) in tissues and body fluids or placenta; detection of parasites from blood or body fluids by mouse inoculation or tissue culture; and other serologic tests. e. Treatment consists of an antiparasitic regimen for a year, including pyrimethamine plus sulfadiazine, and folinic acid to aid in prevention of bone marrow suppression. Gluocorticoids (prednisone) are added when chorioretinitis threatens vision. Clindamycin may be used for infants who develop an allergy to sulfadiazine (Guerina et al., 2013b). f. Isolation procedures: Standard Precautions. 2. R—Rubella virus and Respiratory syncytial virus. a. Congenital rubella syndrome (CRS). (2) Virus found in nasopharyngeal secretions and is transmitted through direct or droplet contact. Most prevalent in winter and early spring. About 25% to 50% of infected individuals are asymptomatic. Outbreaks now occur in those born outside the United States or underimmunized people (AAP, Committee on Infectious Diseases, 2012). Acquiring rubella during pregnancy can result in a wide range of complications, including miscarriage, fetal death, and a group of anomalies described as CRS. Up to 85% of infants will have congenital defects if maternal infection occurs in the first trimester, and 25% to 50% with second-trimester infection (AAP, Committee on Infectious Diseases, 2012). (3) Mild forms of CRS may have few or no apparent clinical symptoms at birth. Associated symptoms of CRS include: cataracts, glaucoma, retinopathy, microphthalmos, myocarditis, patent ductus arteriosus, peripheral pulmonary artery stenosis, hearing impairment, meningoencephalitis, microcephaly, hydrocephalus, behavioral disorders, mental retardation, intrauterine growth restriction, pneumonitis, hepatosplenomegaly, jaundice, thrombocytopenia, bone disease, “blueberry muffin” purpura, and petechiae. (4) Diagnosis is made by detection of specific rubella immunoglobulin M (IgM) antibody. Most congenital cases are IgM positive at birth to 3 months of age, and postnatal cases are positive by 5 days after symptom onset. Congenital infection is also confirmed by stable or rising rubella immunoglobulin G (IgG) titers in serial sera obtained for the first 7 to 11 months. Virus is isolated primarily from throat or nasal secretions, and less consistently from urine, blood, and cataract specimens, with inoculation of appropriate cell culture. Special cell cultures are required for cultivation of rubella virus. (5) Treatment is supportive, and prevention measures are as follows: (a) Pregnant women should be screened for immunity whether or not they have received prior rubella immunization. Susceptible pregnant women should avoid exposure to infected persons. Pregnant women are not given the vaccine because of the small risk of transmission of virus to the fetus (1.3%, of whom 2% had subclinical infection and none had congenital defects) (AAP, Committee on Infectious Diseases, 2012). (b) Seronegative women should be vaccinated postpartum, before discharge from the hospital. Vaccine virus is excreted in breast milk, but breastfeeding is not a contraindication to vaccination. (c) Vaccination in the United States is a live-virus subcutaneous injection combined with measles and mumps vaccines (MMR) or MMR plus varicella (MMRV), recommended at 12 to 15 months of age, and repeated at 4 to 6 years of age. Routine vaccination should be administered to all infants with few exceptions for immunocompromised children and those who have recently received immune globulin (AAP, Committee on Infectious Diseases, 2012). (6) Isolation procedures: Droplet precautions for 7 days after onset of rash, and Contact Precautions in addition to Standard Precautions. Contact Isolation is recommended from birth until ≥ 1 year of age for proven or suspected congenital rubella unless two separate negative cultures 1 month apart after 3 months of age are obtained. b. RSV. (2) Virus is found in oral, nasal, and respiratory secretions. Transmission is by exposure to large-particle droplets or contaminated surfaces. RSV can survive for several hours on environmental surfaces. Incubation period is most commonly 4 to 6 days, but ranges from 2 to 8, and may last as long as 3 to 4 weeks in immunosuppressed people. RSV is one of the most common diseases of early childhood. Most infants are infected in the first year of life, and almost all children have had RSV by age 2. Approximately 20% to 30% of infants develop lower respiratory tract disease and 1% to 3% of infants under 1 year old require hospitalization for bronchiolitis and pneumonia (AAP, Committee on Infectious Diseases, 2012). One in 1000 infants die as a result of RSV infection (Remington et al., 2011). (b) Severe and often fatal in high-risk populations, including premature infants, infants with chronic lung disease or congenital heart disease, or immunocompromised infants. (3) Symptoms may be nonspecific and include poor feeding, lethargy, apnea, and irritability. Symptoms of bronchiolitis may include cough, wheezing, tachypnea, rales, rhonchi, increased work of breathing (retractions, nasal flaring, and use of accessory muscles), pneumonia, cyanosis, and pulmonary infiltrates. (a) May result in respiratory failure. (4) Diagnosis is most commonly made by rapid viral antigen detection isolated from nasopharyngeal aspirate. Molecular testing using reverse transcriptase PCR assays is now commercially available and detects RSV at significantly higher rates than antigen or viral culture, but will detect viral RNA for weeks after the infectious period. (5) Treatment of infants with RSV is primarily supportive care and includes oxygen, clearing airway secretions as indicated, hydration, and isolation. High-risk infants may progress to assisted ventilation because of hypoxemia and hypercapnia. (a) Aerosolized ribavirin has been associated with increased oxygen saturation during the acute phase of infection, but is not recommended for routine use due to potential toxic effects of exposed health care personnel and clinical trials with conflicting efficacy results. May be considered for select cases of potentially life-threatening RSV (AAP, Committee on Infectious Diseases, 2012; Remington et al., 2011). (b) Prophylaxis of RSV is indicated for high-risk infants less than 24 months old with a history of preterm birth (< 35 weeks of gestation), and infants with chronic lung disease or significant congenital heart disease (AAP, Committee on Infectious Diseases, 2012). Number of doses to be received is determined by clinical status and associated risk factors such as attending day care or having one or more siblings under 5 years of age. (i) Palivizumab (Synagis), a humanized mouse monoclonal antibody, is administered intramuscularly once every 30 days during RSV season, and reduces associated hospitalization rates by 39% to 82% (AAP, Committee on Infectious Diseases, 2012). (6) Isolation procedures: Contact Precautions in addition to Standard Precautions. Cohort infected infants to prevent widespread nursery infection. 3. C—Cytomegalovirus (CMV). a. Viral genome double-stranded DNA, of the herpesvirus family Herpesviridae. b. Virus is found in saliva, urine, blood, breast milk, and seminal and cervical fluids. Transmission occurs horizontally from person to person with contaminated secretions, vertically from mother to infant in utero, perinatally or postnatally, and via transfusion or transplantation from infected donors. CMV is the most common congenital viral infection, with approximately 1% of all live-born infants infected in utero and excreting CMV at birth (AAP, Committee on Infectious Diseases, 2012). Severe sequelae are most commonly associated with primary maternal infection in the first trimester, though sequelae can occur regardless of trimester or reinfection. Intrapartum or postpartum infection in term infants is not usually associated with clinical illness, but in preterm or immunocompromised infants, infection after delivery can cause systemic infections. c. Asymptomatic infections are most common in children, but vary with age and immunocompetence of the individual. Congenital infection is usually not evident at birth, with approximately 10% of infected newborn infants presenting with clinical manifestations of the disease (AAP, Committee on Infectious Diseases, 2012). Symptoms of congenital infection include: intrauterine growth restriction, hepatosplenomegaly, jaundice, purpura, pneumonitis, microcephaly, hydrocephalus, intracerebral calcifications, hearing loss, chorioretinitis, and optic atrophy. Prognosis is poor, with one third dying in infancy and up to 90% of survivors having severe neurologic sequelae. Progressive and late-onset sensorineural hearing loss is the most common sequela of congenital CMV. Twenty-one percent of all hearing loss at birth and 25% of all hearing loss at 4 years of age are attributable to CMV. Perinatal and postnatal infections of preterm or immunocompromised infants include lower respiratory tract disease, interstitial pneumonia, prolonged fever, thrombocytopenia, and hepatitis. d. Diagnosis of congenital CMV is made by viral isolation in cell culture from the infant’s urine or saliva, or by a high anti-CMV IgM titer in the first 2 to 4 weeks of life. e. Infants with symptomatic congenital CMV involving the CNS may benefit from treatment with antiviral ganciclovir IV or oral valganciclovir for 6 weeks. Data suggest this therapy is protective against hearing deterioration and possibly decreases developmental delays. Due to possible toxicities, including neutropenia, antiviral therapies are not recommended for all neonates, just those with CNS involvement who are able to start therapy within the first month of life. Current clinical trials involving these antivirals and investigative vaccines are ongoing. f. CMV transmission from blood products can be eliminated by use of CMV antibody–negative donors, by freezing red blood cells in glycerol before administration, or by filtration processes. Breastfeeding is not contraindicated due to low incidence of developing clinical illness and transfer of maternal antibodies. Donor breast milk should be pasteurized, and freezing may also decrease the likelihood of transmission. g. Isolation procedures: Standard Precautions. 4. H—Herpes simplex virus and Hepatitis B virus. (1) Enveloped, double-strand DNA viruses, two distinct types: HSV-1 and HSV-2. (a) HSV-1 usually involves the face and skin above the waist, but can cause genital HSV. (b) HSV-2 usually involves the genital area and skin below the waist in the sexually active, but also can be found above the waist. Causes approximately 75% of neonatal disease (AAP, Committee on Infectious Diseases, 2012). (c) Both viruses establish latency following primary infection and have periodic reactivation that causes recurrent symptomatic disease or viral shedding without symptoms. (2) Transmission to fetus may be by ascending infection through ruptured membranes, but is more common during birth through an infected genital tract, and occurs occasionally postnatally through a nongenital infection such as hands or mouth of a care provider, or from breast lesions during breastfeeding. Causes serious disease in fetus and neonate, with incidence estimated at 1 in 3000 to 1 in 20,000 births (AAP, Committee on Infectious Diseases, 2012). Primary maternal genital infection during pregnancy near the time of delivery accounts for as much as 25% to 60% of neonatal infection. Risk to the neonate of reinfection during the first half of pregnancy is less than 2%. More than 75% of infants who acquire neonatal HSV infection have been born to women who had no history or clinical findings suggestive of active HSV infection during pregnancy (AAP, Committee on Infectious Diseases, 2012). (3) Symptoms of HSV infection include: skin vesicles or scarring, microcephaly, meningoencephalitis, keratoconjunctivitis, and liver failure. (i) Typically presents at 7 to 14 days of life. (ii) Severe liver dysfunction, abnormal CSF findings, seizures, profound sepsis, septic shock, high morbidity and mortality. (b) Localized CNS disease (30% of cases), may have skin involvement. (i) Typically presents at 14 to 21 days of life. (c) Localized skin, eyes, and mouth (SEM) disease (45% of cases); 80% of SEM cases have skin lesions; remainder are localized to eyes and oral mucosal lesions only. (i) Typically presents at 7 to 14 days of life. (4) Diagnosis: Positive culture result with specimen obtained from vesicular fluid, blood, or CSF results in a diagnosis. The diagnostic yield of CSF culture for neonates with CNS disease is less than 50%. The PCR test has a much higher yield in CSF and should be performed if available. Other rapid identification tests include direct fluorescent antibody staining of vesicle scrapings and enzyme immunoassay antigen detection in vesicles or body fluids. (b) Skin swabs of vesicles. (c) Blood and CSF for HSV culture and HSV PCR, serum alanine aminotransferase (ALT) (5) Treatment of choice is acyclovir intravenously for 10 days for asymptomatic infants born to mothers with suspected or proven primary infection with active lesions at delivery, 14 days for SEM infection, or 21 days minimum for disseminated infection or CNS infections. (b) Acyclovir suppressive therapy for 6 months following acute phase improves neurodevelopmental outcomes and helps to suppress skin recurrences. (c) For infants with ocular involvement, a topical ophthalmic drug such as 3% vidarabine, 1% trifluridine, or 0.1% iododeoxyuridine is used in addition to parenteral antiviral therapy. An ophthalmology consultation should be obtained. (6) Active maternal lesions at birth: For asymptomatic infants born to women with recurrent HSV genital lesions at birth, surface cultures and HSV blood PCR should be obtained at approximately 24 hours of life. If infant remains asymptomatic, acyclovir is not indicated at this time. Parents should be educated on signs and symptoms and infant followed closely. Full evaluation and treatment should occur if any culture results become positive or infant becomes symptomatic. If active primary maternal HSV infection is proven or suspected, CSF for cell count, chemistries, HSV PCR, and a serum ALT should be sent in addition to surface cultures and HSV blood PCR. Acyclovir is then initiated. If infant remains asymptomatic with negative cultures and non-indicative CSF and normal ALT, treatment is continued for 10 days. If maternal type-specific serology for HSV-1 and HSV-2 antibodies testing is available and infection proves to be recurrent, treatment can be discontinued at 48 to 72 hours (AAP, Committee on Infectious Diseases and Committee on Fetus and Newborn, 2013). (7) Isolation procedures: Contact Precautions in addition to Standard Precautions. (b) Mothers with active lesions may wear clean covering gown to provide care, and a surgical mask should be worn for oral/cold sore lesions. Breastfeeding is permissible if there are no breast lesions and other lesions are covered. b. HBV. (2) Virus is found in any bodily secretion, including human milk. Transmission to fetus is vertical. There is no added risk to the infant of acquiring HBV infection while breast-feeding if appropriate immunoprophylaxis recommendations are followed. Two billion people worldwide are estimated to have acquired HBV, and more than 350 million are chronically infected (Remington et al., 2011; Tovo et al., 2012). In the United States, over 1 million people have HBV (CDC, Advisory Committee on Immunization Practices, 2013). (3) Infants are usually asymptomatic at birth. Elevated liver enzymes or acute fulminating hepatitis is occasionally present. Infants infected at delivery or after birth may become hepatitis B surface antigen (HBsAg) positive 4 to 12 weeks after birth and are lifelong carriers; the time of onset of symptoms after exposure ranges from 6 weeks to 6 months. Infants who become chronically infected are at risk of having chronic hepatitis, cirrhosis, or hepatocellular carcinoma later in life (Venkatesh et al., 2011). Mortality rate of fulminating hepatitis is around 67%, and many survivors require liver transplantation (Tovo et al., 2012). (4) Routine screening is used for all pregnant women with each pregnancy, and universal screening for all exposed infants should occur at 9 to 18 months of age or 1 to 2 months following primary immunization series. Routine neonatal immunization is with hepatitis B vaccine: (a) Term infant is immunized at discharge and again at 1 to 2 months, and at 6 to 18 months of age (AAP, Committee on Infectious Diseases and Committee on Fetus and Newborn, 2013; CDC, Advisory Committee on Immunization Practices, 2013). (b) Preterm infant is immunized at discharge if weight is ≥ 2 kg or at 2 months of age. (5) Treatment of the infant born to an HBsAg-positive mother is 85% to 90% effective in preventing the development of the hepatitis B carrier state and should include: (a) Administration of hepatitis B immune globulin, in addition to a hepatitis B vaccine ≤ 12 hours after birth (AAP, Committee on Infectious Diseases and Committee on Fetus and Newborn, 2013, CDC, Advisory Committee on Immunization Practices, 2013). (6) Isolation procedures: Standard Precautions. 5. E—Enteroviruses. b. Enterovirus infections occur worldwide and are a common cause of neonatal infection, but clear data are unavailable regarding the incidence of symptomatic congenital and neonatal infections. Infections are most prevalent in the summer and fall in temperate climates, but occur all year in tropical settings. The virus is spread by fecal–oral and respiratory routes and from mother to infant in utero and peripartum, and may also be transmitted by breastfeeding. Polio vaccines have virtually eliminated poliomyelitis in the United States, but it remains endemic in parts of the world. c. Clinical manifestations of Enterovirus infection include but are not limited to: nonspecific febrile illness, respiratory symptoms (including bronchiolitis and pneumonia), pulmonary edema and hemorrhage, hand-foot-and-mouth disease, meningitis, encephalitis, paralysis, vomiting, diarrhea, hepatitis, acute hemorrhagic conjunctivitis, myocarditis, sepsis, and coagulopathy. Neonates present with more severe clinical disease and have increased incidence of long-term morbidities than do older children (Remington et al., 2011). d. Diagnosis of Enterovirus infection is made by PCR assay and cultures from stool, rectal, throat, and conjunctival swabs, as well as tracheal aspirates, urine, blood, CSF, and tissue biopsy. e. No specific treatment other than supportive care. The antiviral drug pleconaril is currently being studied for neonatal enteroviral sepsis by the National Institute of Allergy and Infectious Disease Collaborative Antiviral Study Group. f. Isolation procedures: Standard Precautions, Contact Precautions, and cohorting are used for controlling nursery outbreaks. 6. S—Syphilis. a. Caused by T. pallidum, a thin, motile spirochete. b. Maternally acquired through sexual contact. Vertical transmission across placenta, or via direct contact with active lesion at birth. Congenital infection can occur at any gestation but frequency increases with advancing gestation. Maternal untreated primary or secondary syphilis causes highest risk to fetus, with 60% to 100% transmission (Remington et al., 2011) and up to 30% to 40% spontaneous abortion occurring with early untreated syphilis. Incidence of congenital syphilis is about 0.2 per 1000 live births in the United States and affects an estimated 1 million pregnancies per year worldwide (Dobson, 2013). c. Presentation at birth varies with timing of intrauterine infection. Up to two thirds of infected neonates are asymptomatic at birth. Manifestations may include an edematous “barber’s pole” umbilical cord with stripes of red, light blue, and chalky white; hepatomegaly; splenomegaly; jaundice; nasal rhinitis (“snuffles”); lymphadenopathy; fever; edema; rash; fissures and condylomata lata (wart-like lesions) around mucous membranes of mouth, nares, and anus; long bone abnormalities; pneumonia alba; anemia; thrombocytopenia; and CNS involvement. (2) CNS involvement may be asymptomatic but occurs in approximately 40% of symptomatic infants. Symptoms include progressive hydrocephalus, cranial nerve palsies, optic atrophy, seizures, and neurodevelopmental delay. (3) Periostitis of long bones, with guarding of extremities, or “pseudoparalysis of Parrot” (lack of movement associated with pain from bone lesion). d. Diagnosis is made by serologic testing of maternal blood with Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test early in pregnancy and at the time of delivery. (1) Diagnosis of active disease in the neonate includes: (a) High VDRL titer (≥ 4 times higher than maternal titer). (b) Reactive RPR. (c) Serum IgM level greater than 20 mg/dL. (d) Confirmation with a positive result on darkfield microscopic examination or direct fluorescent antibody staining of suspicious lesions, body fluids, placenta, or umbilical cord. (2) Uninfected infants possess maternally acquired antibodies at concentrations similar to those of infected infants. It may be difficult to interpret neonatal laboratory data; therefore, it is important to determine adequacy of maternal treatment, possibility of reexposure, and family compliance with follow-up. e. Treatment. (2) Treatment of at-risk neonates who are asymptomatic and have nonreactive VDRL or RPR or values less than four-fold the maternal titer varies depending on maternal history and treatment (Dobson, 2012; Remington et al., 2011). (b) May treat with single dose of benzathine penicillin G intramuscularly if mother had adequate treatment. (3) No treatment necessary if all of the following are true: mother adequately treated with appropriate response in titers (decrease four-fold after therapy; VDRL ≤ 1:2 or RPR ≤ 1:4) and levels remained stable and low, maternal treatment occurred more than 4 weeks prior to delivery, and mother has no evidence of relapse or infection. f. Isolation procedures: Standard Precautions. 7. C—Chickenpox. b. Remains one of the most communicable human diseases. Transmission occurs through person-to-person contact of individuals with vesicular lesions, and may occur through infected respiratory tract secretions. Transplacental transmission occurs with maternal infection. Congenital varicella syndrome occurs in approximately 1% to 2% of infants with maternal infection before 20 weeks of gestation. Incubation period may be as short at 2 to 16 days after birth for infants born to actively infected mothers at the time of delivery (AAP, Committee on Infectious Diseases, 2012). Infected individuals are contagious 1 to 2 days before onset of lesions and until all lesions are crusted. c. Infection during the first or second trimester can result in fetal death, limb hypoplasia, cutaneous scarring, eye abnormalities, and CNS derangements. If maternal infection occurs 5 days before to 2 days after delivery, subsequent infant mortality is higher due to the immature immune system of the infant and insufficient maternal antibody transfer. Additional symptoms of chickenpox are a generalized pruritic, vesicular rash, low-grade fever, potential superinfection of skin lesions, pneumonia, thrombocytopenia, and rarely glomerulonephritis, hepatitis, and arthritis. Latent infection can occur, as well as infection after vaccination. d. Diagnosis is made by PCR assay of vesicular fluid or scab, or saliva and buccal swabs, or by tissue culture of vesicles, CSF, or biopsy. e. Acyclovir is commonly used to treat neonatal HSV, but few data are available for its use in treating progressive varicella, and it is not recommended unless the infant has active zoster (Remington et al., 2011). Passive immunization using varicella-zoster immune globulin (VZIG or VariZIG) is recommended for infants born to women who developed active infection 5 days or fewer prior to delivery to 2 days after delivery, given as soon as possible after birth. Additional therapy of the neonate is supportive. f. Isolation procedures: Standard, airborne, and Contact Precautions for hospitalized neonates, continued 21 to 28 days if treated with VZIG. Infants with embryopathy without active lesions do not require isolation. 8. L—Lyme disease. a. Spirochete of the species B. burgdorferi; common vector-borne illness transmitted by ticks. b. Lyme disease occurs throughout the world, and in the United States most cases occur in endemic areas, including New York, New Jersey, Pennsylvania, Minnesota, and Wisconsin. Disease progression can cause severe sequelae, including meningitis and encephalitis, and carditis. Concern regarding transplacental transmission in humans exists due to known transmission and congenital infection of other spirochetes of the Borrelia genus, but documentation of congenital disease or complications of pregnancy due to Lyme disease have not been reported (AAP, Committee on Infectious Diseases, 2012). B. burgdorferi has not been isolated in breast milk. Studies have isolated transplacental transmission in animals with reproductive failure and severe fetal infection (Remington et al., 2011), but studies in humans have shown no adverse effect to the fetus from maternal Lyme disease. c. No diagnostic studies or treatment of the neonate are indicated with confirmed maternal Lyme disease. Parents should be assured there is no evidence suggesting increased risk to the infant. 9. A—Acquired immunodeficiency syndrome/human immunodeficiency virus. a. HIV is a cytopathic lentivirus of the family Retroviridae; human RNA retrovirus. (a) HIV-2 milder disease course with longer time to develop. (2) HIV-1 requires reverse transcriptase enzyme to convert RNA to DNA, then becomes integrated in the host cell genome as a provirus. (3) Suppression of T-helper lymphocytes. This results in B-cell and suppressor T-cell dysfunction, with subsequent defects in cell-mediated immunity and development of opportunistic infections. b. Virus is found in blood, semen, cervicovaginal secretions, and breast milk. Most infants are infected perinatally, accounting for 91% of cases (Remington et al., 2011), though intrauterine transmission may occur. Infants with a positive HIV peripheral blood test within the first 48 hours of life are considered to be infected in utero and tend to have early onset of symptoms. Intrapartum transmission during exposure to maternal blood or genital tract secretions is presumed. The risk of mother-to-child transmission without interventions is 12% to 40%, with an average of 25% to 30% in the United States. The CDC estimates 215 to 370 infants with HIV infection are born each year in the United States (AAP, Committee on Infectious Diseases, 2012; Remington et al., 2011). c. Infants are asymptomatic at birth and are classified as exposed, infected, or seroreverted according to their immunologic status. Disease progression is more rapid in children than in adults, and infected infants may become seriously ill within 2 to 4 weeks of life. Ten percent to 20% of untreated infants die before 4 years of age, with a median age at death of 11 months. Symptoms of infection include: (2) Generalized lymphadenopathy, hepatomegaly, and splenomegaly. (3) Recurrent oral and diaper candidiasis. (4) Systemic bacterial infections. (5) Lymphoid interstitial pneumonitis. (6) Hyperreflexia, hypertonia, floppiness, developmental delay. (7) Parotitis, hepatitis, nephropathy, and cardiomyopathy. (8) Recurrent diarrhea. (9) Opportunistic infections (viral and bacterial). (10) Unexplained fevers. (11) Malignancies. d. The preferred diagnostic test for neonatal HIV-1 is DNA PCR (AAP, Committee on Infectious Diseases and Committee on Fetus and Newborn, 2013). (1) May detect proviral DNA before 48 hours of life with a sensitivity of 55% (Schwarzwald, 2012); considered in-utero transmission if positive. (2) Test is 93% positive by 2 weeks of life, 96% to 99% by 1 month of age, with 97% specificity (AAP, Committee on Infectious Diseases, 2012; Remington et al., 2011). (a) Western blot; confirmatory test, but unreliable for HIV-2. (3) Plasma viral RNA assesses viral load and can aid in monitoring disease progression and therapy efficacy, but is unreliable for diagnosis in the first 2 weeks of life. (4) Viral culture; expensive and long time for results, not recommended. (5) Timing of initial testing varies from less than 48 hours of life to 14 days, and should be repeated at 1 to 2 months of age and again at 4 to 6 months of age. An infant is considered infected with two positive test samples by DNA or RNA PCR (AAP, Committee on Infectious Diseases and Committee on Fetus and Newborn, 2013). e. Initiation of early antiretroviral (ARV) therapy reduces maternal–child transmission, morbidity, and mortality. Maternal therapy should include a combination ARV therapy that includes oral zidovudine (ZDU) beginning at 14 to 34 weeks of gestation and continuing throughout pregnancy. During labor, IV ZDU is recommended, and delivery via cesarean section prior to rupture of membranes is recommended for women with unknown plasma viral loads or greater than 1000 copies/mL (http://aidsinfo.nih.gov/Guidelines). Infants should receive ARV therapy from birth regardless of clinical symptoms, viral load, or immune status. (1) Bathe as soon as possible after birth. (2) Initiate oral ZDU from birth through 6 weeks of life. (3) An additional one- to two-drug regimen is used for infants born to HIV-infected mothers who did not receive any ARVs prior to onset of labor or for infants proven to be infected while on ZDU prophylaxis. Additional medications include lamivudine, nelfinavir, didanosine, stavudine, nevirapine, or lopinavir–ritonavir, but not all may be paired due to adverse reactions. Standards are still evolving, and collaboration with an HIV treatment center is recommended. (4) In the United States, where formula is readily available and safe, breastfeeding is not recommended (John-Stewart, 2013). (5) Follow-up at 2 to 4 weeks is recommended to assure drug regimen compliance and to monitor for ZDU-associated anemia. (6) Long-term follow-up and prompt intervention during bacterial and treatable opportunistic infections. (7) Routine immunization schedule and doses for all inactivated vaccines, with MMR considered for children not severely immunosuppressed as they are at high risk for complications associated with varicella-zoster and measles (Tovo et al., 2012). f. Isolation procedures: Standard Precautions should be strictly followed. 10. P—Parvovirus B19. a. Single-stranded, nonenveloped DNA virus of the family Parvoviridae. b. Common cause of infection worldwide. Transmitted through respiratory secretions and blood, and vertical transmission from mother to fetus. Intrauterine transmission rates range from 25% to 50%, with adverse fetal outcomes less than 10%; highest risk associated with initial infection before 20 weeks of gestation, though may cause fetal demise in all trimesters (Remington et al., 2011). c. Neonatal morbidities associated with primary maternal infection during pregnancy include nonimmune hydrops fetalis, myocarditis, pericardial effusions, meconium ileus, peritonitis, hyperechoic bowel, anemia, and fetal demise, but also has been associated with asymptomatic neonatal infections and normal deliveries. Maternal symptoms include malaise, arthralgia, rash, coryza, or fever. d. Mothers and fetuses with symptoms should be considered high risk and tested for serum IgG and IgM antibodies to parvovirus B19. Presence of IgG but not IgM indicates prior infection as IgG is present for life. Serum PCR assay also can detect low levels of the virus up to 9 months after acute infection. e. Some cases of associated hydrops fetalis have successfully been treated with intrauterine blood transfusions. Other therapy is supportive. f. Isolation precautions: Standard Precautions and droplet precautions for infants with unresolved hydrops fetalis associated with parvovirus B19 at the time of delivery. A. Hospital-acquired infections (HAIs) are a major cause of morbidity and mortality in infants and children. The CDC estimates approximately 5% to 10% of hospitalized patients in the United States develop HAIs, and the estimated cost for each central line–associated infection is $16,550 (The Joint Commission, 2012). In the NICU, HAIs are primarily acquired from the neonate’s own flora, but the NICU environment and hands of health care workers play an important role in transmission. Risk factors are confounded by multiple factors, including birth weight and the immature immune system. LBW remains one of the strongest risk factors, although the presence of indwelling intravascular or transmucosal medical devices has also been identified as one of the greatest risk factors of HAIs. Hand hygiene remains the single most important prevention of HAIs (AAP, Committee on Infectious Diseases, 2012). 1. The CDC has published guidelines and recommendations for the prevention of HAIs, including isolation precautions, guidelines for protecting health care workers, and guidelines for the prevention of postoperative and device-related infections. These guidelines can be found on the CDC website (http://www.cdc.gov/HAI/prevent/prevention.html). Additional resources are available through the Society for Healthcare Epidemiology, the Association for Professionals in Infection Control and Epidemiology, and The Joint Commission. 2. Standard Precautions should be strictly adhered to for all patients regardless of diagnosis, gestational age, or presence of infection. Transmission-Based Precautions should be utilized in addition to Standard Precautions for patients who are infected or colonized with pathogens that are transmitted by airborne, droplet, or direct contact. All health care providers are responsible for following infection control practices and staying informed and educated on the latest guidelines and practices to help prevent HAIs. 3. Central line–associated bloodstream infections (CLABSIs) remain a prominent concern in NICU settings. VLBW infants less than 750 g are at highest risk, with an associated rate of 3.4 per 1000 catheter days, and those less than 1500 g who develop late-onset CLABSI have 3 times higher mortality than those who do not (The Joint Commission, 2012). CDC guidelines for prevention of CLABSIs were established in 2011 (available at: www.cdc.gov/HAI/pdfs/bsi/checklist-for-CLABSI.pdf) and include: a. Prompt removal of unnecessary central lines with daily audits of necessity. b. Proper insertion practices. (2) Strict aseptic technique with maximal sterile barrier precautions, including gown, mask, cap, sterile gloves, and full-body drape. (3) Appropriate skin cleansing. (4) Choose best insertion site to minimize infection and complications. (5) Maintain intact sterile transparent, semipermeable dressings. c. Maintain central lines appropriately. (2) Scrub access port or hub immediately prior to use with antiseptic (e.g., chlorhexidine, povidone–iodine, an iodophor, or 70% alcohol). (3) Access with sterile devices only. (4) Replace soiled, nonocclusive dressings. (5) Dressing changes under aseptic technique using clean or sterile gloves. d. Empower staff to monitor proper procedures. e. Bundle supplies for ease of accessibility. f. Provide checklists for providers to ensure compliance with proper practices. g. Provide adequate access to hand hygiene and monitor adherence to procedure. h. Provide education to all staff regarding insertion, usage, and maintenance. 4. Prevention of ventilator-associated pneumonia (VAP). VAP is the second most common HAI in NICU patients. VAP is associated with increased hospitalization and health care costs. Rates of VAP range from 1 to 4 cases per 1000 ventilator days, but rates greater than 10 cases per 1000 ventilator days have been reported in some neonatal and surgical populations (Coffin et al., 2008). a. Associated with aspiration of secretions, colonization or use of contaminated equipment. b. Risk factors include prematurity, LBW, sedation, paralytic agents, intubation, mechanical ventilation, orogastric/nasogastric tube placement, and use of medications that increase bacterial colonization (e.g., broad-spectrum antibiotics, antacids, or H2 blockers), (Remington et al., 2011). c. Prevention strategies are based upon adult studies (Coffin et al., 2008; Remington et al., 2011) and include: (1) Active surveillance for VAP. (2) Minimize use of mechanical ventilation using weaning protocols and promote use of noninvasive ventilation when possible. (3) Educate providers who work with ventilated patients regarding VAP. (4) Disinfect, sterilize, maintain respiratory equipment; remove condensation from circuit, and change circuit when visibly soiled. (5) Perform regular oral care. (6) Maintain semirecumbent position (30- to 45-degree head-of-bed elevation) unless medically contraindicated. (7) Avoid unplanned extubations. (8) Avoid gastric overdistention. (9) Avoid H2 blockers. (10) Use in-line suctioning devices. 5. Optimal staffing-to-patient ratios have not been established for NICUs, but low nurse–to–high patient ratios have been correlated with increased rates of nosocomial infections. Understaffing has also been correlated with periods of decreased hand hygiene compliance, leading to further infection rates (Remington et al., 2011). B. Vaccination remains a critical component to preventing and controlling the spread of infection nationally and globally. 1. The four major strategies for protecting neonates are: a. Maternal immunization during pregnancy. b. Passive immunization with antibodies or immune globulins. c. Active immunization of neonates. d. Immunization of contacts to prevent transmission (herd immunity). 2. Recommendations and schedules for vaccination use are constantly changing. Health care providers must review current guidelines from regulatory and advisory bodies for current practice standards. a. Guidelines and recommendations are available from the Advisory Committee on Immunization Practices (ACIP) of the CDC and can be found at: http://www.cdc.gov/vaccines/pubs/acip-list.htm. b. The Recommended Immunization Schedules for Persons Aged 0 Through 18 Years are updated annually and approved by the ACIP, the AAP, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists. The 2013 schedule is available at: http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html.
Infectious Diseases in the Neonate
TRANSMISSION OF INFECTIOUS ORGANISMS IN THE NEONATE
RISK FACTORS
Identification of Predisposing Risk Factors for Neonatal Infection
DIAGNOSIS AND TREATMENT
Recognition of Clinical Manifestations of Neonatal Infection
Hematologic Evaluation
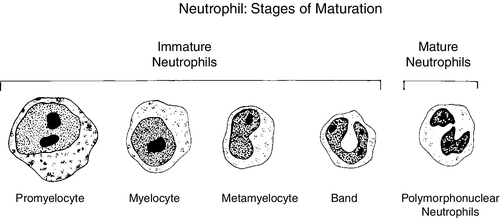
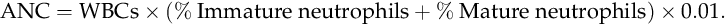
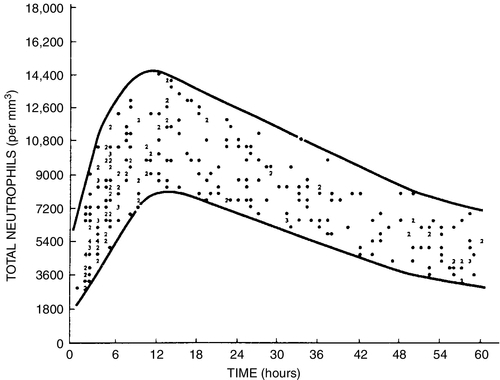
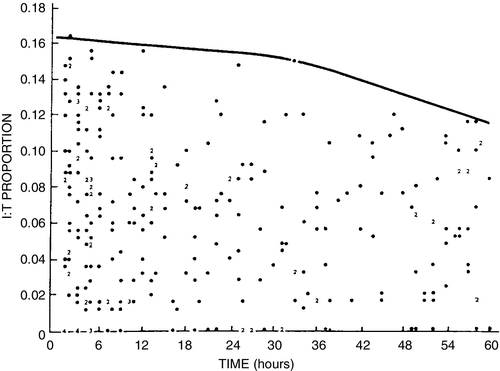
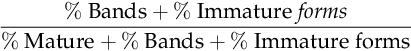
Additional Diagnostic Evaluation
Systemic Inflammatory Response Syndrome (SIRS)
Therapy
Treatment of Septic Shock
Treatment Goals and Prevention Strategies
NEONATAL SEPTICEMIA
Common Focal Sites and Pathogens
INFECTION WITH SPECIFIC PATHOGENS
Group B Streptococcus
Fungal Infection: Candidiasis
ToRCHES CLAP Infections
INFECTION CONTROL
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


