The Child with a Metabolic Condition
Objectives
1. Define each key term listed.
3. List the symptoms of hypothyroidism in infants.
4. Discuss the dietary adjustment required for a child with diabetes insipidus.
5. Compare the signs and symptoms of hyperglycemia and hypoglycemia.
6. Differentiate between type 1 and type 2 diabetes mellitus.
7. List three precipitating events that might cause diabetic ketoacidosis.
10. List three possible causes of insulin shock.
11. Explain the Somogyi phenomenon.
Key Terms
antidiuretic hormone (p. 711)
dawn phenomenon (p. 723)
gestational diabetes mellitus (GDM) (p. 714)
glucagon (p. 723)
glycosuria (glī-kō-SYŪ-rē-ă, p. 714)
glycosylated hemoglobin test (HgbA1c) (glī-kō-sī-lā-tĭd HĒ-mō-glō-bĭn tĕst, p. 714)
hormones (p. 709)
hyperglycemia (hī-pŭr-glī-SĒ-mē-ă, p. 714)
hypoglycemia (hī-pō-glī-SĒ-mē-ă, p. 721)
hypotonia (hī-pō-TŌ-nē-ă, p. 711)
ketoacidosis (kē-tō-ă-sĭ-DŌ-sĭs, p. 715)
lipoatrophy (lĭp-ō-ĂT-rō-fē, p. 720)
polydipsia (pŏl-ē-DĬP-sē-ă, p. 714)
polyphagia (pŏl-ē-FĀ-jhă, p. 714)
polyuria (pŏl-ē-YŪ-rē-ă, p. 714)
Somogyi phenomenon (p. 723)
target organ (p. 709)
vasopressin (vāz-ō-PRĔS-ĭn, p. 711)
![]() http://evolve.elsevier.com/Leifer
http://evolve.elsevier.com/Leifer
Integration of the Nervous and Endocrine Systems
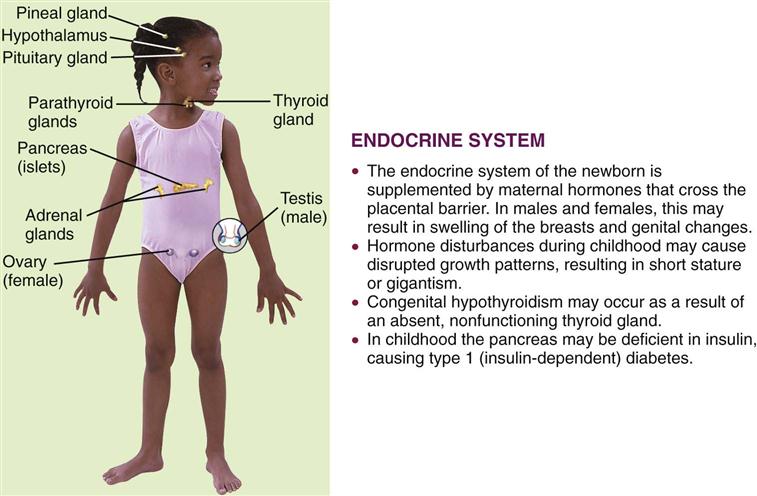
The two major control systems that monitor the functions of the body are the nervous system and the endocrine system. These systems are interdependent. The endocrine, or ductless, glands regulate the body’s metabolic processes. They are primarily responsible for growth, maturation, reproduction, and the response of the body to stress. Figure 31-1 depicts the organs of the endocrine system and outlines how this system in children differs from that in adults. Hormones are chemical substances produced by the glands. They pour their secretions directly into the blood that flows through them. An organ specifically influenced by a certain hormone is called a target organ. Too much or too little of a given hormone may result in a disease state.
Most of the glands and structures of the endocrine system develop during the first trimester of fetal development. Hormonal control is immature until at least 18 months of age, and therefore infants are more prone to problems related to the functioning of the endocrine system. Maternal endocrine dysfunction may affect the fetus; therefore an in-depth maternal history is a valuable tool in data collection.
The absence or deficiency of an enzyme that has a role in metabolism causes a defect in the metabolism process; this can result in illness. Most inborn errors of metabolism can be detected by clinical signs or screening tests that can be performed in utero. Lethargy, poor feeding, failure to thrive, vomiting, and an enlarged liver may be early signs of an inborn error of metabolism in the newborn. When clinical signs are not manifested in the neonatal period, an infection or body stress can precipitate symptoms of a latent defect in the older child. Unexplained mental retardation, developmental delay, convulsions, an odor to the body or urine, or episodes of vomiting may be subtle signs of a metabolic dysfunction. Phenylketonuria (PKU), galactosemia, and maple syrup urine disease are discussed in Chapter 14. Cystic fibrosis is discussed in Chapter 25.
Radiographic studies to determine bone age are valuable diagnostic tools. Serum electrolytes and glucose, hormonal, and calcium level tests may be required. PKU testing of newborns is an important screening device for identifying an enzyme deficiency. Chromosomal studies and tissue biopsy are other diagnostic tools. Sexual maturation and skin texture, pigment, and temperature may be indicators of specific disorders. Thyroid function tests may be required. Ultrasonography is helpful in determining the size and character of adrenal glands, ovaries, and other organs. A 24-hour urine specimen may reveal important data. Genetic counseling can help prevent some disorders. Most endocrine dysfunctions involve chronic problems and call for long-term nursing management. The nurse must assess the effect on growth and development, advocate for early detection and intervention, and promote comprehensive follow-up care that will minimize complications.
Disorders and Dysfunction of the Endocrine System
Inborn Errors of Metabolism
The term inborn errors of metabolism was coined at the turn of the twentieth century by Archibald Garrod. There are literally hundreds of these hereditary biochemical disorders that affect body metabolism. The pattern of inheritance is generally autosomal recessive. These conditions range from mild to severe.
Tay-Sachs Disease
Pathophysiology.
Tay-Sachs disease involves a deficiency of hexosaminidase, an enzyme necessary for the metabolism of fats. Lipid deposits accumulate on nerve cells, causing both physical and mental deterioration. This is a disease found primarily in the Ashkenazic Jewish population. It is an autosomal recessive trait carried by 1 in 30 of the Ashkenazic Jewish population, resulting in an occurrence of 1 in 4000 live births.
Manifestations.
The infant with Tay-Sachs disease is normal until about age 5 to 6 months, when physical development begins to slow. There may be head lag or an inability to sit. The disease progresses, and when deposits occur on the optic nerve, blindness may result. Mental retardation eventually develops because the brain cells become damaged. Most children with Tay-Sachs disease die before 5 years of age from secondary infection or malnutrition.
Treatment and Nursing Care.
There is no treatment for this devastating disease. The nursing care is mainly palliative. Most care is given in the home, with periodic hospitalization for complications such as pneumonia. Chapter 27 discusses the care of the dying child. Carriers can be identified by screening tests in the first trimester of pregnancy. Genetic testing and prenatal counseling have markedly decreased the occurrence of Tay-Sachs disease.
Endocrine Disorders
Hypothyroidism
Pathophysiology.
Hypothyroidism occurs when there is a deficiency in the secretions of the thyroid gland. It may be congenital or acquired. It is one of the more common disorders of the endocrine system in children. The thyroid gland controls the rate of metabolism in the body by producing thyroxine (T4) and triiodothyronine (T3). In congenital hypothyroidism the gland is absent or not functioning. The symptoms of hypothyroidism may not be apparent for several months.
Juvenile hypothyroidism is acquired by the older child. It may be caused by a number of conditions, the most common being lymphocytic thyroiditis. Often it appears during periods of rapid growth. Infectious disease, irradiation for cancer, certain medications containing iodine, and lack of dietary iodine (uncommon in the United States) may predispose the child. The symptoms, diagnosis, and treatment are similar to those for congenital hypothyroidism. Because brain growth is nearly complete by 2 to 3 years of age, mental retardation and neurological complications are not seen in the older child.
Manifestations.
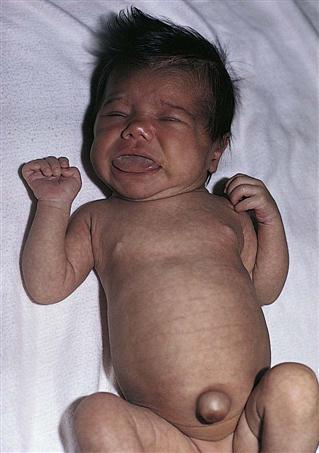
The infant with hypothyroidism is very sluggish and sleeps a lot. The tongue becomes enlarged, causing noisy respiration (Figure 31-2). The skin is dry, there is no perspiration, and the hands and feet are cold. The infant feels floppy when handled. This hypotonia also affects the intestinal tract, causing chronic constipation. The hair eventually becomes dry and brittle. If hypothyroidism is left untreated, irreversible mental retardation and physical disabilities result.
Treatment and Nursing Care.
Early recognition and diagnosis are essential to prevent the developing sequelae. A screening test for hypothyroidism is mandatory in the United States and is performed at birth. This is generally part of an overall screen for other metabolic defects. Treatment involves the administration of the synthetic hormone sodium levothyroxine (Synthroid, Levothroid). Hormone levels are monitored regularly. Therapy reverses the symptoms and prevents further mental retardation but does not reverse existing retardation; therefore early detection of congenital hypothyroidism is very important.
The medication is taken at the same time each day, preferably in the morning. Parents are cautioned not to interchange brands. Children may experience reversible hair loss, insomnia, and aggressiveness, and their schoolwork may decline during the first few months of therapy. This is temporary. It may take 1 to 3 weeks for the medication to reach the full therapeutic effect. Medication is not to be discontinued because hormone replacement for hypothyroidism is lifelong. Parents should be taught the signs and symptoms of overdose, which include rapid pulse rate, dyspnea, irritability, weight loss, and sweating. Signs of inadequate dosage or noncompliance are fatigue, sleepiness, and constipation. Parents are instructed about these issues and are advised to consult their physician before giving other medications.
Common Metabolic Dysfunctions
Other common metabolic dysfunctions, their manifestations, and their treatment are discussed in Table 31-1.
Table 31-1
| GLAND | PROBLEM | INVOLVED HORMONE | MANIFESTATION | THERAPY |
| Pituitary— anterior | Decreased: hypopituitarism | Growth hormone | Short stature, dwarfism | Synthetic growth hormone replacement |
| Increased: hyperpituitarism | Growth hormone | Before epiphyseal closure, gigantism | Cryosurgery, irradiation | |
| After epiphyseal closure, acromegaly | Radioactive implants | |||
| Sexual precocity (puberty before 8 to 9 years of age) | Monthly hormone injection to control secretions until puberty | |||
| Pituitary— posterior | Decreased: hypopituitarism | Decreased antidiuretic hormone | Diabetes insipidus (see p. 711) | |
| Increased: hyperpituitarism (SIADH) | Increased antidiuretic hormone | Fluid restriction and hormone antagonists | ||
| Parathyroid | Decreased: hypoparathyroidism | Decreased parathyroid hormone | Decreased blood calcium and increased phosphorus levels, causing tetany and laryngospasm | Calcium gluconate, vitamin D supplements |
| Increased: hyperparathyroidism | Increased parathormone | Elevated blood calcium and lowered phosphorus levels, causing spontaneous fractures and CNS problems | Restore calcium balance, excise tumor, vitamin D, low phosphorus diet | |
| Adrenal | Decreased: adrenal cortical insufficiency (Addison’s disease) | Decreased steroids, sex steroids, epinephrine | Craving for salt, seizures, neurological and circulatory changes, decreased sexual development | Replace cortisol and aldosterone, genetic sexual assessment |
| Increased: hyperadrenalism (Cushing’s disease) | Increased cortisol | Cushing’s syndrome, hyperglycemia, electrolyte problems, pheochromocytoma | Depends on cause, tumor removal and hormone replacement |
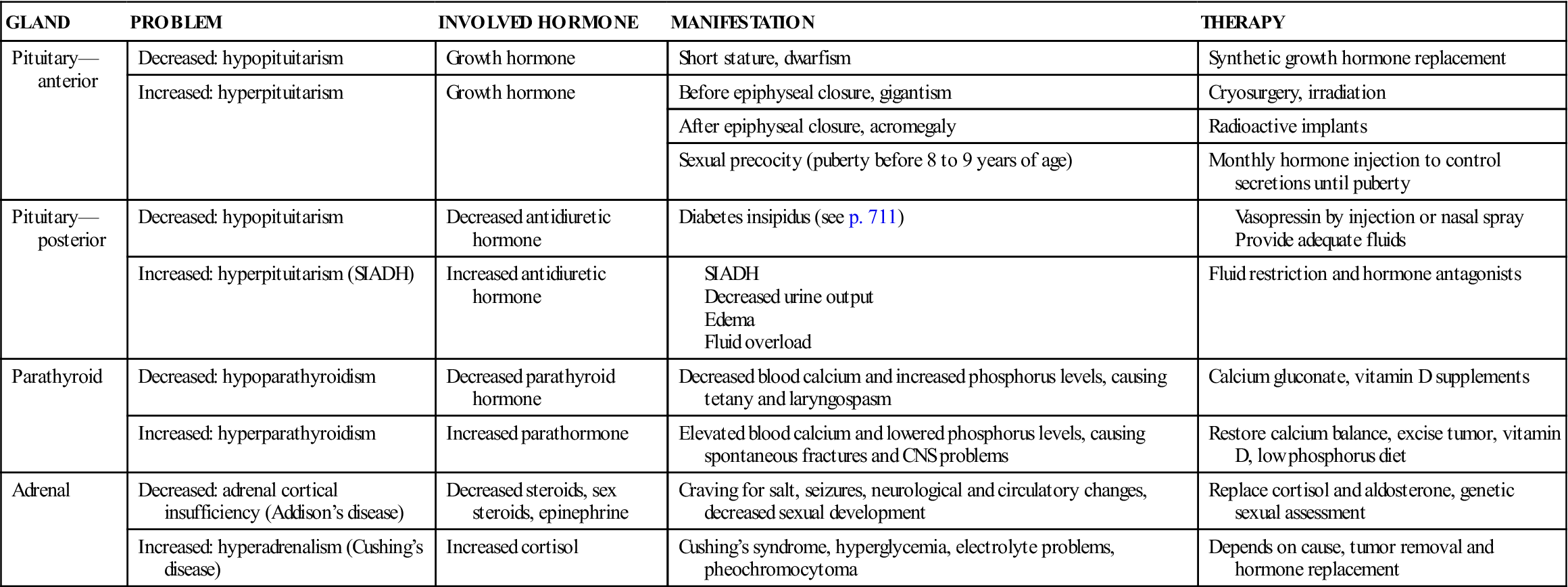
CNS, Central nervous system; SIADH, syndrome of inappropriate antidiuretic hormone.
Diabetes Insipidus
Pathophysiology.
Diabetes insipidus can be hereditary (autosomal dominant) or acquired as the result of a head injury or tumor. It is the consequence of posterior pituitary hypofunction that results in a decreased secretion of vasopressin, the antidiuretic hormone. A lack of antidiuretic hormone results in uncontrolled diuresis. The kidney does not concentrate the urine during dehydration episodes.
Manifestations.
Polydipsia and polyuria are the initial signs. The infant cries and prefers water to milk formula. Loss of weight, growth failure, and dehydration occur rapidly. As the child grows older, enuresis may be a problem. Excessive thirst and the search for water overshadow the desire to play, explore, eat, learn, or sleep. Perspiration is deficient, and the skin is dry.
Treatment and Nursing Care.
Treatment involves hormone replacement of vasopressin in the form of desmopressin by subcutaneous injection or DDAVP (desmopressin acetate) nasal spray. Parents should be taught to monitor for signs of overdose, which include symptoms of water intoxication (edema, lethargy, nausea, central nervous system signs). Children with diabetes insipidus who are admitted to the hospital in an unconscious state and are unable to express thirst are at great risk. A medical identification bracelet should be worn. School personnel should be advised of the child’s needs. School protocol often limits children’s access to bathrooms and water fountains, even during or after physical activity. Such restrictions could be life threatening to a child with diabetes insipidus. The child’s nurse should contact the school nurse and physical education instructors and educate parents concerning the child’s needs and the lifelong administration of the medication. Home care instructions should include recognizing the signs of water intoxication and dehydration.
Diabetes Mellitus
Pathophysiology.
Diabetes mellitus (DM) is a chronic metabolic syndrome (group of symptoms) in which the body is unable to use carbohydrates properly, leading to an impairment of glucose transport (sugar cannot pass into the cells). The body is also unable to store and use fats properly. There is a decrease in protein synthesis. When the blood glucose level becomes dangerously high, glucose spills into the urine, and diuresis occurs. Incomplete fat metabolism produces ketone bodies that accumulate in the blood. This is termed ketonemia and is a serious complication. DM affects the physical and psychological growth and development of children because it requires lifestyle alterations (diet, glucose monitoring, and insulin administration). There are also many long-term complications related to hyperglycemia that result in blindness, circulatory problems, kidney disease, and neuropathy that loom in the future for these children. Treatment is designed to optimize growth and development and to minimize complications.
Classification.
To help eliminate confusion in terminology, the National Institutes of Health appointed an international committee, the National Diabetes Data Group, to classify the carbohydrate intolerance syndromes (Mayfield, 1998). Further refinements in classification will be necessary as more is learned about diabetes (Table 31-2). The following classifications are pertinent to this discussion of pediatric diabetes mellitus:
• Type 2, formerly known as non–insulin-dependent diabetes mellitus (NIDDM) or adult-onset DM or maturity-onset DM: Type 2 DM involves a resistance to insulin. It is often aggravated by a sedentary lifestyle and obesity. It also occurs more frequently in certain ethnic groups, such as African American and Pacific Islanders, especially those who have hypertension and elevated blood lipid levels. Acanthosis nigricans (a dark pigmentation in the flexor creases of the skin) may be a cutaneous marker for patients with type 2 DM. Table 31-3 lists the clinical features of types 1 and 2 DM. Lifestyle intervention is the cornerstone of preventing or delaying the onset of type 2 diabetes mellitus in susceptible individuals. The accepted criterion for diagnosing diabetes mellitus is a fasting blood glucose level of 126 mg/dL or higher (Kliegman et al., 2007).
• Gestational diabetes mellitus (GDM) is the appearance of symptoms for the first time during pregnancy (see Chapter 5).
Table 31-2
Classification of Diabetes Mellitus
| Type 1 | |
| Type 2 | |
| Gestational diabetes | |
| Other genetic defects | Defects in chromosomes 6, 7, 12, and 20 and other genetic disorders are associated with diabetes mellitus syndrome |
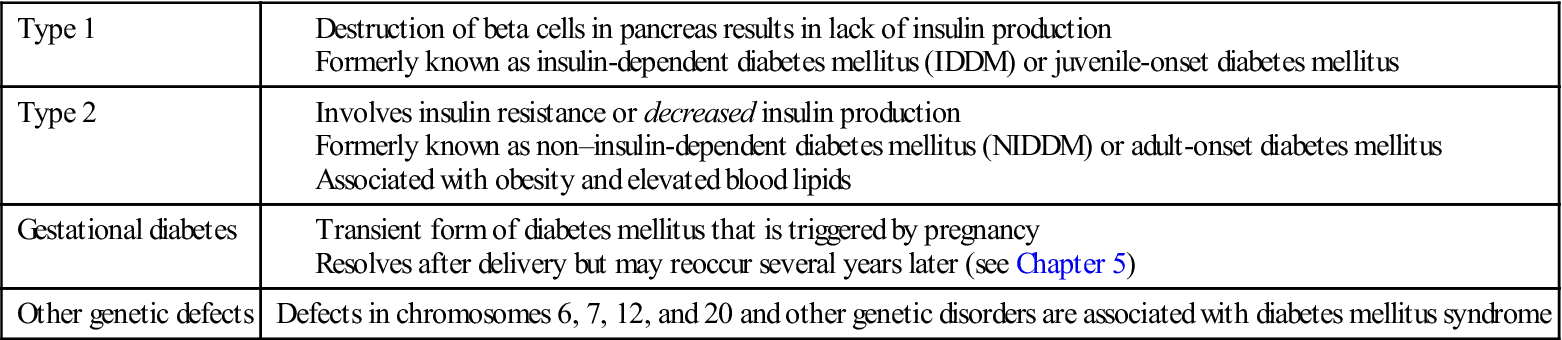
Table 31-3
Clinical Features of Type 1 and Type 2 Diabetes
| FEATURE | TYPE 1 | TYPE 2 |
| Onset | Abrupt; patient can often state week of onset | Insidious; often found by screening tests |
| Body size | Normal or thin | Often obese |
| Blood glucose level | Fluctuates widely with exercise and infection | Fluctuations are less marked |
| Ketoacidosis | Common | Infrequent |
| Sulfonylurea responsiveness | Rare | >50% |
| Insulin required | Almost all | <25% |
| Insulin dosage | Increases until glucose control is stable | May remain stable |
Type 1 Diabetes Mellitus
Incidence.
Approximately 12 million Americans have diabetes mellitus. In the United States, the annual incidence is about 1 newly diagnosed case of type 1 DM per 500 children (Kliegman et al., 2007). The frequency is increasing. Symptoms of type 1 DM may occur at any time in childhood, but the rate of occurrence of new cases is highest among 5- and 7-year-old children and pubescent children 11 to 13 years of age. In the former group, the stress of school and the increased exposure to infectious diseases may be the precipitating event that triggers the onset.
During puberty, rapid growth, increased emotional stress, and insulin antagonism of sex hormones may be implicated as contributing to development of diabetes. DM occurs in both sexes with equal frequency. The disease is more difficult to manage in childhood because the patients are growing, expend a great deal of energy, have varying nutritional needs, and face a lifetime of diabetic management. Young children with type 1 often do not demonstrate the typical “textbook” picture of the disorder. The initial diagnosis may be determined when the child develops ketoacidosis. Therefore the nurse must be particularly astute in subjective and objective observations.
Manifestations.
Children with diabetes mellitus present a classic triad of symptoms: polydipsia, polyuria, and polyphagia. The symptoms appear more rapidly in children. The patient complains of excessive thirst (polydipsia), excretes large amounts of urine frequently (polyuria), and is constantly hungry (polyphagia). An insidious onset with lethargy, weakness, and weight loss is also common. The child who is toilet trained may begin wetting the bed or have frequent “accidents” during play periods, may lose weight, and is irritable. The skin becomes dry. Vaginal yeast infections may be seen in the adolescent girl. There may be a history of recurrent infections. The symptoms may remain unrecognized until an infection becomes apparent or coma results. Laboratory findings indicate glucose in the urine (glycosuria). Hyperglycemia (hyper, “above,” gly, “sugar,” and emia, “blood”) is also apparent. Hyperglycemia occurs because glucose cannot enter the cells without the help of insulin, and therefore glucose remains in the bloodstream. The cells use protein and fat for energy; therefore protein stores in the body are depleted. The lack of glucose in the cells triggers the body to develop polyphagia and the increase in glucose intake further increases glucose levels in the blood. Hyperglycemia is the cause of the many complications associated with uncontrolled diabetes mellitus.
The honeymoon period.
When type 1 is initially diagnosed and the child is stabilized by insulin dosage, the condition may appear to improve. Insulin requirements decrease and the child feels well. This phenomenon supports the parent’s phase of “denial” in accepting the long-term diagnosis of DM for their child. The “honeymoon period” lasts a short time (a few months), and parents must be encouraged to closely monitor blood glucose levels to prevent complications.
Diagnostic Blood Tests
Blood glucose.
A random blood glucose level may be obtained at any time and requires no preparation of the patient. The results should be within the normal limits for both nondiabetic patients and diabetic patients who have good control of their disease.
Fasting blood glucose.
A fasting blood glucose level is a standard and reliable test for diabetes. The blood glucose level is measured in the fasting patient, usually immediately upon awakening in the morning. The results of the test will not be accurate if the patient is receiving a dextrose intravenous solution. If the child is known to have diabetes, food and insulin are withheld until after the test. If a person’s fasting blood glucose level is greater than 126 mg/dL on two separate occasions and the history is positive, the patient is considered to have diabetes mellitus and requires treatment.
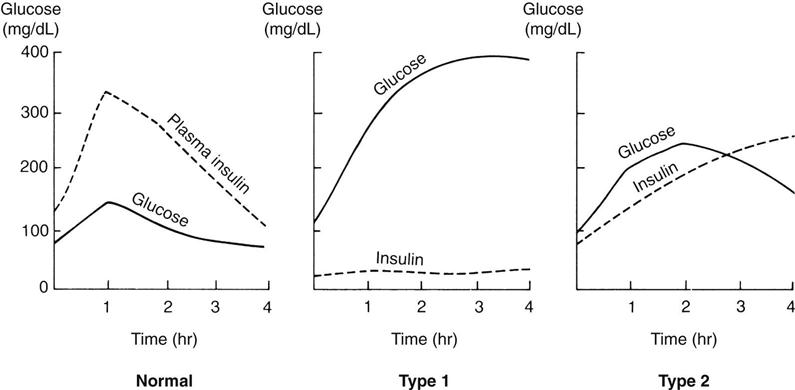
Glucose tolerance test.
Another test to determine the amount of glucose in the blood is the glucose tolerance test (GTT). The results are plotted on a graph (Figure 31-3). This procedure is time-consuming, and therefore is no longer in routine use since the glycosylated hemoglobin test is fast and accurate and reveals longer-term elevation of glucose levels. A blood glucose concentration greater than 200 mg/dL is considered positive. Normal values may not return for more than 3 hours.
Glycosylated hemoglobin test.
The glycosylated hemoglobin test (HgbA1c) reflects glycemic levels over a period of months. Values are found to be elevated in virtually all children with newly diagnosed diabetes. This study also helps to confirm the results of blood and urine tests done either at home or by the physician. Glucose in the bloodstream constantly enters red blood cells and links with, or glycosylates, molecules of hemoglobin. The more glucose in the blood, the more hemoglobin becomes coated with glucose. The red blood cells carry this glucose until they are replaced by cells with fresh hemoglobin. This process takes about 3 to 4 months. Values vary according to the measurement used. Values of 6% to 9% represent very good metabolic control. Values above 12% indicate poor control.
The future of testing.
The Human Genome Project has identified the genes responsible for many diseases. Because DM is considered an autoimmune response, it is possible to predict the occurrence risk of DM by use of genetic, immunological, and metabolic markers. Research is ongoing concerning the screening techniques and preventive immunotherapy. C-peptide (connecting chains of insulin peptides) may also be measured to determine how much insulin the body is producing (endogenous). This is of particular value during the honeymoon period of the disease.
Diabetic Ketoacidosis.
Diabetic ketoacidosis (DKA) is also referred to as diabetic coma, although a person may have DKA with or without being in a coma. It may result if a patient with diabetes contracts a secondary infection and does not follow proper self-care. It may also occur if the disease proceeds unrecognized; this happens fairly often in children with diabetes. Even minor infections, such as a cold, increase the body’s metabolic rate and thereby change the body’s demand for insulin and the severity of diabetes. Ketoacidosis is the end result of the effects of insulin deficiency.
Symptoms of ketoacidosis are compared with those of hypoglycemia in Table 31-4; signs and symptoms include a fruity odor to the breath, nausea, decreased level of consciousness and dehydration. Lab values include ketonuria, decreased serum bicarbonate concentration (decreased CO2 levels) and low pH, and hypertonic dehydration. Diabetic teaching should include this information. The symptoms range from mild to severe and occur within hours to days.
Table 31-4
Hyperglycemia and Hypoglycemia
| HYPERGLYCEMIA (KETOACIDOSIS) | HYPOGLYCEMIA |
| Cause | |
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |