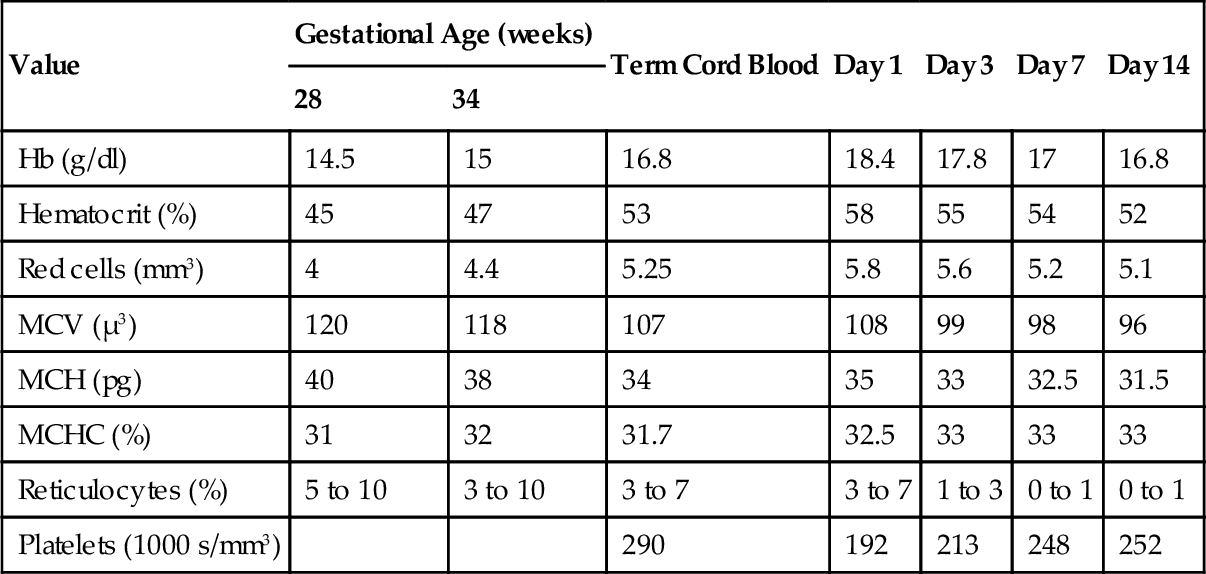
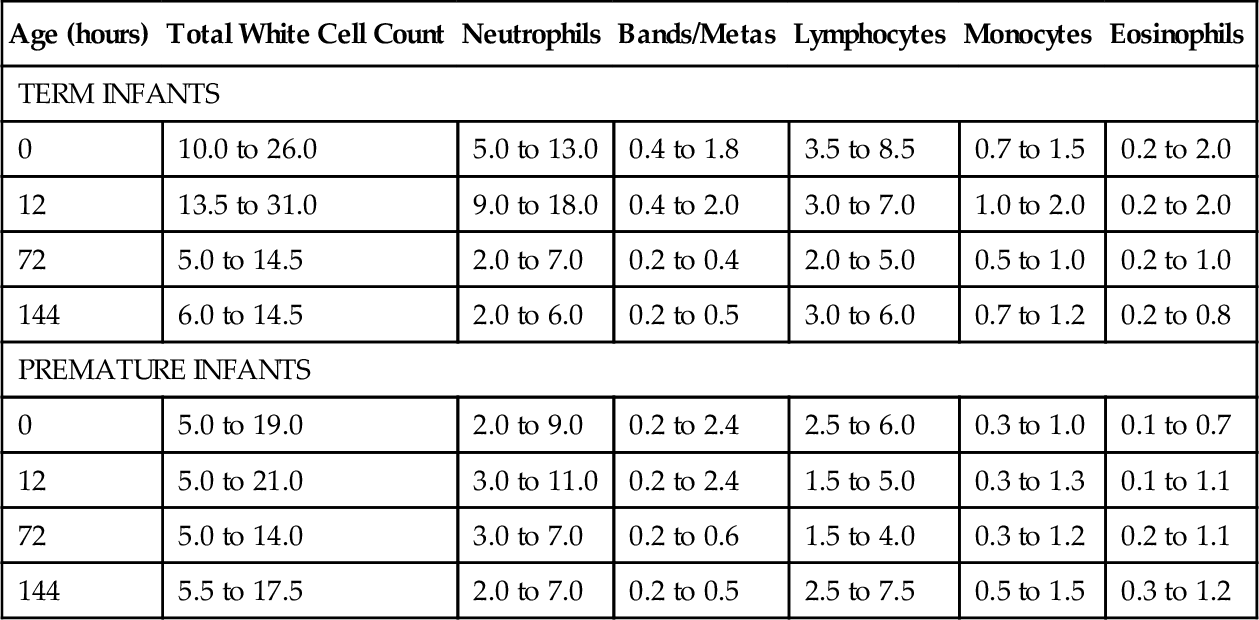
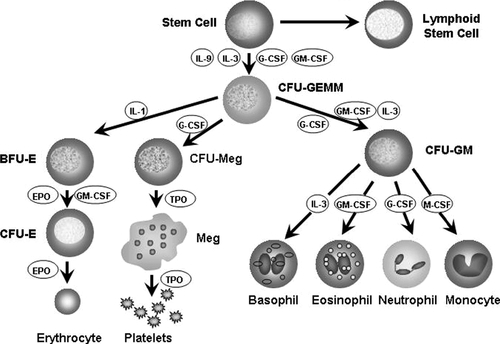
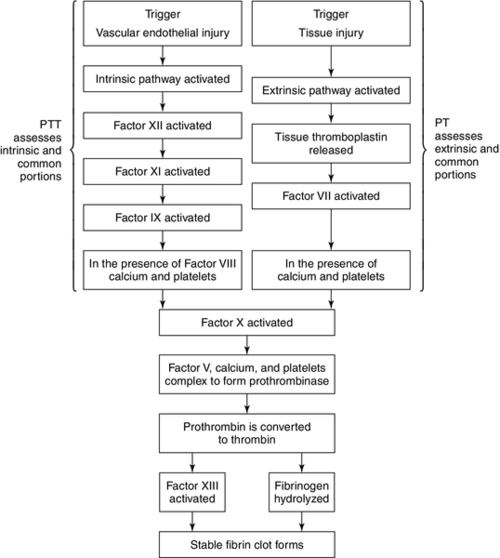
CHAPTER 31 William Diehl-Jones; Debbie Fraser 1. Understand the processes of hematopoiesis and erythropoiesis. 2. Recall erythrocyte and leukocyte development from pluripotent stem cells. 3. Relate the consequences of anemia to the management of the infant. 4. Evaluate the clinical presentation of disseminated intravascular coagulation in relation to the coagulation consumption and fibrinolysis. 5. Describe the etiologic factors of hemorrhagic disease of the newborn. 6. Describe key indicators for nursing assessment of the thrombocytopenic infant. 7. Evaluate the neonatal consequences of maternal immune thrombocytopenic purpura. 8. Discuss the role of partial exchange transfusion in the treatment of neonatal polycythemia. 9. Describe current recommendations for use of blood components. 10. Analyze the components of the complete blood cell count and describe the usefulness of each in the determination of neonatal sepsis. To meet the objectives, this chapter presents an overview of blood cell development and coagulation factors, and includes normal birth values and common diagnostic tests. Blood products and transfusion therapies are discussed with current recommendations for use. Common hematologic problems and therapies affecting the newborn infant are outlined. An evaluation of the red blood cell (RBC) indices, useful for diagnosis of hematologic disorders, is included. A. Hematopoiesis: formation, production, and maintenance of blood cells (Blackburn, 2013; Blanchette et al., 2005; Brugnara and Platt, 2009; Luchtman-Jones and Wilson, 2011; Ohls, 2011). B. Fetal hematopoiesis: occurs in three distinct phases, mesoblastic, hepatic, and myeloid. 2. Primitive erythroblasts arising from megaloblasts are detectable in circulating blood between 21 and 35 days of gestation. 3. Extravascular liver hematopoiesis begins with migration of pluripotent stem cells from the yolk sac, well established by week 5 or 6 of gestation. 4. Liver hematopoiesis peaks at 3 to 6 months of gestation and then slowly regresses as medullary (bone marrow) hematopoiesis predominates from 22 weeks of gestation. 5. Sites of extramedullary hematopoiesis (spleen, lymph nodes, thymus, kidneys) aid production of cells during fetal life when long bones are small. 6. Pluripotent cells develop into either colony-forming unit–granulocyte, erythrocyte, monocyte, macrophage (CFU-GEMM), colony-forming unit–erythroid (CFU-E), or colony-forming unit–granulocyte-macrophage (CFU-GM) cell lines, which evolve into specific cell lines (Fig. 31-1). 7. Hypoxia, bacterial infection, and other forms of physiologic stress can influence the rate of differentiation of pluripotent cells. 8. Hematopoietic factors include interleukins (ILs; e.g., IL-1 through IL-4, IL-6 through IL-8, and IL-10 through IL-12), growth and differentiation factors such as granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), thrombopoietin, and erythropoietin. C. Erythropoiesis: production of erythrocytes (RBCs). 2. Erythropoiesis and synthesis of hemoglobin are regulated by a hormone, erythropoietin, which is in turn regulated by hypoxia-sensing mechanisms in the fetal liver and kidney. 3. Erythropoietin is produced postnatally in the kidneys, but during fetal life extrarenal sites (liver, submandibular glands) predominate. 4. Erythropoietin levels are increased in response to anemia and low oxygen availability to tissues and are decreased in response to hypertransfusion. 5. Erythropoietin levels are also elevated in infants with Down syndrome or intrauterine growth restriction, and those born to women with diabetes or pregnancy-induced hypertension. D. Hemoglobin: major iron-containing component of the RBCs. 1. Hemoglobin carries oxygen from the lungs to the tissue cells through the circulation. 2. Hemoglobin synthesis begins around 14 days of embryonic life. 3. Transition from predominant production of fetal hemoglobin (HbF) to production of adult hemoglobin (HbA) begins at the end of fetal life. RBCs contain 70% to 90% HbF at birth. The switch to production of HbA is delayed in cases of maternal hypoxia or fetal growth restriction, and in infants of diabetic mothers. 4. Hemoglobin binds with 2,3-diphosphoglycerate (2,3-DPG), releasing an oxygen molecule. a. HbF has far less affinity for 2,3-DPG than does HbA, resulting in a greater affinity for oxygen. b. Levels of 2,3-DPG are directly proportional to gestational age. 5. Normal birth values (Table 31-1). b. Peripheral vasoconstriction and stasis yield higher values from capillary samples. c. Hemoglobin levels are higher in newborns, and decrease by the end of the first week of life to values similar to cord blood. d. Increased PaO2 following delivery and increase in HbA causes a decrease in erythropoietin, leading to a gradual decline in hemoglobin (Luchtman-Jones and Wilson, 2011). E. Hematocrit: percentage of RBCs in a unit volume of blood. 1. Values rise immediately after birth and then decline to cord levels in the first week. 2. Normal birth values (see Table 31-1). b. Peripheral vasoconstriction and stasis yield higher values from capillary samples. F. RBCs. 1. The erythrocyte BFU-E differentiates, under hormonal control, to form a CFU-E, which loses its nucleus as it forms erythrocytes (see Fig. 31-1). 2. The CFU-Es (or reticulocytes), in the absence of physiologic stress, mature 1 to 2 days in the bone marrow and then another day in the circulation before maturing to erythrocytes. a. Reticulocyte count is inversely proportional to gestational age at birth (see Table 31-1) but falls rapidly to less than 2% by 7 days. b. Persistent reticulocytosis may indicate chronic blood loss or hemolysis. 3. RBC function. a. Oxygen transport via oxyhemoglobin. b. Carbon dioxide transport via carboxyhemoglobin. c. Carbon dioxide reacts with water to form carbonic acid; reaction catalyzed by carbonic anhydrase in the cytoplasm of RBCs. d. Carbonic acid dissociation to form bicarbonate ions. e. Buffering of protons via binding with hemoglobin to form acid hemoglobin and by reaction with bicarbonate ions. 4. RBC count. a. Number of circulating mature RBCs per cubic millimeter (see Table 31-1). b. Count equals production versus destruction or loss. c. RBC life span proportional to gestational age. (2) Term infant: 60 to 70 days. (3) Premature infant: 35 to 50 days. d. Nucleated RBCs are circulating immature (prereticulocyte) RBCs. (1) Number is inversely proportional to gestational age and declines rapidly in the first week. (2) Increase may indicate hemolysis, acute blood loss, hypoxemia, congenital heart disease, or infection. 5. RBC indices: measure of RBC size and hemoglobin content used for designation of anemias (see Table 31-1). a. Mean corpuscular volume (MCV): average size and volume of a single RBC. (2) Increased MCV: RBCs referred to as macrocytes. (3) Decreased MCV: RBCs referred to as microcytes. b. Mean corpuscular hemoglobin (MCH): average amount (by weight) of hemoglobin in each RBC. (1) A decrease in MCH parallels a decrease in MCV. (2) Increased MCH: RBCs appear hyperchromic. (3) Decreased MCH: RBCs appear hypochromic. c. Mean corpuscular hemoglobin concentration (MCHC): average concentration of hemoglobin per single RBC, calculated from the amount of hemoglobin per deciliter of cells. (1) Adult values for MCHC reached by 6 months. (2) Increased MCHC: RBCs appear hyperchromic. (3) Decreased MCHC: RBCs appear hypochromic. d. Erythrocyte mass: total mass of erythrocytes. (2) Direct correlation between erythrocyte mass and hemoglobin concentration. (3) Gold standard is use of chromium-labeled erythrocytes. G. White blood cells (WBCs). 1. Leukocyte precursors mature in the bone marrow and lymphatic tissues, in the absence of physiologic stress, through the CFU-GEMM and CFU-GM stages (see Fig. 31-1). 2. WBCs can leave the circulation to enter the extravascular tissues, where they function as an important part of the immunologic system in reaction to foreign protein. 3. Granulocytes, lymphocytes, and monocytes are types of WBCs. a. Granulocytes: include basophils, eosinophils, and neutrophils. (a) Important in allergic and inflammatory responses. (b) Least numerous of the granulocytes: 0.5% to 1% of total WBC count. (2) Eosinophils. (a) Perform similar functions as neutrophils but are less effective in response. (b) Unlike neutrophils, can survive for prolonged periods in extravascular space. (c) Important in allergic and anaphylactic responses and most effective granulocyte for parasitic destruction. (d) Benign eosinophilia of prematurity, inversely proportional to gestational age, may reflect immaturity of barrier mechanisms in the gastrointestinal and/or respiratory tract (Blanchette et al., 2005). (e) Normally comprise 1% to 3% of total WBC count. (3) Neutrophils. (b) Physiologic stress can increase production and bone marrow release of immature forms. (c) Neutrophils are increased at birth but decrease during the first week to reach percentages approximately equal to those of lymphocytes. b. Lymphocytes. (2) Bone marrow–derived (B) lymphocytes: important in the production and secretion of immunoglobulins and antibodies. c. Monocytes. (1) Circulating immature macrophages. (2) Transformed into macrophages in tissues (i.e., lung—alveolar macrophages; liver—Kupffer cell macrophages). (3) Responsible for clearance of old blood cells, cellular debris, opsonized bacteria, antigen–antibody complexes, and activated clotting factors from the circulation. 4. WBC count. a. WBC count is the number of circulating WBCs per cubic millimeter (Table 31-2). b. WBC count is proportional to gestational age, with the total counts of premature infants approximately 30% to 50% lower than those of term infants. H. Platelets. 1. Small, nonnucleated, disk-shaped cells aid in hemostasis, coagulation, and thrombus formation. a. Platelets are derived from megakaryocytes in the bone marrow. b. Disrupted endothelium stimulates platelet plug formation and initiates hemostasis. 2. After release into the bloodstream, platelets will circulate 7 to 10 days before removal by the spleen. In the absence of injury, they circulate freely, without wall adhesion or aggregation with other platelets. 3. Normal range is 150,000 to 400,000/mm3 in the term infant. Premature infants have a lower platelet count with a broader range of normal (100,000 to 450,000) (Nguyen-Vermillion and Juul, 2012). Counts are 20% to 25% lower in infants who are small for gestational age. 4. Neonatal platelets are hypoactive in the first few days after birth; this property protects against thrombosis but may increase risk of bleeding and coagulopathy. I. Blood volume. 1. Volume of blood is measured in milliliters per kilogram of body weight. 2. Factors affecting blood volume are as follows: (1) Term infant: approximately 80 to 100 mL/kg. (2) Preterm infant: approximately 90 to 105 mL/kg. b. Placental transfusion. (2) Position of infant relative to placenta (above or below) before cord clamping. (3) Timing and strength of uterine contractions. (4) Onset of respiration and decrease in pulmonary vascular resistance. (5) Cord compression. c. Maternal–fetal or fetal–maternal transfusion. d. Twin-to-twin transfusion. e. Placenta previa or abruptio placentae. f. Nuchal cord. g. Iatrogenic loss. Hemostasis is accomplished by biochemical and physiologic events initiated to stop the flow of blood when vessel injury occurs (Young, 2012). A. Deficiencies in newborn clotting mechanisms. 1. Transient diminished platelet function. 2. Transient deficiency of clotting factors II, VII, IX, X, XI, and XII. Neonatal levels are approximately 50% of adult levels in the early weeks after birth (Blackburn, 2013). a. Immaturity of hepatic enzymes responsible for production. b. Transient deficiency of vitamin K, needed for synthesis of factors II, VII, IX, and X. c. Factor concentrations: proportional to gestational age. B. Hemostatic mechanisms. 1. Vascular: damaged vessel contracts, minimizing blood loss. 2. Intravascular: platelet plug formation. Platelet function is stimulated by exposure to damaged endothelial lining. Platelets: a. Swell and develop thornlike projections. b. Adhere to subendothelial fibers. c. Secrete adenosine diphosphate to trigger swelling and adhesiveness in nearby platelets. d. Aggregate and form platelet plug. 3. Extravascular. a. Compression by surrounding tissue. b. Release of tissue thromboplastin by injured tissue. C. Coagulation process. 1. Cascade of events, requiring both cellular and plasma components (Fig. 31-2). 2. Culminates in the formation of fibrin-based clots, and requires serial activation of precursor zymogens. 3. Calcium, iron, and phospholipids are key components of the coagulation cascade. a. Extrinsic system triggered by tissue injury and exposure of cell membrane tissue factor. b. Intrinsic system triggered by vascular endothelial injury; amplifies factor X activation, which is a cofactor common to intrinsic and extrinsic pathways. c. Factor X activation begins the process of prothrombin-to-thrombin conversion. Conversion hydrolyzes fibrinogen (soluble protein in plasma) to fibrin (insoluble, threadlike polymer) and activates factor XIII, stabilizing fibrin threads into a meshwork to trap platelets and other cells to form the clot. 4. Intravascular clotting is balanced by concurrent fibrinolysis. b. Plasmin begins fibrin clot dissolution, releasing fibrin degradation products (FDPs), also called fibrin split products (FSPs), into the circulation. c. FDPs exert an anticoagulant effect by interfering with clot formation and the function of platelets, thrombin, and fibrinogen. D. Coagulation tests (Kenet et al., 2010) (Table 31-3). 1. Platelet count is used to assess platelet number. 2. Prothrombin time (PT) is used to assess extrinsic and common portions of the coagulation cascade. In neonates, a prolonged PT reflects decreased vitamin K–dependent clotting factors. 3. Partial thromboplastin time (PTT) is used to assess intrinsic and common portions of the coagulation cascade. A prolonged PTT in a neonate reflects a decrease in both vitamin K–dependent factors and contact factors (XI, XII, prekallikrein). 4. Fibrinogen level is used to assess the circulating level of this protein substrate, required for clot formation. The presence of fetal fibrinogen may result in falsely elevated fibrinogen levels in the newborn. 5. FDP/FSP level is used to assess fibrinolytic activity. 6. Individual clotting factors may be assayed, depending on results of the tests cited above. TABLE 31-3 Normal Values for Tests of Hemostasis From Nathan, D.G., Orkin, S.H., Ginsberg D., and Look, A.T. (Eds.): Nathan and Oski’s hematology of infancy and childhood (6th ed.). Philadelphia, 2003, W.B. Saunders, p. 1855. Ag, Antigenic value; APTT, activated partial thromboplastin time; c, coagulant activity; HMWK, high-molecular-weight kininogen; INR, International Normalized Ratio; PK, protein kinase; PT, prothrombin time; TCT, thrombin clotting time. Values are the mean, followed in parentheses by the lower and upper boundaries including 95% of the population. * P = 0.05. † P = 0.01. E. Physiologic anemia of infancy (Aher et al., 2008; Kates and Kates, 2007). Low hemoglobin concentration and/or decreased number of RBCs diminishes the oxygen-carrying capacity of the blood and the level of oxygen available to the tissues. Anemia at birth can be classified into three major causes: (1) the result of blood loss (hemorrhage); (2) shortened RBC survival, including hemolysis; or (3) underproduction of erythrocytes (Manco-Johnson et al., 2011). 1. Hemorrhage: accounts for 5% to 10% of severe neonatal anemia (Aher et al., 2008). (2) Traumatic amniocentesis. (3) External cephalic version. b. Twin-to-twin: 15% to 30% of monochorionic twins (Manco-Johnson et al., 2011). (1) Monozygotic, monochorionic (single) placenta. (2) Hemoglobin difference between twins greater than 5 g/dL (50 g/L). c. Placental/cord. (2) Cord or placental hematoma. (3) Anomalous cord insertion. (4) Rupture of anomalous vessels of cord or placenta. (5) Accidental incision of cord or placenta. (6) Placenta previa or abruptio placentae. d. Internal. (1) Intracranial (subdural, subarachnoid, intraventricular), subgaleal. (2) Organ rupture (liver, spleen, adrenal, kidney). (3) Pulmonary. e. External. (2) Iatrogenic (e.g., catheter losses). 2. Hemolysis. a. Blood group incompatibilities. (1) Rh incompatibility: erythroblastosis fetalis (Liley, 2009; Manco-Johnson et al., 2011). (b) Predisposing factors. (i) Previous pregnancy or abortion. (ii) Fetal–maternal hemorrhage during pregnancy. (iii) Delivery (vaginal, breech, cesarean). (iv) Amniocentesis, chorionic villus sampling. (v) External version. (vi) Manual removal of placenta. (c) Infant presentation. (i) Anemia (caused by hemolysis, resulting in increased production of very immature RBCs). (ii) Tissue hypoxia, acidosis (decreased RBC count and decreased oxygen-carrying capacity of immature cells). (iii) Congestive heart failure and hydrops fetalis (fetus attempts to expand blood volume and cardiac output, resulting in generalized edema). (iv) Ascites, pleural effusion (fluid collecting in large cavities). (v) Hepatosplenomegaly (increased extramedullary hematopoiesis). (vi) Petechiae (thrombocytopenia accompanying severe anemia). (vii) Hypoglycemia (increased RBC destruction stimulates insulin secretion, resulting in hyperplasia of pancreatic islets and hyperinsulinemia). (viii) Positive direct Coombs’ test result. (d) Prophylactic therapy: anti-D immune globulin. (ii) Destruction of fetal RBCs in maternal circulation, blocking maternal antibody production. (iii) Ninety percent effective in prevention of sensitization. (iv) Recommended administration at 28 weeks of gestation, within 72 hours after delivery, and after amniocentesis, chorionic villus sampling, percutaneous umbilical blood sampling, or evidence or possibility of fetal–maternal hemorrhage. (2) ABO incompatibility (Manco-Johnson et al., 2011; Matthews and Glader, 2012). (a) More frequently occurring but less severe hemolytic disease than with Rh incompatibility. (b) Most often seen in mothers with O blood type (absence of antigen) carrying fetus with A or B blood type (see Table 31-4 for other potential incompatibilities). TABLE 31-4 Potential Maternal–Fetal ABO Incompatibilities (c) Maternal exposure to naturally occurring A and B antigens in food, bacteria, and pollen initiates maternal production of anti-A, anti-B antibodies and accounts for occurrence of disease with first pregnancy. (d) ABO incompatibility protects against fetal Rh disease because of rapid destruction of fetal A/B cells, preventing Rh antigen exposure and maternal antibody production. (e) Infant presentation includes the following: (i) Mild hemolysis, anemia, reticulocytosis. (ii) Hyperbilirubinemia (occasionally requiring exchange). b. Enzymatic defect: glucose-6-phosphate dehydrogenase (G6PD) deficiency (see also Chapter 29). (2) Interaction of intracellular abnormality (deficiency of RBC enzyme) and extracellular factor (exposure to oxidant stress: drugs, infection), causing hemolysis and shortened erythrocyte life. (3) Most common occurrence in African American infants (10% to 15%) and in infants of Mediterranean, African, and Asian descent. c. Hemoglobin disorders (Matthews and Glader, 2012; Steiner and Gallagher, 2007). (a) Deletion of one or more of the four α-globin genes. (b) Severity of expression is related to the number of globin genes missing. (i) Silent carrier—single gene deletion, asymptomatic. (ii) α-Thalassemia trait—deletion or dysfunction of two genes resulting in mild microcytic anemia. (iii) Hemoglobin H disease—absence or nonfunction of three globin genes. Mild to moderate hemolytic anemia exacerbated by oxidant stresses. (iv) α-Thalassemia (homozygous)—deletion of all four globin genes results in hydrops fetalis and, often, fetal death as a result of severe intrauterine hemolytic anemia. (c) Most common in infants of Southeast Asian, Middle Eastern, and Mediterranean descent. (2) β-Thalassemia. (a) Occurs as a result of deletion of β-globin gene. (b) Also most common in infants of Southeast Asian, African, or Mediterranean descent. (c) Neonates occasionally present with hemolytic anemia. (d) Does not usually present before 2 months of age because of the presence of HbF. d. Infection. Intrauterine (viral, protozoan, spirochetal) and postnatal (bacterial) infection may cause neonatal hemolysis, anemia, thrombocytopenia, and disseminated intravascular coagulation (DIC). 3. Anemia of prematurity. a. Hemoglobin concentration at birth varies only slightly in relation to gestational age. b. During the first 2 to 3 months, hemoglobin concentration falls to the lowest value that occurs at any developmental period. c. Anemia of prematurity is considered physiologic because it is characteristic of healthy infants. d. Associated factors. (1) Rates of decline and nadir are inversely proportional to gestational age. (2) Iron concentration is low because of decreased blood volume and decreased concentration of circulating hemoglobin iron. (3) Improved extrauterine oxygen delivery causes a temporarily inactive stage of erythropoiesis. (4) Erythropoietin production in response to anemia is diminished. (5) Shortened RBC life span decreases RBC mass. (6) Growth causes dilutional anemia as a result of decreased hemoglobin concentration with expanding blood volume. (7) Despite rapid hemoglobin fall, tissue oxygenation is maintained by events responsible for right shift of the hemoglobin–oxygen dissociation curve. e. Some infants do manifest symptoms of hypoxemia (poor feeding and weight gain, dyspnea, tachypnea, tachycardia, diminished activity, pallor) in the absence of other problems and require transfusion. 4. Iatrogenic postnatal phlebotomy. Critically ill infants who require frequent monitoring may have excessive amounts of blood removed for diagnostic studies, thereby inducing anemia. Removal of greater than 20% of the blood volume over 24 to 48 hours can produce anemia; in a 1500-g infant this represents approximately 25 mL (Blanchette et al., 2005). B. Clinical presentation: varies with the volume of hemorrhage and the time period over which the blood is lost. a. Pallor initially, and then cyanosis and desaturation. b. Shallow, rapid, irregular respirations. c. Tachycardia. d. Weak or absent peripheral pulses. e. Low or absent blood pressure, low venous pressure. f. Hemoglobin concentration may be normal initially, with rapid decline over 4 to 12 hours with hemodilution. 2. Chronic blood loss. a. Pallor without signs of acute distress. b. Possible signs of congestive heart failure with hepatomegaly. c. Normal blood pressure, normal or elevated venous pressure. d. Low hemoglobin concentration. C. Clinical assessment. a. Bleeding, anemia, splenectomy. b. Consanguinity. c. Ethnic and geographic origins. d. Blood group incompatibilities. e. Previous child with anemia or jaundice. 2. Maternal history. b. Late third-trimester bleeding. D. Physical examination. 1. Signs of acute or chronic blood loss, as above. 2. Jaundice. 3. Cephalohematoma. 4. Abdominal distention or mass: liver, spleen, adrenal, kidney rupture. 5. Petechiae, purpura. 6. Cardiovascular abnormalities: tachycardia, murmur, gallop rhythm. 7. Hydropic changes. E. Diagnostic studies. 1. Hemoglobin concentration: Normal hemoglobin values are dependent on gestational age, site of sampling, and timing of sampling (Brugnara and Platt, 2009). Values vary according to birth weight and postnatal age (Table 31-5). TABLE 31-5 Serial Hemoglobin Values in Low Birth Weight Infants
Hematologic Disorders
DEVELOPMENT OF BLOOD CELLS
COAGULATION
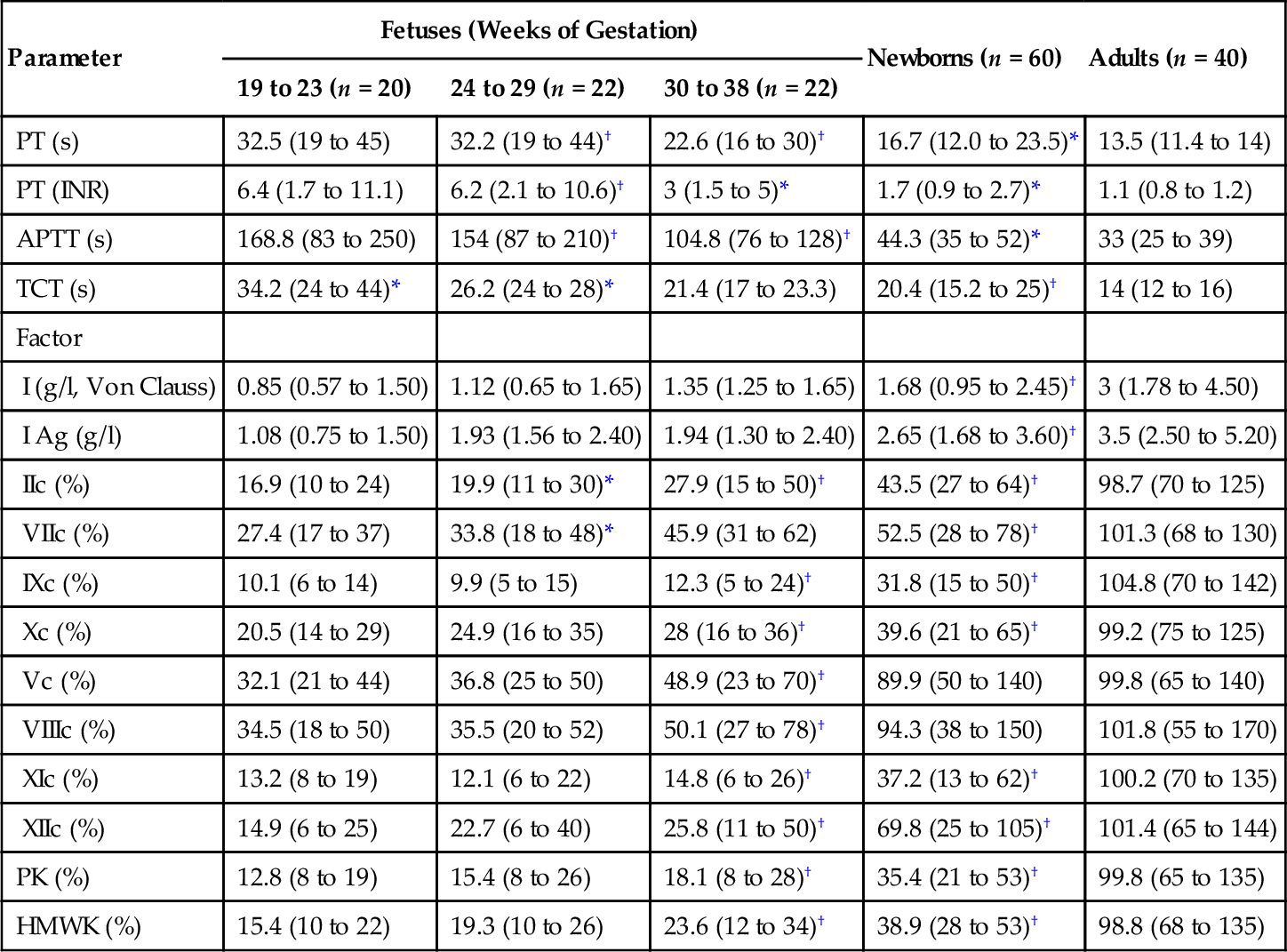
Parameter
Fetuses (Weeks of Gestation)
Newborns (n = 60)
Adults (n = 40)
19 to 23 (n = 20)
24 to 29 (n = 22)
30 to 38 (n = 22)
PT (s)
32.5 (19 to 45)
32.2 (19 to 44)†
22.6 (16 to 30)†
16.7 (12.0 to 23.5)*
13.5 (11.4 to 14)
PT (INR)
6.4 (1.7 to 11.1)
6.2 (2.1 to 10.6)†
3 (1.5 to 5)*
1.7 (0.9 to 2.7)*
1.1 (0.8 to 1.2)
APTT (s)
168.8 (83 to 250)
154 (87 to 210)†
104.8 (76 to 128)†
44.3 (35 to 52)*
33 (25 to 39)
TCT (s)
34.2 (24 to 44)*
26.2 (24 to 28)*
21.4 (17 to 23.3)
20.4 (15.2 to 25)†
14 (12 to 16)
Factor
I (g/l, Von Clauss)
0.85 (0.57 to 1.50)
1.12 (0.65 to 1.65)
1.35 (1.25 to 1.65)
1.68 (0.95 to 2.45)†
3 (1.78 to 4.50)
I Ag (g/l)
1.08 (0.75 to 1.50)
1.93 (1.56 to 2.40)
1.94 (1.30 to 2.40)
2.65 (1.68 to 3.60)†
3.5 (2.50 to 5.20)
IIc (%)
16.9 (10 to 24)
19.9 (11 to 30)*
27.9 (15 to 50)†
43.5 (27 to 64)†
98.7 (70 to 125)
VIIc (%)
27.4 (17 to 37)
33.8 (18 to 48)*
45.9 (31 to 62)
52.5 (28 to 78)†
101.3 (68 to 130)
IXc (%)
10.1 (6 to 14)
9.9 (5 to 15)
12.3 (5 to 24)†
31.8 (15 to 50)†
104.8 (70 to 142)
Xc (%)
20.5 (14 to 29)
24.9 (16 to 35)
28 (16 to 36)†
39.6 (21 to 65)†
99.2 (75 to 125)
Vc (%)
32.1 (21 to 44)
36.8 (25 to 50)
48.9 (23 to 70)†
89.9 (50 to 140)
99.8 (65 to 140)
VIIIc (%)
34.5 (18 to 50)
35.5 (20 to 52)
50.1 (27 to 78)†
94.3 (38 to 150)
101.8 (55 to 170)
XIc (%)
13.2 (8 to 19)
12.1 (6 to 22)
14.8 (6 to 26)†
37.2 (13 to 62)†
100.2 (70 to 135)
XIIc (%)
14.9 (6 to 25)
22.7 (6 to 40)
25.8 (11 to 50)†
69.8 (25 to 105)†
101.4 (65 to 144)
PK (%)
12.8 (8 to 19)
15.4 (8 to 26)
18.1 (8 to 28)†
35.4 (21 to 53)†
99.8 (65 to 135)
HMWK (%)
15.4 (10 to 22)
19.3 (10 to 26)
23.6 (12 to 34)†
38.9 (28 to 53)†
98.8 (68 to 135)
ANEMIA
Maternal Blood Group
Incompatible Fetal Blood Group
O
A or B
B
A or AB
A
B or AB
Birth Weight (g)
Hemoglobin Concentration (g/dL) by Age
2 weeks
4 weeks
6 weeks
8 weeks
10 weeks
800 to 1000
16 ± 0.6
10.2 ± 3.2
8.7 ± 1.5
8 ± 0.9
8 ± 1.1
1001 to 1200
16.4 ± 2.3
12.8 ± 2.5
10.5 ± 1.8
9.1 ± 1.3
8.5 ± 1.5
1201 to 1400
16.2 ± 1.3
13.4 ± 2.8
10.9 ± 1.2
9.9 ± 1.9
—
1401 to 1500
15.6 ± 2.2
11.7 ± 1
10.5 ± 0.7
9.8 ± 1.4
— ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


31: Hematologic Disorders
FIGURE 31-1 ■ Hematopoiesis and selected growth factors. BFU-E, Burst-forming unit, erythroid; CFU-E, colony-forming unit–erythroid; CFU-GEMM, colony-forming unit–granulocyte, erythrocyte, monocyte, macrophage; CFU-GM, colony-forming unit–granulocyte-macrophage; CFU-Meg, colony-forming unit–megakaryocyte; EPO, erythropoietin; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IL-1, interleukin-1; IL-3, interleukin-3; IL-9, interleukin-9; M-CSF, macrophage colony-stimulating factor; Meg, megakaryocyte; TPO, thrombopoietin. (Adapted from Israels, L.G. and Israels, S.J.: Mechanisms in hematology [3rd ed.]. Toronto, 2002, Core Health Sciences, Inc., p. 402.)
FIGURE 31-2 ■ Fibrin clot formation through activation of intrinsic or extrinsic pathways of coagulation process.