Health information infrastructure and systems
Meryl Bloomrosen
Objectives
After reading this chapter, the reader will be able to do the following:
Key words
Health information technology
EHR systems
National Health Information Network
Regional Health Information Organization
Abbreviations
AHRQ—Agency for Healthcare Quality and Research
AHIC—American Health Information Community
ARRA—American Recovery and Reinvestment Act
ASTM International—
CMS—Centers for Medicare and Medicaid Services
CDS—Clinical Decision Support
CCHIT—Commission on the Certification for Health Information Technology
CHI—Consolidated Health Informatics Standards
CHT—Center for Health Transformation
CITL—Center for Information Technology Leadership
CMS—Centers for Medicare and Medicaid
CPOE—Computerized Physician Order Entry
CSI—Commission on Systemic Interoperability
DHHS—Department of Health and Human Services
DICOM—Digital Imaging Communications in Medicine
DOQ-IT—Doctors’ Office Quality Information Technology
DSS—Decision Support System
EHRs—Electronic Health Records
EMR—Electronic Medical Record
ERX—Electronic Prescribing
FACA—Federal Advisory Committee Act
FAH—Federal Health Architecture
FDA—Food and Drug Administration
GAO—General Accounting Office
GPO—Government Printing Office
HIE—Health Information Exchange
HIPAA—Health Insurance Portability and Accountability Act
HISPC—Health Information Security and Privacy Collaboration
HISSB—Health Information and Surveillance Systems Board
HIT—Health Information Technology
HITECH—Health Information Technology for Economic and Clinical Health Act
HL7—Health Level 7
HRSA—Health Resources and Services Administration
HGNC—Human Gene Nomenclature
IOM—Institute of Medicine
IEEE 1073—Institute of Electrical and Electronics Engineers 1073
LOINC—Logical Observation Identifier Name Codes
MIPPA—Medicare Improvements for Patients and Providers Act
MMA—Medicare Modernization Act
NCPDP—National Council on Prescription Drug Programs
NCVHS—National Center for Vital and Health Statistics
NDF-RT—National Drug File Reference Terminology
NEHC—National eHealth Collaborative
NHII—National Health Information Infrastructure
NHIN—Nationwide Health Information Network
NPRM—Notice of Proposed Rule Making
NRC—National Resource Center
OAT—Office for Advancement of Telehealth
OMB—Office of Management and Budgets
ONC—Office of the National Coordinator for Health Information Technology
ORHP—Office of Rural Health Policy
PHI—Personal Health Information
PHR—Personal Health Record
QIO—Quality Improvement Organization
RFI—Request for Information
RHIOs—Regional Health Information Organizations
RIO—Return on Investments
SNO—Sub Network Organization
SDE—State Designated Entity
SDOS—Standards Development Organizations
XML—Extensible Markup Language
Student Study Guide activities for this chapter are available on the Evolve Learning Resources site for this textbook. Please visit http://evolve.elsevier.com/Abdelhak.
When you see the Evolve logo  , go to the Evolve site and complete the corresponding activity, referenced by the page number in the text where the logo appears.
, go to the Evolve site and complete the corresponding activity, referenced by the page number in the text where the logo appears.
Health information technology in health care
Every aspect of the health care industry is highly fragmented, which has led to untenable levels of administrative complexity and compromises in quality of care. One of the top-ranking challenges faced by most providers is the timeliness and efficiency of access to patient information. Patient data are most frequently recorded and stored in paper-based systems and shared across organizations every day through low-technology modes such as fax, mail, and courier, leading to unnecessarily high labor and nonlabor expenses. Patients are misdiagnosed and mistreated because of a lack of timely and easily available information at the point of care.*†1
The earliest activities devoted to health information technology (HIT) implementation focused on the automation of administrative functions such as billing and patient registration. Subsequently, attention has moved to address reporting requirements such as those needed for patient safety and quality assurance reporting. Later, attention turned to automating other record keeping and administrative functions such as laboratory results reporting or order entry. Other efforts have focused on automating various clinical care processes such as computerized physician order entry (CPOE) and clinical decision support (CDS). Most recently, activities are addressing how to provide for the exchange of clinical and administrative information and to facilitate industry use of more standardized electronic communications across organizations.
Efforts to date have largely operated independent of each other. Today, numerous efforts are underway to coordinate and integrate activities and to leverage lessons learned from one activity to another.2 For example, since the late 1990s, we have seen the implementation of nationwide efforts toward administrative simplification under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the related rise in electronic claims processing. In February 2009, Congress passed the American Recovery and Reinvestment Act (ARRA), which includes provisions relating to Health Information Technology. Title XIII of Division A and Title IV of Division B together are known as the Health Information Technology for Economic and Clinical Health Act or the HITECH Act. The ARRA legislation provided an unprecedented level of funding to the federal government agencies to help stimulate the U.S. economy. The federal government will develop standards for HITECH, and the new HITECH law states that ‘[T]he standards, implementation specifications, and certification criteria recommended under this subsection shall be consistent with the standards for information transactions and data elements adopted pursuant to section 1173 of the Social Security Act [Standards to Enable Electronic Exchange]” [Section 3003(b)(1)(D) Consistency, Congressional Record-House, February 12, 2009, p. H1340]. The referenced standards include the HIPAA standards.
It has long been recognized that providers need data to treat their patients and to choose among treatment modalities: payers require data to verify eligibility for treatment and to determine medical necessity for care, researchers need data for various outcomes measurement projects, and regulators and policy makers require data to make prudent and cost-effective decisions that will ensure the public health and well-being of our country’s citizens. The national policy agenda for HIT, health information exchange (HIE), and electronic health records (EHRs) in the public and private sectors has been developing rapidly and is seen as a top priority.3 These policy discussions have concluded that health care data and information should be universally digitized. Improving patient safety and increasing the quality of health care are primary motivating factors in most public and private sector efforts. Increasingly multiple and previously diverse activities are being coordinated and leveraged together so that ideally we can all yield the health, economic, and social benefits that are optimistically promised.
There is an increasing body of literature discussing the benefits and challenges in HIT implementations in various settings.1–3 Sometimes the research has focused on “stand-alone” functions, other times as a component of an EHR.
Reaping the benefits of an EHR application is in part dependent on successful implementation, and implementation raises a host of technical, clinical, and organizational issues that must be confronted before users are likely to accept and adopt the EHR.
This peak in activity seemed to be propelled with President George Bush’s State of the Union Address in 2004, followed by the creation of the Department of Health and Human Services (DHHS) Office of the National Coordinator for HIT (ONC) and the Release of the Strategic Framework, the federal funding of HIT projects by several agencies of the DHHS, and the numerous bills introduced in Congress that address various aspects of HIT and HIE. The National Health Information Infrastructure concept envisions providing electronically the information necessary for decision making at the time it is needed, in the place where it is needed, and to the people who need it. It would connect physicians, hospitals, health care purchasers and payers, researchers, public health professionals, and consumers. It is a comprehensive knowledge-based network of interoperable systems of clinical, public health, and personal health information.4 Early attention to the importance of improving access to health information led to increasing efforts for the creation of a National Health Information Network (NHIN), an endeavor working toward a vision of an effective, efficient, high-quality health care system that harnesses the power of shared data to improve the health of all Americans. Subsequently, the concept of the NHIN was defined by DHHS as an Internet-based architecture that links disparate health care information systems together to allow patients, physicians, hospitals, community health centers, and public health agencies across the country to share clinical information securely.5
The NHIN is a collection of standards, protocols, legal agreements, specifications, and services that enables the secure exchange of health information over the Internet. ONC began to develop the NHIN in 2004. The first phase included development of prototype architectures, and the second phase developed specifications and services and developed working constructs. As of December 2009, the NNHIN operates as a “network of networks” that connects diverse entities that need to exchange health information, such as state and regional health information exchange organizations (RHIOs), integrated delivery systems, federal agencies, and other networks. The NHIN is a key component of the nationwide health information technology strategy and will provide a common platform for health information exchange across diverse entities, within communities and across the country, helping to achieve the goals of the HITECH Act. This critical part of the national HIT agenda will enable health information to follow the consumer, be available for clinical decision making, and support appropriate use of health care information beyond direct patient care to improve public health.

The NHIN is evolving to meet the emerging needs of those needing to exchange health information securely over the Internet. The desired outcome is to promote a more effective marketplace, greater competition, and increased choice through accessibility to accurate information on health care costs, quality, and outcomes.
In the Trial Implementation stage, the American Health Information Community (AHIC) use cases became priorities for the NHIN. The program focused on identifying and establishing standards and policies with which to lay a foundation for interoperable health information exchange. An initial set of specifications of those standards and policies has been developed and adopted by several vendors.
The Federal Health Architecture (FHA) is an E-Government Line of Business initiative managed by the Office of the National Coordinator for Health IT6. FHA was formed to coordinate health IT activities among the more than 20 federal agencies that provide health and health care services to citizens.
FHA and its federal partners are helping build a federal health information technology environment that is interoperable with private sector systems and supports the President’s plan to enable better point-of-service care, increased efficiency, and improved overall health in the U.S. population.
CONNECT is an open source software solution that supports health information exchange—both locally and at the national level. CONNECT uses Nationwide Health Information Network (NHIN) standards and governance to make sure that health information exchanges are compatible with other exchanges being set up throughout the country.
This software solution was initially developed by federal agencies to support their health-related missions, but it is now available to all organizations and can be used to help set up health information exchanges and share data using nationally-recognized interoperability standards.7 The CONNECT solution was built by federal agencies in response to their need to share health data among themselves and with other levels of government and the private sector using the Nationwide Health Information Network (NHIN).
Rather than have each federal agency independently build its own NHIN-compliant gateway solution, they banded together through the Federal Health Architecture (an E-Gov initiative) to build CONNECT. The CONNECT project team brought together more than 20 federal agencies to define project needs, it developed the solution, demonstrated its viability for connecting federal and non-federal health organizations, and it made the solution available to the public in less than a year. The Nationwide Health Information Network “trust fabric” is established via the combination of operating procedures, the data use and reciprocal sharing agreement (DURSA) and the Nationwide Health Information Network service interface specifications. The DURSA is the legal basis for the trust fabric, the operating procedures encapsulate Nationwide Health Information Network-specific operating policies forming the operational and management basis for trust, and the Nationwide Health Information Network service interface specifications are the technical basis of trust in the Nationwide Health Information Network. Federal agencies using CONNECT must adhere to FISMA (Federal Information Security Management Act of 2002) requirements in addition to meeting the HIPAA requirements.
Background
For decades, the need for an improved HIT infrastructure has been a recurring theme in the Institute of Medicine (IOM) reports, recommendations by the National Committee on Vital and Health Statistics (NCVHS), and innumerable testimonies before congressional committees.
In the IOM’s report, “Crossing the Quality Chasm”8 the members of the institute, among other things, recommended the following:
Recommendation 19: Congress, the executive branch, leaders of health care organizations, public and private sector purchasers, and health informatics associations and vendors should make a renewed national commitment to building an information infrastructure to support health care delivery, consumer health, quality measurement and improvement, public accountability, clinical and health services research, and clinical education. This commitment should lead to the elimination of most handwritten clinical data by the end of the decade.
In particular, HIT was identified as a critical environmental force that would significantly improve health care quality. Numerous activities in both the public and private sectors were quickly expanded.9,10 Some of the more notable events are described subsequently.
In July 2003, the DHHS Agency for Healthcare Research and Quality (AHRQ) convened a diverse group of approximately 50 experts who helped the agency to identify gaps in knowledge relating to the use of HIT and provided recommendations on important thematic areas. Among the panel’s many recommendations were the need for more research on the impact of HIT on important health-related outcomes; more research on HIT in diverse health care settings; the need to support local and regional HIT collaborative projects that would lead to standards-based data sharing across health care delivery sites; the need to demonstrate the value of HIT in improving patient safety and quality of care, including direct/indirect and tangible/intangible benefits; the need to study incentives and disincentives to the adoption and use of HIT; the need for technical assistance to providers, organizations, and communities to implement HIT successfully in their environment; and the need to develop and disseminate evidence-based, executable knowledge content and decision-support tools to support clinical decision making. The panel also encouraged collaboration between AHRQ and other federal agencies, such as the Office for Rural Health Policy and the Office for the Advancement of Telehealth (OAT) at the Health Resources and Services Administration (HRSA) and the Center for Medicare and Medicaid Services (CMS), to leverage the resources, expertise, and experiences of these diverse federal agencies and increase the program’s success. Finally, the panel stressed the need for developing collaborative partnerships and HIT programs that are viable and sustainable.11
The IOM has studied these topics and released several reports such as “Crossing the Quality Chasm” that detail these needs, and various other organizations have focused industry attention on electronic health information and information technology.12–17
Many components of the federal government touch on health care, and increasingly, federal leadership in HIT is more focused and coordinated. Although there is some integration of these efforts, until recently there has been neither a single voice for this effort nor a holistic set of goals for change. The ONC has been given the responsibility for coordinating HIT efforts throughout the federal government. As part of the outreach effort, the programs, projects, and policies that involve HIT are more focused and coordinated by one Federal component.
Federal involvement in health information technology
Congress
Congress plays a significant leadership role—bipartisan support of the use of HIT to improve health care, and their efforts have snowballed recently. Multiple bills have been introduced by both parties that include significant components related to HIT and HIE. On February 13, 2009, Congress passed the American Recovery and Reinvestment Act (ARRA) of 2009. ARRA was signed into law by President Obama on February 17, 2009. This $787 billion Recovery plan includes federal tax cuts and incentives, an expansion of unemployment benefits, and other spending on social entitlement programs. In addition, federal agencies used Recovery funds to award contracts, grants, and loans around the country. The Recovery Act’s longer-term economic investment goals included the goal to initiate a process to computerize health records to reduce medical errors and save on health care costs.
Department of health and human services
The DHHS’s involvement in HIT and health information standards has been long-standing. The goal is to develop and implement a nationwide interoperable HIT infrastructure, improve the quality and efficiency of health care, and help consumers manage their care and safety. For many years, the federal government has supported research into medical informatics and the use of computers in clinical practice with the objective of increasing the use of computers in health care. Most recently, a number of initiatives have gained considerable momentum to establish messaging and data content standards. Table 3-1 provides an overview of these activities.18
Table 3-1
SELECTED HIT/HIE FEDERAL INITIATIVES
| Consolidated Health Informatics Initiative (CHI) | DHHS |
| The CHI is one of several projects launched as part of the president’s e-Government initiative, which aims to improve government efficiency, effectiveness, and responsiveness to citizens while making it easier for citizens to obtain services and interact with the federal government. The CHI involves all federal agencies relating to health care, including the Department of Defense, Department of Veterans’ Affairs, the CMS, the Centers for Disease Control and Prevention, and the National Institutes for Health. This project will provide the basis for a simplified and unified system for sharing and revising medical information among government agencies and their private health care providers and insurers, ultimately leading to a single mechanism for making records accessible. | |
| Health Alert Network | CDC |
| HAN is a strong national program, providing vital health information and the infrastructure to support the dissemination of that information at the State and Local levels, and beyond. A vast majority of the State-based HAN programs have over 90% of their population covered under the umbrella of HAN. The HAN Messaging System currently directly and indirectly transmits Health Alerts, Advisories, and Updates to over one million recipients. The current system is being phased into the overall PHIN messaging component.a | |
| National Electronic Disease Surveillance System | CDC |
| NEDSS (National Electronic Disease Surveillance System) is an Internet-based infrastructure for public health surveillance data exchange that uses specific PHIN (Public Health Information Network) and NEDSS Data Standards. NEDSS also relies heavily on industry standards (including: standard vocabulary code sets such as LOINC, SNOMED, and HL7), policy-level agreements on data access, and the protection of confidentiality. NEDSS represents an ongoing close collaboration between the CDC and its public health partners. NEDSS is not a single, monolithic application, but a system of interoperable subsystems, components, and systems modules that include software applications developed and implemented by the CDC; those developed and implemented by State and Local health departments and those created by commercial services and vendors.b | |
| National Vaccine Program Office (NVPO)c | CDC |
| The National Vaccine Program Office is responsible for coordinating and ensuring collaboration among the many Federal agencies involved in vaccine and immunization activities. NVPO provides leadership and coordination among Federal agencies as they work together to carry out the goals of the National Vaccine Plan. The National Vaccine Plan provides a framework, including goals, objectives, and strategies, for pursuing the prevention of infectious diseases through immunizations. Additionally, NVPO staffs the National Vaccine Advisory Committee (NVAC), which was established to comply with Section 2105 of the Public Health Service Act (42 U.S. Code 300aa-5). Its purpose is to advise and make recommendations to the Director of the National Vaccine Program (currently the Assistant Secretary for Health) on matters related to program responsibilities. | |
| National Immunization Program (NIP) | |
| The program is committed to promoting the development and maintenance of state- and community-based computerized registries that capture immunization information on all children. Working with the National Vaccine Advisory Committee, NIP has identified minimum core immunization data elements that enable consistent data collection by immunization registry systems. |
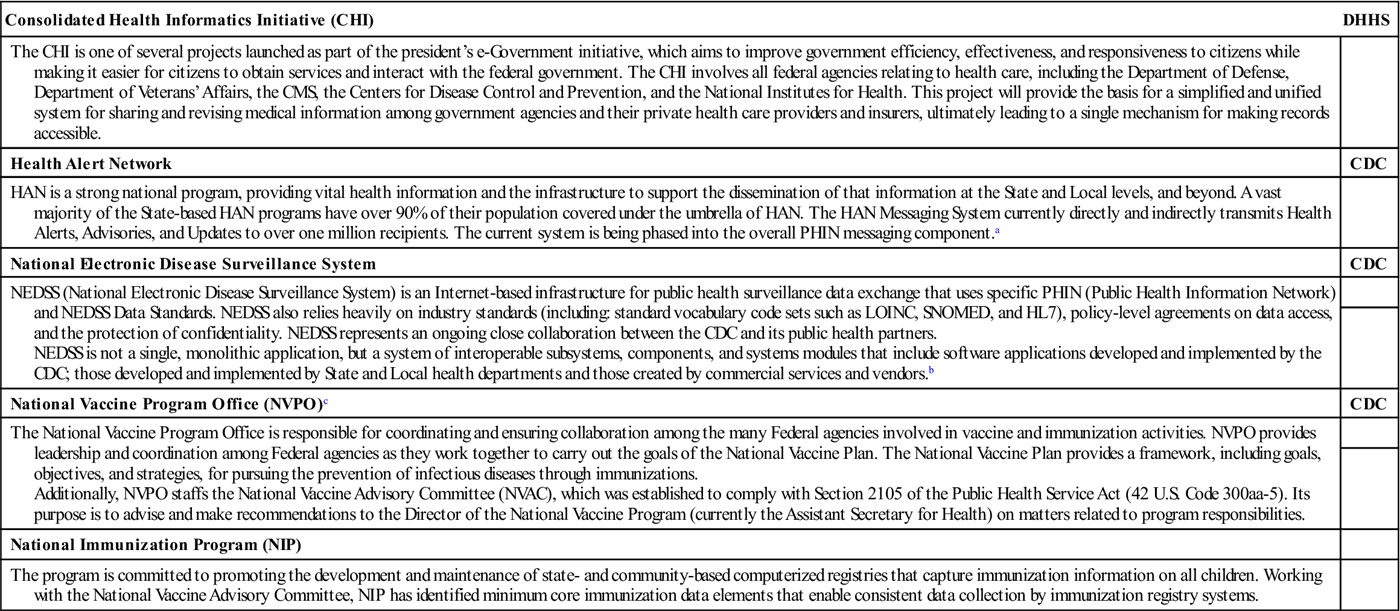
CDC, Centers for Disease Control and Prevention; DHHS, Department of Health and Human Services.
ahttp://www2a.cdc.gov/han/index.asp. Accessed 08/04/10.
bhttp://www.cdc.gov/phin/activities/applications-services/nedss/index.html. Accessed 08/04/10.
chttp://www.hhs.gov/nvpo/index.html. Accessed 08/04/10.
DHHS agencies such as the AHRQ, the Food and Drug Administration (FDA), the Health Resources and Services Administration (HRSA), the CMS, and others have made adoption of technologies a priority (please see Figure 1-1 for an updated DHHS organizational chart). In a general sense these efforts have the following purposes:
• Foster the development of data standards
• Advance the use of EHRs and accelerate secure sharing of health information among providers
The DHHS has played a leadership role in fostering the development of data standards. Standards and certification are needed to identify and harmonize technical standards related to health information exchange. To accomplish this, there is a need to oversee the development and presentation of use cases, to coordinate work with the Health IT Standards Panel (HITSP) and the NHIN effort, and to support the EHR certification efforts of groups such as the Certification Commission for Healthcare Information Technology (CCHIT). In June 2010, DHHS issued 45 CFR Part 170 – The Health Information Technology: Initial Set of Standards, Implementation Specifications, and Certification Criteria for Electronic Health Record Technology which established updated certification programs for the purposes of testing and certifying health information technology.
Eligible professionals and eligible hospitals who seek to qualify for incentive payments under the Medicare and Medicaid EHR Incentive Programs are required by statute to use Certified EHR Technology. Once certified, Complete EHRs and EHR Modules would be able to be used by eligible professionals and eligible hospitals, or be combined, to meet the statutory requirement for Certified EHR Technology.
In collaboration with ONC, the National Institute of Standards and Technology (NIST) is developing the functional and conformance testing requirements, test cases, and test tools to support the proposed Health IT Certification Programs. These conformance test methods (test procedures, test data, and test tools) will help ensure compliance with the meaningful use technical requirements and standards.
In July, 2010, DHHS Secretary Kathleen Sebelius and the Office for Civil Rights (OCR) announced that the 1996 HIPAA Privacy and Security Rules will be modified to add new protections. The proposed new regulations will improve patient privacy and security in health information exchange by extending OCR’s enforcement, giving patients the right to receive their medical information electronically, and setting new limits on the use of protected health information.
Prior efforts included the Consolidated Health Informatics (CHI) Initiative and support for the National Health Information Infrastructure. Both activities have resulted in the adoption of standards for federal programs. The CHI initiative is one of the Office of Management and Budget’s (OMB) eGov initiatives. CHI is a collaborative effort to adopt health information interoperability standards, particularly health vocabulary and messaging standards, for implementation in federal government systems. About 20 departments and agencies including the DHHS, the Department of Defense, and the Department of Veteran’s Affairs, are active in the CHI governance process. The CHI began as an eGov initiative that establishes a portfolio of existing health information interoperability standards (health vocabulary and messaging) enabling all agencies in the federal health enterprise to “speak the same language” based on common enterprise-wide business and information technology architectures.19 CHI is currently managed under the Office of the National Coordinator for Health Informational Technology’s (ONC) Federal Health Architecture (FHA) Program Management Office.
The United States Health Information Knowledgebase (USHIK) is a metadata registry of health care–related data standards funded and directed by the AHRQ with management support in partnership with the CMS.
AHRQ provides and maintains this metadata registry of health information data element definitions, values, and information models that enables browsing, comparison, synchronization, and harmonization within a uniform query and interface environment.
The U.S. Health Information Knowledgebase is populated with the data elements and information models of Standards Development Organizations (SDOs) and other health care organizations, in such a way that public and private organizations can harmonize information formats with existing and emerging health care standards.
USHIK also contains data element information for government initiatives that support the use and implementation of data standards such as HIPAA, CHI initiative, and the HITSP.
USHIK employs a metadata registry methodology based on international standards in order to promote interoperability and comparability.
USHIK is housed and funded by the AHRQ with CMS and VA as strategic interagency partners.20
CHI adopted 20 uniform standards for electronic exchange of clinical information to be used across the federal health enterprise. On March 21, 2003, the DHHS and the Departments of Defense and Veterans Affairs announced the first set of uniform standards for the electronic exchange of clinical health information to be adopted across the federal government. The standards all federal agencies will adopt are as follows:
On May 6, 2004, the DHHS and the Departments of Defense and Veterans Affairs announced the adoption of 15 additional standards agreed to by the CHI initiative to allow for electronic exchange of clinical information across the federal government. The 15 new standards build on the existing set of five standards adopted by the DHHS in March 2003. The new standards agreed to by federal agencies will be used as agencies develop and implement new information technology systems.
The specific new standards were as follows:

In July 2003, DHHS asked the IOM and HL7 to design a functional model and standard for the EHR. DHHS also announced that the department has signed an agreement with the College of American Pathologists to license the college’s standardized medical vocabulary system and make it available without charge throughout the United States. The IOM issued its report, titled “Key Capabilities of an Electronic Health Record System,” on July 31, 2003. In October 2003, the General Accounting Office released a report called “Information Technology. Benefits Realized for Selected Health Care Functions.”
In September 2004, the AHRQ awarded $139 million in contracts and grants to promote the use of HIT through the development of networks for sharing clinical data and projects for planning, implementing, and demonstrating the value of HIT. The goals of these research projects are as follows:
• Improve patient safety by reducing medical errors
• Increase health information sharing among providers, laboratories, pharmacies, and patients
• Help patients transition between health care settings
• Reduce duplicative and unnecessary testing
In July 2004, DHHS released the “Framework for Strategic Action, The Decade of Health Information Technology: Delivering Consumer-Centric and Information-Rich Health Care.” The goals of this 10-year plan are to transform the delivery of health care by building a new health information infrastructure, including EHRs, and a new network to link health records nationwide. The plan established three interrelated core strategies:
• Promoting EHR adoption by clinicians
• Supporting the creation of regional health care information organizations
AHRQ’s HIT Portfolio (research initiative) on HIT is critical to the nation’s 10-year strategy to bring health care into the 21st century by advancing the use of information technology.
The broad mission of AHRQ’s health IT initiative is to improve the quality of health care for all Americans. The Agency has focused its health IT activities on the following three goals:
To address the mission, AHRQ has invested over millions of dollars in contracts and grants to hundreds of communities, hospitals, providers, and health care systems throughout the U.S. to promote access to and encourage the adoption of health IT.
The AHRQ National Resource Center for Health Information Technology (the National Resource Center [NRC]), which was launched in September 2004, continues to play a pivotal role in achieving the goals of DHHS to modernize health care through the best and most effective use of information technology.21 The NRC encourages adoption of HIT by sharing the knowledge and findings that result from the real-world laboratory created in AHRQ’s HIT initiatives, as well as from other resources. The NRC supports the work of the HIT projects funded by AHRQ and other federal partners and will provide direct technical assistance and consulting services to individual projects during all phases of the work to develop and use HIT. Particular focus is placed on providing services to support challenges facing HIT implementation in rural settings.

The Commission on Systemic Interoperability, which was authorized by the Medicare Modernization Act, held its first meeting in January 2005. The commission developed a strategy to make health care information instantly accessible at all times by consumers and their health care providers. The final report was released in October 2005.22
In May 2005, DHHS Secretary Mike Leavitt issued a report citing investment in information technology as an essential high priority for the American health care system and the U.S. economy. The report is titled “Health Information Technology Leadership Panel: Final Report.” The panel identified three key imperatives for HIT23:
• Widespread adoption of interoperable HIT should be a top priority for the U.S. health care system.
American Health Information Community and Its Workgroups
In June 2005, the DHHS secretary announced the formation of a national collaboration, the American Health Information Community (AHIC), to advance efforts to reach President Bush’s call for most Americans to have EHRs within 10 years. The purpose of the AHIC was to help bring about the nationwide transition to EHRs—including common standards and interoperability—in a smooth, market-led way. The AHIC was formed under the auspices of the Federal Advisory Committee Act and provided input and recommendations to the DHHS on how to make health records digital and interoperable and ensure that the privacy and security of those records are protected.24 AHIC was a federal advisory body, chartered in 2005 to make recommendations to the secretary of the U.S. DHHS on how to accelerate the development and adoption of health information technology. From its inauguration in 2005 to its conclusion in November 2008, the AHIC advanced more than 200 recommendations over the course of 25 meetings in either Washington, DC, or other locations (see http://healthit.hhs.gov). The AHIC was transitioned from a Federal Advisory Committee to a private-public organization, the National eHealth Collaborative (NeHC). NeHC was established through a grant from the ONC to build on the accomplishments of the AHIC (see http://www.nationalehealth.org) NeHC operates in partnership with the ONC, DHHS, the HITSP, the CCHIT, and the NHIN Collaborative, to establish nationwide health information technology priorities and initiatives.
Health IT policy committee (2009–present)
In 2009, the American Recovery and Reinvestment Act of 2009 (ARRA) provided that the Health IT Policy Committee be created under the Federal Advisory Committee Act (FACA) and be charged with making recommendations to the National Coordinator for Health IT on a policy framework for the development and adoption of a nationwide health information infrastructure, including standards for the exchange of patient medical information. Seven HIT Policy Committee workgroups have been formed as sub-committees to the parent FACA. These workgroups meet periodically to discuss their topics, present their findings at HIT Policy Committee meetings, and make recommendations to the HIT Policy Committee.
Health IT standards committee
In 2009, the ARRA also provided that the Health IT Standards Committee be created under FACA and be charged with making recommendations to the National Coordinator for Health Information Technology on standards, implementation specifications, and certification criteria for the electronic exchange and use of health information. Initially, the HIT Standards Committee focused on the policies developed by the HIT Policy Committee. Four HIT Standards Committee workgroups have been formed as sub-committees to the parent FACA. These workgroups meet periodically to discuss their topics, present their findings at HIT Standards Committee meetings, and make recommendations to the HIT Standards Committee.

National committee on vital and health statistics
The NCVHS advises the secretary of DHHS in the area of health data, statistics, and national health information policy. In that capacity, the committee provides advice and assistance to the department and serves as a forum for interaction with interested private sector groups on a variety of key health data issues.
Centers for medicare and medicaid services
Under the direction of CMS, the Quality Improvement Organization (QIO) program consists of a national network of 53 QIOs, responsible for each U.S. state, territory, and the District of Columbia. QIOs work with consumers and physicians, hospitals, and other caregivers to refine care delivery systems to make sure patients get the right care at the right time, particularly patients from underserved populations. The program also safeguards the integrity of the Medicare Trust Fund by ensuring that payment is made only for medically necessary services and investigates beneficiary complaints about quality of care. The Doctor’s Office Quality Information Technology initiative promoted the adoption of EHRs and information technology in small- to medium-sized physician offices nationwide. The program aimed to help physicians increase access to patient information, decision support, and reference data and to improve patient-clinician communications. CMS created the DOQ-IT program as part of the QIO 8th Scope of Work (SOW). Each state’s QIO was required to recruit at least 5% of the state’s primary care physicians to work on implementing electronic health records (EHR) and improve on care management skills. This consulting service was at no charge to the practice. Throughout the United States, 4,186 physician offices participated in this program. Over 3,000 of those practices had not yet implemented or signed a contract with an EHR vendor, 395 had a signed contract, and 716 had already implemented a system.25,26 In August 2008, work began on the QIO Program’s 9th Statement of Work (SOW), which extends through July 31, 2011.
The inclusion of electronic prescribing in the Medicare Modernization Act (MMA) of 2003 gave momentum to the movement, and the July 2006 Institute of Medicine report on the role of e-prescribing in reducing medication errors has received widespread publicity, helping to build awareness of the role of e-prescribing in enhancing patient safety. DHHS regulations required electronic prescriptions for Medicare concurrent with the prescription drug benefit (January 2006). On November 7, 2005, CMS published foundation standards that became effective on January 1, 2006. These standards apply to all electronic prescribing done under Part D of the MMA. MMA required CMS to implement pilot projects to test additional standards. These additional standards were pilot tested in 2006. The results of the pilot test were announced in a report to Congress in April 2007 and were the basis for an NPRM proposing additional standards that was published on November 16, 2007. The e-prescribing regulations adopted standards for the following:
Section 132 of the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) authorized a new and separate incentive program for eligible professionals who are successful electronic prescribers (e-prescribers) as defined by MIPPA. The program began January 1, 2009, and provides incentives for eligible professionals who are “successful e-prescribers” (see: http://www.cms.hhs.gov/ERXIncentive).
Table 3-2 depicts various DHHS activities. An organizational chart of the DHHS is included as Figure 1-1. ONC is responsible for coordinating federal activities relating to HIT.
Table 3-2
SELECTED ACTIVITIES BY ORGANIZATION
| Selected DHHS Agencies/Organizations | Representative HIT/HIE-Related Activities |
| AHRQ DHHS agency | AHRQ’s health information technology (health IT) initiative is part of the Nation’s strategy to put information technology to work in health care. By developing secure and private electronic health records for most Americans and making health information available electronically when and where it is needed, health IT can improve the quality of care, even as it makes health care more cost-effective. The broad mission of AHRQ’s health IT initiative is to improve the quality of health care for all Americans. To address the mission, AHRQ has invested over $300 million in contracts and grants to over 200 communities, hospitals, providers, and health care systems in 48 States to promote access to and encourage the adoption of health IT. AHRQ’s National Resource Center (NRC) for Health IT supports the Agency’s mission of developing and disseminating evidence and evidence-based tools on how health IT can improve health care quality, safety, and efficiency. AHRQ initially established the NRC for Health IT in 2004 as a way of communicating and delivering technical assistance to its grantees. Since then, AHRQ has made the NRC available as a public resource for sharing research findings, best practices, lessons learned, and funding opportunities with health IT researchers, implementers, and policymakers. More than 10,000 documents, presentations, articles, and tools are freely available on the NRC. Traffic on the NRC has steadily grown since its inception, with recent usage averaging more than 40,000 unique site visits per month.a |
| AHIC Advisory body | The American Health Information Community (AHIC) was a federal advisory body, chartered in 2005 to make recommendations to the Secretary of the U.S. Department of Health and Human Services on how to accelerate the development and adoption of health information technology. AHIC was formed by the Secretary to help advance efforts to achieve President Bush’s goal for most Americans to have access to secure electronic health records by 2014. Since its formation in 2005, the AHIC identified four initial areas with potential for early breakthroughs in the advancement of standards that will lead to interoperability. The AHIC organized four workgroups to pursue recommendations in these areas, and delivered their first set of recommendations to the Secretary in May of 2006. Three additional workgroups were subsequently formed to address a wider range of issues, and the seven workgroups delivered recommendations to the Secretary in 2007 and 2008. The AHIC held its final meeting in November 2008.b |
| CMS DHHS agency | On Feb. 17, 2009, President Obama signed the American Recovery and Reinvestment Act of 2009 (Recovery Act), a critical measure to stimulate the economy. Among other provisions, the new law provides major opportunities for the Department of Health and Human Services (DHHS), its partner agencies, and the States to improve the nation’s health care through health information technology (HIT) by promoting the meaningful use of electronic health records (EHR) via incentives. The HIT provisions of the Recovery Act are found primarily in Title XIII, Division A, Health Information Technology, and in Title IV of Division B, Medicare and Medicaid Health Information Technology. These titles together are cited as the Health Information Technology for Economic and Clinical Health Act or the HITECH Act. Under Title IV, funding is available to certain eligible professionals (EPs) and hospitals, as described below. Funds will be distributed through Medicare and Medicaid incentive payments to EPs, physicians, and hospitals who are “meaningful EHR users.” In addition, with regard to the Medicaid program, federal matching funds are also available to States to support their administrative costs associated with these provisions. CMS also worked with the Office of the National Coordinator for Health Information Technology (ONC) in developing standards, implementation specifications, and certification criteria for EHR technology. Patient privacy and security is an important consideration in implementing the EHR incentive programs. CMS is also working with the Office for Civil Rights (OCR) and ONC to address the privacy and security protections under HITECH Act.c |
| CDC DHHS agency | The CDC is one of the 13 major operating components of the DHHS, which is the principal agency in the U.S. government for protecting the health and safety of all Americans and for providing essential human services, especially for those people who are least able to help themselves. The PHIN is CDC’s vision for advancing fully capable and interoperable information systems in the many organizations that participate in public health. PHIN is a national initiative to implement a multiorganizational business and technical architecture for public health information systems. |
| Commission on Systemic Interoperability Advisory Body | The Commission on Systemic Interoperability, authorized by the Medicare Modernization Act, held its first meeting on January 10, 2005. Congress created the commission as part of the Medicare Modernization Act of 2003 and charged the commission with developing recommendations, priorities, and a timeline for implementing an electronic HIE network. On October 25, 2005, the commission released its report, titled “Ending the Document Game: Connecting and Transforming Your Healthcare Through Information Technology. The Commission was comprised of eleven members. The Senate Majority Leader, the Senate Minority Leader, the Speaker of the House of Representatives, and the House Minority Leader each appointed two representatives to the Commission. The President appointed three members. The Commission represented a broad mix of individuals from different geographic locales |
| HRSA DHHS Agency | The Health Resources and Services Administration (HRSA), an agency of the U.S. Department of Health and Human Services, is the primary Federal agency for improving access to health care services for people who are uninsured, isolated, or medically vulnerable.d Comprising six bureaus and 13 offices, HRSA provides leadership and financial support to health care providers in every state and U.S. territory. HRSA grantees provide health care to uninsured people, people living with HIV/AIDS, pregnant women, mothers, and children. They train health professionals and improve systems of care in rural communities. HRSA oversees organ, bone marrow, and cord blood donation. It supports programs that prepare against bioterrorism, compensates individuals harmed by vaccination, and maintains databases that protect against health care malpractice and health care waste, fraud, and abuse. Since 1943 the agencies that were HRSA precursors have worked to improve the health of needy people. HRSA was created in 1982, when the Health Resources Administration and the Health Services Administration were merged. |
| NCVHS Advisory body | The National Committee on Vital and Health Statistics was established by Congress to serve as an advisory body to the Department of Health and Human Services on health data, statistics, and national health information policy. It fulfills important review and advisory functions relative to health data and statistical problems of national and international interest, stimulates or conducts studies of such problems, and makes proposals for improvement of the Nation’s health statistics and information systems. In 1996, the Committee was restructured to meet expanded responsibilities under the Health Insurance Portability and Accountability Act of 1996 (HIPAA).e Over its 55-year history, the Committee has stimulated a host of improvements in national and international health data and statistics. The Committee has been associated with ground-breaking contributions in such areas as disease classification, health surveys, uniform health data sets and other data standards, data needs for minority and other special populations, mental health statistics, State and community health data needs, and privacy protection for health information. The Committee is composed of 18 individuals distinguished in the fields of health statistics, electronic interchange of health care information, privacy and security of electronic information, population-based public health, purchasing or financing health care services, integrated computerized health information systems, health services research, consumer interests in health information, health data standards, epidemiology, and the provision of health services. Sixteen of the members are appointed by the Secretary of HHS for terms of four years each; with about four new members being appointed each year. |
| NIH DHHS agency | The National Institutes of Health (NIH), a part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important medical discoveries that improve health and save lives. NIH is the largest source of funding for medical research in the world, creating hundreds of thousands of high-quality jobs by funding thousands of scientists in universities and research institutions in every state across America and around the globe. NIH is made up of 27 Institutes and Centers, each with a specific research agenda, often focusing on particular diseases or body systems. All but three receive their funding directly from Congress and administrate their own budgets. More than 80% of the NIH’s budget goes to more than 300,000 research personnel at over 3,000 universities and research institutions. In addition, about 6,000 scientists work in NIH’s own laboratories, most of which are on the NIH main campus in Bethesda, Maryland. The main campus is also home to the NIH Clinical Center, the largest hospital in the world totally dedicated to clinical research. NIH supports many innovative training programs and funding mechanisms that foster scientific creativity and exploration. The goal is to strengthen our nation’s research capacity, broaden our research base, and inspire a passion for science in current and future generations of researchers.f |
| ONC DHHS agency | Serves as the secretary’s principal advisor on the development, application, and use of HIT; coordinates DHHS HIT programs; ensures that DHHS health information technology policy and programs are coordinated with those of other relevant executive branch agencies; and to the extent permitted by law, develops, maintains, and directs the implementation of a strategic plan to guide the nationwide implementation of interoperable HIT in both the public and private health care sectors that will reduce medical errors, improve quality, and produce greater value for health care expenditures, and coordinates outreach and consultation by the relevant executive branch agencies with the public and private sectors. |
ahttp://healthit.ahrq.gov/portal/server.pt/community/about/562. Accessed 08/04/10.
bhttp://www.hhs.gov/healthit/community/background
chttps://www.cms.gov/apps/media/press/factsheet.asp?Counter=3466&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date.
dhttp://www.hrsa.gov/about/index.html.
ehttp://www.ncvhs.hhs.gov/intro.htm.
In the fall of 2005, DHHS awarded several multimillion dollar contracts to public-private groups to accelerate the adoption of HIT and the secure portability of health information across the United States. These groups have strategic partnerships to develop the building blocks necessary for achieving the president’s goal of widespread adoption of interoperable EHRs within 10 years.
The HIT partnerships were created to do the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


