1 Explain the impact of the placebo effect and nocebo effect. 2 Identify the importance of drug dependence and drug accumulation. 3 Discuss the effects of age on drug absorption, distribution, metabolism, and excretion. 5 List the definitions of the use-in-pregnancy categories A, B, C, D, and X. 7 Discuss the role of genetics and its influence on drug action. gender-specific medicine ( placebo effect ( nocebo effect ( placebo ( tolerance ( drug dependence ( drug accumulation ( carcinogenicity ( passive diffusion ( hydrolysis ( intestinal transit ( protein binding ( drug metabolism ( metabolites ( therapeutic drug monitoring ( polypharmacy ( teratogens ( genetics ( genome ( polymorphisms ( pharmacogenetics ( Patients often say the following: “That drug really knocked me out!” or “That drug didn’t touch the pain!” The effects of drugs are unexpectedly potent in some patients, whereas other patients show little response at the same dosage. In addition, some patients react differently to the same dosage of a drug that is administered at different times. Because of individual patient variation, exact responses to drug therapy are difficult to predict. The following factors have been identified as contributors to a variable response to drugs. Infants and the very old tend to be the most sensitive to the effects of drugs. There are important differences with regard to the absorption, distribution, metabolism, and excretion of drugs in premature neonates, full-term newborns, and children. The aging process brings about changes in body composition and organ function that can affect the older patient’s response to drug therapy. The age of the patient can have a significant impact on drug therapy. When discussing the effect of age on drug therapy, it is helpful to subdivide the population into the following categories: Considerably overweight patients may require an increase in dosage to attain the same therapeutic response as the general population. Conversely, patients who are underweight as compared with the general population tend to require lower dosages for the same therapeutic response. It is extremely important to obtain accurate heights and weights of patients, because the dosage of medicine may be calculated with the use of these parameters. Most pediatric dosages are calculated by milligrams of drug per kilogram (mg/kg) of body weight to adjust for growth rate. The dosages of other medicines, particularly chemotherapeutic agents, are ordered on the basis of the body surface area (see Gender also affects drug therapy. Men and women respond to medications differently. Gender-specific medicine is a developing science that studies differences in the normal function of men and women and addresses how people of each gender perceive and experience disease. Unfortunately, few scientific data exist to document differences in the pharmacokinetics of most drugs in men as compared with women. In 1993, the U.S. Food and Drug Administration (FDA) issued guidelines stating that drug development must evaluate the effects on both genders. Testing is also needed to assess differences in pharmacokinetic parameters between men and women. In the women’s studies, the research must distinguish between premenopausal and postmenopausal women as well as among women in different phases of the menstrual cycle. Substantial new information has been gained, including the following: Patients with a higher-than-average metabolic rate tend to metabolize drugs more rapidly, thus requiring larger doses or more frequent administration. The converse is true for those with lower-than-average metabolic rates. Chronic smoking enhances the metabolism of some drugs (e.g., theophylline), thereby requiring larger doses to be administered more frequently for a therapeutic effect. Pathologic conditions may alter the rate of absorption, distribution, metabolism, and excretion. For example, patients who are in shock have reduced peripheral vascular circulation and will absorb intramuscularly or subcutaneously injected drugs more slowly. Patients who are vomiting may not be able to retain a medication in the stomach long enough for dissolution and absorption. Patients with diseases such as nephrotic syndrome or malnutrition may have reduced amounts of serum proteins in the blood that are necessary for adequate distribution of drugs. Patients with kidney failure must have significant reductions in the dosages of medications that are excreted by the kidneys. Attitudes and expectations play a major role in a patient’s response to therapy and in his or her willingness to take the medication as prescribed. Patients with diseases that have relatively rapid consequences if therapy is ignored (e.g., type 1 [insulin-dependent] diabetes) usually have a good rate of compliance. Patients with “silent” illnesses (e.g., hypertension) tend to be much less compliant with the treatment regimen. Other psychological considerations are the placebo effect and the nocebo effect. It is well documented that a patient’s positive expectations about treatment and the care received can positively affect the outcome of therapy; this is a phenomenon known as the placebo effect (from Latin, meaning “I will please”). Although more difficult to prove because of ethical considerations, it is also believed that negative expectations about therapy and the care received can have a nocebo effect (from Latin, meaning “I will harm”), which results in less-than-optimal outcomes of therapy. It is thought that the nocebo effect plays a major role in psychogenic illness, especially in stress-related problems, because the patient may worry about his or her condition or treatment. Caregivers can help to diminish the nocebo effect by having a positive mental attitude and emphasizing the positive aspects of therapy. A placebo is a drug dosage form (e.g., tablet, capsule) that has no pharmacologic activity because the dosage form has no active ingredients. However, when the placebo is taken, the patient may report a therapeutic response. Placebos are frequently used in studies of new medicines to measure the pharmacologic effects of a new medicine as compared with the inert placebo. The American Pain Society and the Agency for Health Care Policy and Research recommend the avoidance of the deceitful use of placebos in current clinical practice guidelines for pain management. It is thought that the deceitful use of placebos in pain management violates a patient’s right to receive the highest quality of care possible. Tolerance occurs when a person begins to require a higher dosage to produce the same effects that a lower dosage once provided. An example is the person who is addicted to heroin. After a few weeks of use, larger doses are required to provide the same “high.” Tolerance can be caused by psychological dependence, or the body may metabolize a particular drug more rapidly than before, thereby causing the effects of the drug to diminish more rapidly. Drug dependence, which is also known as addiction or habituation, occurs when a person is unable to control his or her desire for ingestion of drugs. The dependence may be physical, in which the person develops withdrawal symptoms if the drug is withdrawn for a certain period, or psychological, in which the patient is emotionally attached to the drug. Drug dependence occurs most commonly with the use of the scheduled or controlled medications listed in Chapter 1 (e.g., opiates, benzodiazepines). Many people, especially older adults, worry about becoming addicted to pain medication and therefore may not take their pain medication, even when it is needed. The nurse needs to assure these individuals that studies have shown that less than 1% of patients using opioids for pain relief become addicted and that it is important for their overall well-being to be as free of pain as possible. (For more information, see Chapter 49.) A drug may accumulate in the body if the next dose is administered before the previously administered dose has been metabolized or excreted. Excessive drug accumulation may result in drug toxicity. An example of drug accumulation is the excessive ingestion of alcoholic beverages. A person becomes “drunk” or “inebriated” when the rate of consumption exceeds the rate of metabolism and the excretion of the alcohol. Carcinogenicity is the ability of a drug to induce living cells to mutate and become cancerous. Many drugs have this potential, so all drugs are tested in several animal species before human investigation to eliminate this potential. Drug absorption refers to the process by which drugs are absorbed in the body. This occurs by way of different routes through which the drugs are administered. For example, the most common way to administer a drug is orally (by mouth), and then the drug is absorbed by the gastrointestinal tract (enterally). Other routes include intramuscular (in the muscle) or intravenously (in the vein). The rate of absorption is dependent on various factors (e.g., blood flow to the area in which the drug has been administered). Pediatric and geriatric patients each require special considerations for medication administration. Medicines given intramuscularly (IM) are usually erratically absorbed in neonates and older adults. Differences in muscle mass, blood flow to muscles, and muscle inactivity in patients who are bedridden make absorption unpredictable. Topical administration with percutaneous absorption is usually effective for infants, because their outer layer of skin (i.e., the stratum corneum) is not fully developed. Because the skin is more fully hydrated at this age, water-soluble drugs are absorbed more readily. Infants who wear plastic-coated diapers are also more susceptible to skin absorption, because the plastic acts as an occlusive dressing that increases the hydration of the skin. Inflammation (e.g., diaper rash) also increases the amount of drug that is absorbed. Transdermal administration in geriatric patients is often difficult to predict. Although dermal thickness decreases with aging and may enhance absorption, factors that may diminish absorption can be seen, including drying, wrinkling, and a decrease in the number of hair follicles. With aging, decreased cardiac output and diminishing tissue perfusion may also affect transdermal drug absorption. In most cases, medicines are administered orally. Infants and older adults often lack a sufficient number of teeth for chewable medicines. Chewable tablets should not be given to children or to any patient with loose teeth. Geriatric patients often have reduced salivary flow, which makes chewing and swallowing more difficult. However, tablet and capsule forms are often too large for pediatric or geriatric patients to swallow safely. It is often necessary to crush a tablet for administration with food or to use a liquid formulation for easier and safer administration. Taste also becomes a factor when administering oral liquids, because the liquid comes into contact with the taste buds. Timed-release tablets (pp. 124-125), enteric-coated tablets (p. 125), and sublingual tablets (p. 113) should not be crushed, because this will increase their absorption rate and thus the potential for toxicity. The GI absorption of medicines is influenced by various factors, including gastric pH, gastric emptying time, the motility of the GI tract, enzymatic activity, the blood flow of the mucous lining of the stomach and intestines, the permeability and maturation of the mucosal membrane, and concurrent disease processes. Absorption by passive diffusion across the membranes and gastric emptying time depend on the pH of the environment. Newborns and geriatric patients have reduced gastric acidity and prolonged transit time as compared with adults. Premature infants have a high gastric pH (6 to 8) as a result of the immature acid-secreting cells in their stomachs. In a full-term newborn, the gastric pH is also 6 to 8, but, within 24 hours, the pH decreases to 2 to 4 in response to gastric acid secretion. At 1 year old, the child’s stomach pH approximates that of an adult (i.e., 1 to 2 when empty, up to 5 when full). Geriatric patients often have a higher gastric pH because of the loss of acid-secreting cells. Drugs that are destroyed by gastric acid (e.g., ampicillin, penicillin) are more readily absorbed in older adults because of the decrease in acid production which results in higher serum concentrations. By contrast, drugs that depend on an acidic environment for absorption (e.g., phenobarbital, acetaminophen, phenytoin, aspirin) are more poorly absorbed, thereby resulting in lower serum concentrations in older adults. Premature infants and geriatric patients also have a slower gastric emptying time, partly because of their reduced acid secretion. A slower gastric emptying time may allow the drug to stay in contact with the absorptive tissue longer, thereby allowing for increased absorption with a higher serum concentration. There is also the potential for toxicity caused by extended contact time in the stomach for ulcerogenic drugs (e.g., nonsteroidal anti-inflammatory drugs). Another factor that affects drug absorption in the newborn is the absence of the enzymes needed for hydrolysis. Hydrolysis is the process that uses water to initiate a chemical reaction. Infants cannot metabolize palmitic acid from chloramphenicol palmitate (an antibiotic), thereby preventing the absorption of the chloramphenicol. Oral phenytoin dosages are also greater in infants who are less than 6 months old because of poor absorption (i.e., in neonates, the rate is 15 to 20 mg/kg/24 hr as compared with infants and children, in whom the rate is 4 to 7 mg/kg/24 hr). The intestinal transit refers to the speed at which the intestine moves foods, secretions and other ingested matter along and this rate varies with age. Premature and full-term newborns have a slower transit time. As the healthy newborn matures into infancy, the GI transit rate increases to a relatively standard rate by about 4 months of age. Older adults develop decreased GI motility and intestinal blood flow. This has the potential for altering the absorption of medicines as well as for causing constipation or diarrhea, depending on the medicine. Generally, a woman’s stomach empties solids more slowly than a man’s does, and it may have greater gastric acidity, thus slowing the absorption of certain types of medicines (e.g., aspirin). A slower gastric emptying time may allow the drug to stay in contact with the absorptive tissue longer, thereby allowing for more absorption and a higher serum concentration. There is also an increased potential for toxicity caused by more contact time in the stomach for potentially ulcerogenic drugs (e.g., nonsteroidal anti-inflammatory drugs). Women also have lower gastric levels of the enzyme alcohol dehydrogenase, which is needed to metabolize ingested alcohol. Thus, larger amounts of ingested alcohol may be absorbed instead of metabolized in the stomach, thereby leading to a higher blood alcohol level in a woman than in a man for equal amounts of ingested alcohol. Other factors, such as body weight and drug distribution (see the next section of this chapter), may aggravate the higher blood alcohol level and state of intoxication in women as compared with men. The term distribution refers to the ways in which drugs are transported by the circulating body fluids to the sites of action (receptors), metabolism, and excretion. Distribution is dependent on pH, body water concentrations (i.e., intracellular, extracellular, and total body water), the presence and quantity of fat tissue, protein binding, cardiac output, and regional blood flow. Most medicines are transported either dissolved in the circulating water (i.e., in blood) of the body or bound to plasma proteins within the blood. Body water composition as a percentage of weight changes substantially with age (Table 3-1). Note that the total body water content of a preterm infant is 83%, whereas that of an adult man is 60%; this drops to 50% in older persons. The significance of this is that infants have a larger volume of distribution for water-soluble drugs and thus require a higher dose on a milligram-per-kilogram (mg/kg) basis than an older child or an adult. Table 3-1 *Developmental changes from birth to adulthood. Extracellular and intracellular water values are expressed as percentages of total body weight. Data from Friis-Hansen B: Body composition during growth, Pediatrics 47(Suppl 2):264, 1971. With aging, lean body mass and total body water decrease, and total fat content increases. The body weight of a preterm infant may be composed of 1% to 2% fat, whereas a full-term newborn may have 15% fat. Adult total body fat ranges from 18% to 36% for men and 33% to 48% for women between the ages of 18 and 35 years. Drugs that are highly fat soluble (e.g., antidepressants, phenothiazines, benzodiazepines, calcium channel blockers) require a longer onset of action and accumulate in fat tissues, thereby prolonging their action and increasing the potential for toxicity. For water-soluble drugs (e.g., ethanol, aminoglycoside antibiotics), a woman’s greater proportion of body fat produces a higher blood level as compared with that of a man when given as an equal dose per kilogram of body weight. In the case of ethanol, this effect tends to cause a higher level of ethanol in the brain cells, which results in greater intoxication. Highly fat-soluble medicines (e.g., diazepam) must be given in smaller mg/kg dosages to low-birth-weight infants, because there is less fat tissue to bind the drug, thereby leaving more drug to be active at receptor sites. Drugs that are relatively insoluble are transported in the circulation by being bound to plasma proteins (albumin and globulins), especially albumin. Protein binding is reduced in preterm infants because of decreased plasma protein concentrations, lower binding capacity of protein, and decreased affinity of proteins for drug binding. Drugs that are known to have lower protein binding in neonates than in adults include phenobarbital, phenytoin, theophylline, propranolol, lidocaine, penicillin, and chloramphenicol. Because serum protein binding is diminished, the drugs are distributed over a wider area of the neonate’s body, and a larger loading dose is required than is needed in older children to achieve therapeutic serum concentrations. Several drugs that are used to treat neonatal conditions may compete for binding sites. Sulfisoxazole is well known for displacing bilirubin from protein-binding sites; this allows the bilirubin to accumulate and pass into the brain, thereby causing kernicterus (i.e., the degeneration of brain nerve cells that is caused by the binding of bilirubin to cells). Little difference exists between albumin protein in men and women, although there are some differences between the globulin proteins (i.e., corticosteroid-binding and sex-hormone–binding globulins). In adults who are more than 40 years old, the composition of body proteins begins to change. Although the total body protein concentration is unaffected, albumin concentrations gradually decrease, and other protein levels (e.g., globulins) increase. As albumin levels diminish, the level of unbound active drug increases. Increased levels of naproxen, diflunisal, salicylate, and valproate have been found in older adults, presumably as a result of decreased albumin levels. Disease states such as cirrhosis, renal failure, and malnutrition can lower albumin levels. Initial doses of highly protein-bound drugs (e.g., warfarin, phenytoin, tolbutamide, propranolol, diazepam) should be reduced and then increased slowly if there is evidence of decreased serum albumin. Lower protein binding may also lead to a greater immediate pharmacologic effect because more active drug is available; however, the duration of action may be reduced because more of the unbound drug is available for metabolism and excretion. Drug metabolism is the process whereby the body inactivates medicines. It is controlled by factors such as genes, diet, age, health, and the maturity of enzyme systems. Enzyme systems, primarily in the liver, are the major pathways of drug metabolism. All enzyme systems are present at birth, but they mature at different rates, taking several weeks to a year to fully develop. Liver weight, the number of functioning hepatic cells, and hepatic blood flow decrease with age; this results in the slower metabolism of drugs in older adults. Reduced metabolism can be seriously aggravated by the presence of liver disease or heart failure. Drugs that are extensively metabolized by the liver (e.g., morphine, lidocaine, propranolol) can have substantially prolonged durations of action if hepatic blood flow is reduced. Dosages usually must be reduced or the time interval between doses extended to prevent the accumulation of active medicine and potential toxicity. Drug metabolism can also be affected at all ages by genetics, smoking, diet, gender, other medicines (Table 3-2), and diseases (e.g., hepatitis, cirrhosis). No specific laboratory tests are available for measuring liver function to adjust drug dosages; renal function (creatinine clearance) must be assessed and dosages adjusted accordingly. Medications That Require Hepatic Monitoring*† *This is a list of the more common drugs that require periodic liver function tests, usually at the beginning of therapy and then every few weeks to months thereafter. See individual monographs. †Enzymes that are routinely monitored for liver function are alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase. If the patient’s levels become elevated, the physician should be notified for individualized treatment. Data from Tice SA, Parry D: Medications that require hepatic monitoring, Hosp Pharm 36(4):456-464, 2001; Tice SA, Parry D: Medications that require hepatic monitoring, Hosp Pharm 39(6):595-606, 2004; Porter RS, Kaplan JL (eds.): The Merck manual of diagnosis and therapy, ed 19, Whitehouse Station, NJ, 2012, Merck.
Drug Action Across the Life Span
Objectives
Key Terms
 ) (p. 21)
) (p. 21)
 ) (p. 21)
) (p. 21)
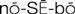 ) (p. 21)
) (p. 21)
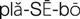 ) (p. 21)
) (p. 21)
 ) (p. 21)
) (p. 21)
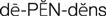 ) (p. 22)
) (p. 22)
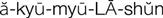 ) (p. 22)
) (p. 22)
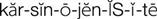 ) (p. 22)
) (p. 22)
 ) (p. 22)
) (p. 22)
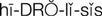 ) (p. 23)
) (p. 23)
 ) (p. 23)
) (p. 23)
 ) (p. 24)
) (p. 24)
 ) (p. 24)
) (p. 24)
 ) (p. 24)
) (p. 24)
 ) (p. 25)
) (p. 25)
 ) (p. 28)
) (p. 28)
 ) (p. 30)
) (p. 30)
 ) (p. 32)
) (p. 32)
 ) (p. 32)
) (p. 32)
 ) (p. 32)
) (p. 32)
 ) (p. 32)
) (p. 32)
Factors That Affect Drug Therapy
![]() http://evolve.elsevier.com/Clayton
http://evolve.elsevier.com/Clayton
Age
Age
Stage
<38 wk gestation
Premature
0-1 mo
Newborn, neonate
1-24 mo
Infant, toddler
3-5 yr
Young child
6-12 yr
Older child
13-18 yr
Adolescent
19-54 yr
Adult
55-64 yr
Older adult
65-74 yr
Elderly
75-84 yr
The aged
85 yr or older
The very old
Body Weight
![]() Appendix B); this calculation requires both height and weight. To ensure accurate measurements, the patient’s weight should be taken at the same time of day and while the patient is wearing similar-weight clothing; this should be done at admission and at intervals as ordered by the physician throughout the provision of care.
Appendix B); this calculation requires both height and weight. To ensure accurate measurements, the patient’s weight should be taken at the same time of day and while the patient is wearing similar-weight clothing; this should be done at admission and at intervals as ordered by the physician throughout the provision of care.
Gender
Metabolic Rate
Illness
Psychology
Tolerance
Dependence
Cumulative Effect
Factors That Influence Drug Actions
Absorption
Age
Gender
Distribution
Age and Gender
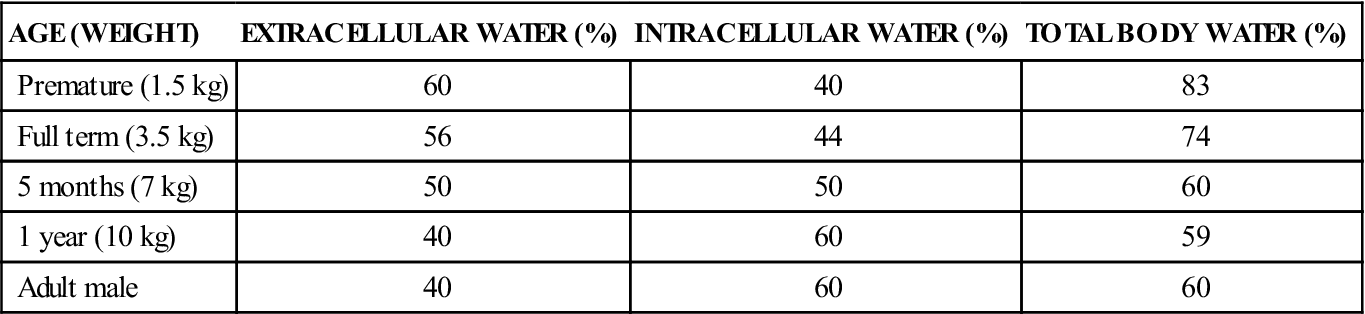
AGE (WEIGHT)
EXTRACELLULAR WATER (%)
INTRACELLULAR WATER (%)
TOTAL BODY WATER (%)
Premature (1.5 kg)
60
40
83
Full term (3.5 kg)
56
44
74
5 months (7 kg)
50
50
60
1 year (10 kg)
40
60
59
Adult male
40
60
60
Metabolism
Age
![]() Table 3-2
Table 3-2
GENERIC NAME
BRAND NAME
acetaminophen
Tylenol
amiodarone
Cordarone
atorvastatin
Lipitor
azathioprine
Imuran
carbamazepine
Tegretol
diclofenac
Voltaren
efavirenz
Sustiva
ethosuximide
Zarontin
ethotoin
Peganone
felbamate
Felbatol
fenofibrate
Tricor
fluvastatin
Lescol
gemfibrozil
Lopid
griseofulvin
Gris-PEG
indinavir
Crixivan
isoniazid
Nydrazid
ketoconazole
Nizoral
lamivudine
Epivir
leflunomide
Arava
lovastatin
Mevacor
meloxicam
Mobic
methsuximide
Celontin
methotrexate
Rheumatrex
naproxen
Naprosyn
nevirapine
Viramune
niacin
Niaspan
oxcarbazepine
Trileptal
pemoline
Cylert
pentamidine
Pentam
pioglitazone
Actos
pravastatin
Pravachol
piroxicam
Feldene
rifampin
Rifadin
ritonavir
Norvir
rosiglitazone
Avandia
rosuvastatin
Crestor
simvastatin
Zocor
tacrine
Cognex
terbinafine
Lamisil
tizanidine
Zanaflex
tolcapone
Tasmar
valproic acid
Depakote
3. Drug Action Across the Life Span
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


