CHAPTER 27. Intraspinal Access and Medication Administration
Candace K. Stearns, MN, FNP-BC, ONP-C and Jeannine M. Brant, PhD, APRN, AOCN∗
Spinal Anatomy, 525
Intraspinal Routes, 527
Methods of Drug Administration, 531
Medications, 532
Complications of Intraspinal Access Devices, 533
Nursing Considerations, 536
Summary, 538
Intraspinal access devices are defined as those in the epidural, intrathecal, or ventricular spaces (INS, 2006). The infusion of medications into the intraspinal spaces, a practice employed for decades, involves a multitude of medications, including chemotherapeutic agents, opiates, local anesthetics, alpha 2-adrenergic blockers, and N-type calcium channel blockers, as well as others. The infusion of analgesic agents into the intraspinal spaces is sometimes considered the fourth step in the World Health Organization’s (WHO) ladder for pain management, that is, the step after other treatment options have been exhausted. Nurses may encounter the intraspinal route of medication administration and access devices in a variety of practice settings from acute care to outpatient and home care (e.g., postoperatively, women in labor, chronic malignant and nonmalignant pain, spasticity control), and as such should demonstrate competency when caring for a patient with this type of device (INS, 2006). The purpose of this chapter is to provide an overview of the intraspinal route of administration. Spinal anatomy, neuropharmacology, assessment of placement and function of the access device, care and maintenance practices, and potential complications shall be addressed. There is a wide array of intraspinal uses; however, this chapter will focus on the infusion of analgesic medications, the most common use of the intraspinal route.
Throughout this chapter, it is important to keep in mind that each state varies in regards to the nursing scope of practice and in the management of intraspinal infusions. Nurses must take responsibility for consulting their individual state board of nursing rules and regulations to ensure actions are within the scope of practice for their individual education level. Some activities are limited to nurses with advanced training, such as Certified Registered Nurse Anesthetists (CRNA), Clinical Nurse Specialists (CNS), or Nurse Practitioners (NP). In addition, the placement of an intraspinal access device is considered a surgical procedure, and placement is dictated by practice laws as well. Additionally, the nurse must follow individual organizational guidelines and attain and maintain competency for the management of intraspinal infusions and devices (INS, 2006). The following summary describes spinal anatomy and the locations in which intraspinal analgesia and other medications are delivered.
SPINAL ANATOMY
SPINAL STRUCTURE
The spine is divided into four regions—cervical, thoracic, lumbar, and sacral. The spinal column consists of 33 individual vertebrae that are referred to by their location in 1 of 5 regions. The spinal column consists of 7 cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 4 fused coccygeal vertebrae. Each vertebra consists of an anterior body—the lamina—that protects the lateral spinal cord, and spinous processes that project posteriorly and outwardly from the laminae (FIGURE 27-1 and FIGURE 27-2).
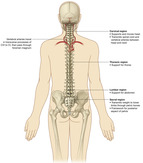 |
| FIGURE 27-1 Relationships of the back to other regions. (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
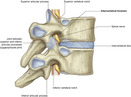 |
| FIGURE 27-2 Intervertebral foramina. (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
The spinal cord itself begins at the base of the skull (foramen magnum) and passes through the vertebral canal of the spinal column. In adults, the spinal cord terminates at the first or second lumbar vertebra. Here, it splits into the cauda equina, which are the lumbar and sacral nerve roots. The spinal cord is located within the bony vertebral column and connective tissues, providing protection from outside injury. The cord consists of a central region of gray matter surrounded by bundles of white matter. The butterfly-shaped gray matter consists of two dorsal (posterior) horns that extend toward the dorsolateral surfaces of the cord and two thicker ventral (anterior) horns that extend toward the ventrolateral surfaces. The right and left regions of gray matter are connected by a gray transverse band of nerve fibers that crosses the middle of the cord. In the middle of the gray band of nerve fibers is the central canal, which is filled with cerebrospinal fluid. The size of the white and gray matter varies according to the function of the spinal segment. White matter is made of approximately 80% lipids. The dorsal horn of the spinal cord is part of the gray matter and is rich in opioid receptors (see FIGURE 27-3 and FIGURE 27-4) (Saladin and Porth, 1998 and Ghafoor et al., 2007).
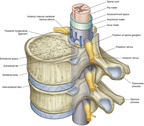 |
| FIGURE 27-3 Vertebral canal. (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
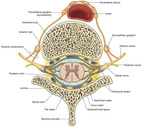 |
| FIGURE 27-4 Spinal nerves (transverse section). (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
SPINAL NERVES
The spinal cord gives rise to 31 pairs of spinal nerves (Figure 27-5). The portion of the spinal cord connected to each pair of nerves is called a segment of the cord. Dermatomes are delineated areas of skin innervated by a spinal cord segment (FIGURE 27-6 and FIGURE 27-7). Each spinal nerve except C1 receives sensory input from these specific areas of the skin. This model is used to monitor the level of anesthesia and monitor sensations arising from each level. Dermatomes may overlap at their edges by as much as 50%. Therefore anesthetizing one sensory nerve root does not entirely deaden sensation from a dermatome. It is necessary to anesthetize or deaden three successive spinal nerves to produce a total loss of sensation from a dermatome (Saladin and Porth, 1998 and McCaffery and Pasero, 1999). The nurse needs to be familiar with the dermatomes to adequately assess patients who receive intraspinal analgesia.
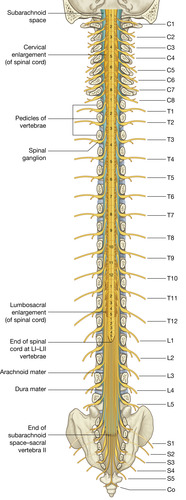 |
| FIGURE 27-5 Vertebral canal, spinal cord, and spinal nerves. (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
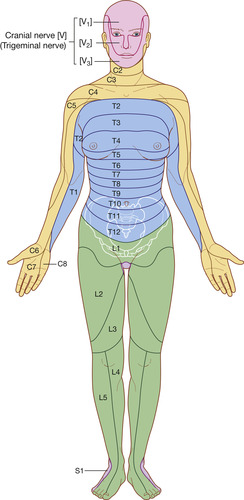 |
| FIGURE 27-6 Dermatomes (anterior view). (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
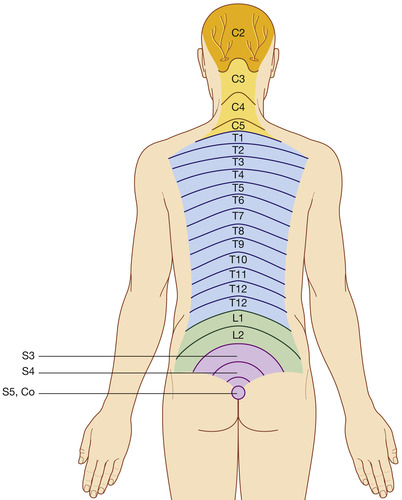 |
| FIGURE 27-7 Dermatomes innervated by posterior rami of spinal nerves. (From Drake RL, Vogl W, Mitchell AMW: Gray’s anatomy for students, London, 2005, Churchill Livingstone.) |
SPINAL MENINGES
The connective tissues that provide protection for the spinal cord are also called meninges. The three protective meninges are the dura mater, the arachnoid mater, and the pia mater. The outermost layer is the dura mater, which is the toughest membrane and consists of dense fibrous connective tissue. The arachnoid mater is a thin membrane covering the brain and spinal cord. The arachnoid mater is separated from the dura mater by the extradural or epidural space. The pia mater is the layer closest to the spinal cord and is composed of delicate connective tissue. This tissue clings tightly to the brain and spinal cord (Saladin and Porth, 1998). The epidural space is the area surrounding the spinal cord and its coverings (dura mater, arachnoid, and pia mater). It contains fatty tissue, veins, spinal arteries, and spinal nerves. The space extends from the foramen magnum to the sacrococcygeal membrane. Below the arachnoid space is the subarachnoid or intrathecal space, containing cerebrospinal fluid (CSF) (Saladin and Porth, 1998, McCaffery and Pasero, 1999 and Maher et al., 2002). The cerebrospinal fluid that circulates in the intrathecal space is forced down the dorsal surface of the spinal cord and up the ventral side by the pulsatile flow of blood in the central nervous system, and has an important role in distribution of medications (Ghafoor et al, 2007). Drugs are carried upward or rostrally (toward the brain), which may increase the medications’ effects away from the targeted area. This concept is referred to as rostral spread (see Figure 27-7) (McCaffery and Pasero, 1999 and Ghafoor et al., 2007).
INTRASPINAL ROUTES
EPIDURAL
Epidural opioids are administered adjacent to the spinal cord, and diffuse across the dura mater and into the spine where they bind directly with opioid receptors to block the transmission of pain. Some of the analgesia is lost in the epidural vasculature during the diffusion process, leading to systemic absorption and increased sedation with greater rostral spread (Sloan, 2007). Most typically this type of access is used on a short-term basis (e.g., for postoperative pain management, during labor) (McCaffery and Pasero, 1999). Epidural devices should be aspirated to ascertain the absence of spinal fluid and blood before medication administration (INS, 2006). The doses of medications administered through the epidural route are much lower than systemic doses, approximately one tenth of an intravenous dose and 10 times higher than with the intrathecal route (American Pain Society, 2003).
INTRATHECAL
Intrathecal opioids are administered directly inside the spinal cord, eliminating the need to cross the lipid membrane (dura). This leads to faster action, thereby requiring lower doses of medications when administration is intrathecal rather than epidural (Brant, 1995). Intrathecal administration is often associated with long-term drug administration through catheters that have been surgically implanted. Nurses with advanced training and physicians may inject drugs into these catheters (Potter and Perry, 2007). A drug given intrathecally comes into direct contact with the spinal cord and therefore is effective at a much smaller dose than would be given epidurally. Intrathecal devices should be aspirated to ascertain the presence of spinal fluid and the absence of blood before medication administrations (INS, 2006).
INTRAVENTRICULAR ACCESS
Intraventricular access is usually obtained through a surgically implanted reservoir and provides direct access to ventricular cerebrospinal fluid. This type of access is used primarily to consistently and predictably deliver medications, most often antineoplastic drugs or pain medications, directly into the subarachnoid space and CSF. The majority of chemotherapeutic agents do not cross the blood-brain barrier, so the use of this type of access device allows more targeted delivery to the source of the cancer. Most typically this type of access is used to target cancers of the head, neck, or spine, or for pain unrelieved by conventional methods (Brant, 1995 and Burke et al., 2001). The use of this reservoir also allows samples of the CSF to be extracted for pathological examination and permits the measurement of CSF pressure, which avoids repeated lumbar punctures for the purposes of medication or sampling of CSF (Burke et al, 2001). Usually the volumes of medications administered are small (15 mL or less) and should be injected slowly. Patients receiving medications through this route must be monitored closely for neurotoxicity. Most often physicians will administer the medications through this access. An intraventricular reservoir is rarely removed once implanted unless the device malfunctions or the body develops an infection that cannot be resolved with the device in place. If removal is necessary, the intraventricular reservoir must be removed in surgery (Yarbro et al., 2000 and Burke et al., 2001).
EPIDURAL/INTRATHECAL CATHETERS AND ADMINISTRATION SYSTEMS
The choice of short-term or long-term systems for delivery of intraspinal infusion is based on patient needs, contraindications, and cost-benefit evaluations. Externalized systems are typically used for those patients with life expectancy less than 3 months. An implanted system is preferred when life expectancy is greater than 3 months (Krames, 2002, DuPen, 2005 and Stearns et al., 2005). Pumps are available in programmable and nonprogrammable forms with reservoir capacities of between 10 mL and 50 mL. Programmable pumps allow immediate adjustments and bolus dosing for patients. Nonprogrammable pumps require dosage adjustments to be made by changing medication concentrations and do not allow rapid medication adjustments for pain control (Krames, 2002 and DuPen, 2005). Pumps may be inserted using a local, spinal, or general anesthetic (see Figure 27-8). Additionally, placement is typically aided with the use of fluoroscopic guidance (Krames, 2002 and Stearns et al., 2005). Site care of the implanted system is dependent on the individual agency’s standards regarding postoperative wound management and the length of time since implantation. It is the nurse’s responsibility to ensure familiarity and competency are maintained regarding individual agency pumps, and all users should be aware of factors that may affect the accuracy of medication delivery (Skyryabina and Dunn, 2006).
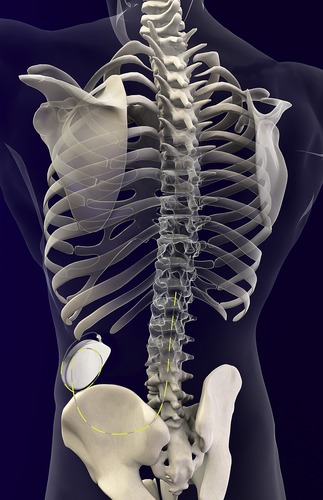 |
| FIGURE 27-8 Spine with implanted pump. (Reprinted with permission from Medtronic, Inc. ©2008.) |
Intraspinal catheters may be temporary or permanent. Temporary, or “trialing,” catheters are typically used for no longer than 7 days, such as in epidural or postoperative analgesia (Figure 27-9). However, they have been used for as long as 11 days for trials before placement of permanent catheters (Figure 27-10) (DuPen, 2005). Permanent catheters may be used for weeks to months. The permanent catheter is tunneled and has a Dacron cuff to aid in the stabilization of the catheter to the tissues. Many have a second Vita cuff impregnated with silver to reduce antimicrobial activity. These catheters are wire guided, which allows for tip advancement and precise positioning. Catheters that are expected to be used longer than 3 months should be internalized. Catheters may break, disconnect at the site, migrate out of the intrathecal space or epidural space, or disconnect from the pump (Gooch et al., 2003 and Albright et al., 2004).
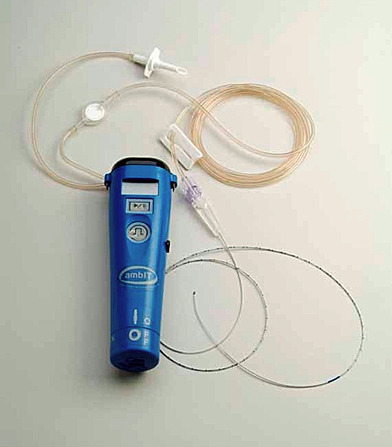 |
| FIGURE 27-9 Trailing pump. (Reprinted with permission from Medtronic, Inc. ©2008.) |
 |
| FIGURE 27-10 Programmable pump with catheter. (Reprinted with permission from Medtronic, Inc. ©2008.) |
Implanted ports may be used in an effort to decrease infection rates although there are no data to support this. Ports must still be continuously accessed for medication delivery. Care involves weekly transparent dressing change over the firmly secured noncoring needle.
Pump and catheter failures should be reported to allow for improvement in pumps and allow patients to make informed decisions (Gooch et al., 2003 and Infusion Nurses Society, 2006). In children, more complications were found in patients who had implanted pumps with access ports.
MANAGEMENT OF PUMP DEVICES/CATHETERS/PORTS
Management of the pump device is entirely dependent on the type of pump device used, and whether the pump device is implanted or external, programmable or nonprogrammable, and the manufacturer’s instructions. Regardless of the type of pump used, all pumps should prevent free-flow of medications to avoid overadministration (INS, 2006).
METHODS OF DRUG ADMINISTRATION
Infusion of intraspinal agents may be provided on a short-term or long-term basis. The infusion may be a one-time injection or may be a continuous infusion. Catheters themselves may be internal or external. If long-term intraspinal infusions are used, an implanted device and internal catheter may be warranted to avoid potential complications with infections, malfunctioning of the pump, and/or dislodgement of catheters (Stearns et al, 2005). Patients may receive continuous or bolus medications, or a combination of both depending on the patient situation. A combination of medications may be provided. Additionally, patients may control the infusion (patient-controlled epidural analgesia [PCEA]). The expected length of time of infusion, method of delivery (intrathecal, intraventricular, or epidural), and patient condition will dictate the prescribed therapy.
Nurses must be aware of relative and absolute contraindications to the use of intraspinal analgesia. Relative contraindications may include spinal column deformities, laminectomy or low back pain, severe headaches or backaches, patients unable to cooperate, and unstable neurological disease. Absolute contraindications include patient refusal, infection at the puncture site, sepsis, allergy to local anesthetics and/or medications to be infused, coagulopathies, uncorrected hypovolemia, and active neurological disease (Brogan, 2006 and Sloan, 2007). Contraindications when identified must be communicated by nurses to other members of the health care team. Nurses must also provide patient education, ensure informed consent is completed, and document actions and interventions according to organizational policy and standards of nursing care.
MEDICATIONS
Medications administered may include opioids, alpha 2-adrenergics, local anesthetics, and chemotherapeutic agents. Regardless of the medication used, only medications that are preservative-free should be injected into the intraspinal space to avoid neurotoxicity (Prager, 2002 and Infusion Nurses Society, 2006). Any drug packaged in a multidose vial likely has preservatives added and should not be used for intraspinal access (Prager, 2002). Epidural and intrathecal medications must diffuse from the cerebrospinal fluid to the dorsal horn of the spinal cord to produce analgesia (McCaffery and Pasero, 1999 and Ghafoor et al., 2007). Doses required to obtain analgesic effects from intraspinal medications are much lower than those required to obtain analgesia from systemic administration, leading to decreased side effects (Kedlaya et al., 2002, Prager, 2002, Farrow-Gillespie and Kaplan, 2006, Ghafoor et al., 2007 and Cohen and Dragovich, 2007). Doses are dependent on administration technique (i.e., one-time infusion, continuous), but must be adjusted based on age, weight, patient’s opiate tolerance, and condition (American Pain Society, 2003). The onset, peak, and duration of action vary according to the pharmacokinetics of the particular medication.
OPIOIDS
Morphine is considered the standard medication for starting spinal opioid therapy and the agent to which all others are compared. Morphine is a hydrophilic-type opiate and has high affinity for mµ-receptors in the dorsal horn of the spinal cord. Hydrophilic opioids, like morphine, decline more slowly than lipophilic opioids in cerebrospinal fluid, which accounts for more rostral spread. This may also lead to delayed respiratory depression with initiation of this drug, and increased dermatomal analgesia during long-term administration (Pasero, 2003, Heitz and Viscusi, 2005, Cohen and Dragovich, 2007, Ghafoor et al., 2007 and Sloan, 2007). Tolerance can develop when used for extended periods, which may lead to the need for dosage increases (Heitz and Viscusi, 2005). Epidural morphine is 5 to 10 times more potent than intravenous delivery (Kedlaya et al., 2002 and American Pain Society,).
Hydromorphone, also hydrophilic, may be used as an alternative to morphine (e.g., intolerability to morphine side effects, titrated doses continue to lack optimal analgesia) (Krames, 2002 and Stearns et al., 2005). Hydromorphone has less rostral spread than morphine and an intermediate onset and duration of action (Kedlaya et al., 2002 and American Pain Society,). Given epidurally, it is five times more potent than the intravenous formulation (Kedlaya et al, 2002). The use of hydromorphone may produce less pruritus in comparison to morphine (Golembiewski et al, 2005).
Fentanyl and sufentanil are lipophilic in nature; therefore they have more rapid onset of analgesia and less rostral spread (Ghafoor et al, 2007). Fentanyl may have limited access to opiate receptors because of rapid clearing of the drug and more systemic uptake. This issue may lead to little advantage over intravenous infusions. Epidural fentanyl is considered to be equivalent to intravenous administration (Kedlaya et al., 2002 and Heitz and Viscusi, 2005).
LOCAL ANESTHETICS
Local anesthetics are sodium channel antagonists and produce analgesia in the epidural space by diffusing across the dura mater to anesthetize nerve roots and the spinal cord (Kedlaya et al., 2002 and Heitz and Viscusi, 2005). Many local anesthetics are suitable for epidural infusion, but lidocaine, bupivacaine, and ropivacaine are used most commonly in current practice (Ghafoor et al, 2007). Lidocaine has a fast onset but the shortest duration of action, and is therefore more useful for rescue dosing and catheter testing than for ongoing pain control. Repeated dosing of lidocaine has also been associated with tachyphylaxis (Heitz and Viscusi, 2005). Bupivacaine is often co-administered with morphine and may produce synergistic effects and therefore decrease morphine doses (Ghafoor et al, 2007). Bupivacaine also has the longest duration of action and has been the most extensively used. It does, however, carry a higher risk of cardiotoxicity (Heitz and Viscusi, 2005). Additionally, side effects may include motor blockade, hypotension, diarrhea, and urinary retention (Ghafoor et al, 2007). Ropivacaine, a newer agent, has been shown to have less motor blockade and less potential for cardiotoxicity (Heitz and Vuscusi, 2005).
ALPHA 2-ADRENERGIC AGONISTS
The primary effect of alpha 2-adrenergic drugs is activation of ascending inhibitory pathways on postsynaptic nociceptors in the dorsal horn, thereby blocking pain (Ghafoor et al, 2007). Clonidine, the most widely used alpha 2-adrenergic agonist, is a centrally acting drug that was initially approved for use as an antihypertensive medication; however, because of sedating side effects, it has been more frequently used as an analgesic. Side effects may include hypotensive effects and dose-limiting toxicity when used as an analgesic. It is most commonly used in conjunction with morphine or bupivacaine (Heitz and Viscusi, 2005 and Cohen and Dragovich, 2007). Besides hypotension, other side effects include sedation, nausea, dry mouth, and bradycardia. It is thought to be more effective than opiates in treating neuropathic pain (Kedlaya et al., 2002 and Ghafoor et al., 2007).
GABA INHIBITORS
Activation of γ-aminobutyric acid (GABA) receptors results in a decrease in the release of neurotransmitters, and decreased opening of calcium channels to decrease pain. The most commonly used GABA inhibitor is baclofen (Cohen and Dragovich, 2007). Intrathecal administration of baclofen acts directly at the receptor sites in the spinal cord, yielding less systemic toxicity and better therapeutic effect (Zahavi et al, 2004). Baclofen is primarily used in the treatment of spasticity, but may be combined with other medications for pain control. Side effects may include hypotonia, sedation, constipation, erectile dysfunction, loss of sphincter control, and respiratory depression (Ghafoor et al., 2007 and Cohen and Dragovich, 2007).
SELECTIVE N-TYPE CALCIUM CHANNEL BLOCKER
Ziconotide is a non-opioid analgesic that blocks pain transmission by binding with selective N-type voltage-sensitive calcium channels (Ghafoor et al, 2007). This drug must be administered intrathecally to maximize effectiveness. It is used to treat refractory pain in patients with cancer, autoimmune deficiency syndrome (AIDS), and/or chronic pain (Staats et al, 2004). Adverse effects include dizziness, nausea, asthenia, somnolence, diarrhea, confusion, and ataxia (Rauck et al, 2006).
COMPLICATIONS OF INTRASPINAL ACCESS DEVICES
Regardless of the method of delivery (epidural, intrathecal, or intraventricular) employed, complications of nonvascular access devices can be categorized as those related to the procedure, medication side effects, or complications related to the device itself. Familiarity with the type of infusions, catheters, and pumps as well as medication side effects is integral to nursing care provided to the patient, and to manage expected effects and complications should they arise.
MEDICATION SIDE EFFECT MANAGEMENT
Pruritus
Pruritus is common with intraspinal analgesia infusions. This side effect may be a result of an allergic-type reaction or may be caused by the stimulation of histamine release secondary to opiate pain medication. The incidence of pruritus has been identified to be higher with epidural analgesia than with systemic, intramuscular, or intravenous routes (Choi et al., 2003 and Dolin and Cashman, 2005). Nursing actions for pruritus include providing diversional activities, using cool linens or clothing, or administering medications such as Benadryl, nalbuphine, naloxone, or ondansetron (Slowikowski and Flaherty, 2000, Choi et al., 2003, Rathmell et al., 2005 and Kim et al., 2007). Low doses of naloxone, an opioid antagonist, have been shown to reverse pruritus without affecting the analgesic properties of epidural morphine (Choi et al, 2000). Nalbuphine is an opioid agonist-antagonist used for the treatment of pruritus. Titration of both naloxone and nalbuphine must be completed carefully and slowly to avoid reversal of analgesic effects of the opiate pain medications (Choi et al., 2000, Slowikowski and Flaherty, 2000 and Kim et al., 2007). If an allergic reaction is suspected, Benadryl can be given to interfere with the histamine release causing the pruritus, leading to cessation of scratching, but Benadryl is rarely effective if the pruritus is not allergic (Slowikowski and Flaherty, 2000 and Rathmell et al., 2005). Pruritus is rarely seen with chronic intraspinal opioid administration as tolerance commonly develops.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


