CHAPTER 27 1. Examine briefly common neonatal ECMO pathophysiology. 2. Discuss indications and contraindications for neonatal ECMO. 3. Discuss the criteria used to determine an infant’s need for neonatal ECMO. 4. Examine common perfusion techniques in neonatal ECMO. 5. Review the technical and mechanical aspects of the neonatal ECMO procedure. 6. Review the physiology of neonatal extracorporeal circulation. 7. Discuss the general care given to infants undergoing the ECMO procedure and the support provided to their families. 8. Review follow-up and outcome of neonatal ECMO survivors. Extracorporeal life support (ECMO) was developed initially by surgeon John Gibbon as a heart–lung bypass machine for use in adult open heart surgeries. Currently, neonatal ECMO has been in use for over three decades, and has transitioned from a rarely utilized lifesaving modality to a routine practice in major children’s centers across the nation. Employed initially to manage newborn respiratory failure in 1975 (Bartlett, 2010), neonatal ECMO presently aids in the management of multiple pathologies from cardiac anomalies to overwhelming sepsis. In fact, the Extracorporeal Life Support Organization (ELSO), founded in 1989, has created a national registry for ECMO cases; ELSO reported in 2010 that 20,000 of the over 40,000 ECMO cases were newborns (Bartlett, 2010). With this increase in use and understanding, survival rates have improved and there has been an ushering in of new neonatal ECMO technology, including membrane lungs, safe centrifugal pumps, and vascular access. A. ELSO International Registry Report (Neonatal ECMO Registry Report, 2008). 2. After a peak of ECMO cases during the years 1989 to 1995 (927 to 1181 cases per year), the number of ECMO cases for neonates has decreased (472 to 571 cases per year) for the years 2003 to 2008, while the survival rate has declined to 63% to 68%. This decline in both cases and survival reflects a change in the kinds of neonates who require ECMO support, having failed the more sophisticated ventilator management, surfactant, and use of inhaled nitric oxide that is currently standard practice (Ford, 2006). B. New technology (2008). 1. Roller pumps began to be replaced by safer centrifugal pumps (Bartlett, 2010). 2. A new generation of membrane lungs developed. 3. Increased capabilities of vascular access. When discussing neonatal ECMO, the practitioner must keep in mind a strong understanding of fetal circulation, including the three primary shunts: the ductus venosus, patent ductus arteriosus (PDA), and patent foramen ovale (PFO). Additionally, the practitioner should also have a firm understanding of the most common disease processes associated with the utilization of neonatal ECMO, including but not limited to the management of the underlying pulmonary hypertension, related to sepsis, MAS, pneumonia, CDH, or idiopathic PPHN (Shelley and Rees, 2010). Pulmonary hypertension in the newborn period is characterized by elevated pulmonary vascular resistance forcing deoxygenated blood to shunt right to left through a PDA or PFO. Subsequently, there is mixing of oxygenated and deoxygenated blood in the aorta followed by systemic hypoxia and acidosis resulting in a persistent state of increased pulmonary vascular resistance (Short, 2010). Pulmonary vascular resistance can be decreased primarily through an increase in arterial oxygenation (PaO2), use of nitric oxide, and a correction in acidosis. Additionally, the patient may require correction of polycythemia, hypocalcemia, and systemic hypotension, as well as the need for gentle ventilation and sedation. Once these methods have been employed and failed, the patient may require being placed on ECMO (Short, 2010). The goal is to properly identify patients who would benefit from ECMO while simultaneously limiting those patient populations in which the potential risks outweigh the benefits. Thus, inclusion criteria were developed to aid centers in ECMO candidate selection. These criteria are guidelines and are not mandates (Shelley and Rees, 2010). A. Neonatal ECMO patient criteria. 1. Gestational age greater than 34 weeks. 2. Birth weight greater than 2000 g; small-for-gestational-age infants should not be excluded. 3. Reversible lung disease. 4. No significant coagulopathy or uncontrolled bleeding—should be corrected prior to initiating ECMO, if possible. 5. Intracranial hemorrhage grade 2 or less. 6. Failure of optimal medical management. 7. No lethal anomalies or brain injuries. 8. No major cardiac malformations (except in infants requiring stabilization and life support before or after surgery). 9. Must have decision to provide “full support” (Shelley and Rees, 2010). B. Acute and reversible pathology. 1. Respiratory distress syndrome. 2. MAS. 3. PPHN. 4. CDH. 5. Sepsis. 6. Pneumonia. 7. Life support before or after cardiac surgery. 8. Acute respiratory distress syndrome. C. Cranial and cardiac ultrasonography findings ruling out severe intracranial hemorrhage and cyanotic congenital heart disease. D. Objective criteria for final selection predictive of greater than 80% mortality rate (Box 27-1). To achieve specificity, each ECMO center must determine its own mortality indicators and criteria. Criteria may differ in different disease states, such as septic shock, CDH, or severe air leak caused by barotrauma. E. Pre-ECMO stabilization, including optimal ventilatory management and trial of high- frequency ventilation, volume support, vasopressors, vasodilator medications, surfactant, and nitric oxide if indicated. These should be used at a center where ECMO can be initiated quickly if the infant does not respond adequately (Shelley and Rees, 2010). Prior to placing a patient on ECMO, a multidisciplinary team must decide what modality of perfusion delivery will be best for the patient and the patient’s particular disease process. The two most common approaches are venoarterial (VA) and venovenous (VV). VA support, the most widely used ECMO modality in the United States, is a technique whereby the cannulas are inserted to permit drainage of deoxygenated blood from a large vein or veins (Heard et al., 2010a). Subsequently, the blood is circulated through an artificial lung for oxygenation, and returned to the body through a major artery such as the aorta. Conversely, VV ECMO, which is gaining ground in the neonatal population, utilizes only the venous system for both drainage and return of blood to the patient. A. Technique for VA perfusion. 2. Venous cannula must be a size that is capable of delivering total cardiac output (120 to 150 mL/kg/min) to the membrane lung; cannulas of largest possible internal diameter (8 F to 14 F) are inserted. 3. Oxygenated blood is returned through a cannula placed into the ascending aorta via the right common carotid artery. 4. Achieves approximately 60% to 80% of normal cardiac output (Heard et al., 2010a). B. Advantages. 2. Positive pressure ventilation can be reduced to minimal parameters: peak inspiratory pressure, 15 to 25 cm H2O; positive end-expiratory pressure, 5 to 10 cm H2O; respiratory rate, 10 to 20/minute; and fractional concentration of oxygen in inspired gas (FiO2), 21% to 30%. C. Disadvantages. 1. Emboli (air or particulate) could be infused directly into the arterial circulation. 2. Ligation of carotid artery may be permanent and subsequently affect cerebral perfusion. 3. Greater potential for left ventricular “cardiac stun” (Fukuda et al., 1999). A. Technique for VV perfusion. 2. Blood is returned through the arterial limb, also located in the right atrium, with side holes positioned at the tricuspid valve. 3. Blood flow is directed across the valve, into the right ventricle, and through the pulmonary circulation. It returns to the left atrium before entering the systemic circulation via the aorta. B. Advantages (Heard et al., 2010b). 1. No ligation of the carotid artery is necessary. 2. Oxygenated blood flows through pulmonary circulation, which may help reverse pulmonary hypertension. 3. Oxygenated blood is provided to the coronary arteries. 4. Emboli (air or particulate) are less likely to result in severe compromise to the infant because blood is not returned directly to the arterial circulation. 5. May eliminate potential for left ventricular cardiac stun. C. Disadvantages. 2. Recirculation of oxygenated blood can occur. Oxygenated blood returned to the right atrium may be emptied again into the venous side of the double-lumen cannula, rather than across the tricuspid valve. 3. Use of somewhat higher ventilatory support may be required because lower flow rates are achieved with the smaller lumens of the double-lumen cannula. 4. Vasopressor therapy may need to be continued to support blood pressure and ECMO flow. 5. Greater potential for right ventricular cardiac stun. Neonatal care and equipment are constantly being modified and improved, and neonatal ECMO equipment is not different. Rather than use the circuits utilized for short runs of cardiac bypass, novel biocompatible circuits and improved centrifugal pumps controlled by highly advanced computerized servo-regulated systems are emerging. Additionally, vascular access cannulas are being improved to provide reliable care and enhance outcomes (Harris et al., 2010). 2. Some manufacturers have developed surface bonding materials for circuit tubing and cannulas, in an effort to minimize the activation of complement, platelets, and inflammatory mediators, and these are thought to be less thrombogenic (Harris et al., 2010). 3. Blood circulates throughout all components of the ECMO circuit; parenteral fluids, medications, and blood products are administered into the venous side of the circuit (before the membrane oxygenator). 4. Only platelets are infused on the arterial side of the circuit, or into the patient directly, to prevent adherence to the membrane oxygenator. 5. Precautions and guidelines for placing medications and blood products into the circuit are the same as those for safely administering medications and blood products directly to patients. B. Pumps. 1. Roller/occlusive: uses the principle of fluid displacement utilizing two rollers placed opposite of one another to pull blood from the right atrium and push blood forward through the circuits tubing (Harris et al., 2010). a. Servo-regulation of venous return: bladder reservoir, pressure monitoring with roller pump. b. Collapsible silicone bladder distends with returning venous blood. c. If using a roller head pump, inadequate flow (decreased venous return) into the ECMO circuit causes the bladder to collapse, which triggers a microswitch and an audible alarm and stops the roller head pump. d. When the bladder reexpands, the microswitch engages the pump and normal pump operation continues. e. Adequate venous return is critical for maintaining cardiorespiratory support; therefore, the cause of decreased return must be recognized and corrected immediately. f. Servo-mechanism regulation of ECMO flow can also be achieved by transducers placed in the circuits. Premembrane (venous) pressure and postmembrane (arterial) pressure may be monitored continuously to signal extracorporeal flow problems before collapse of the silicone bladder. This allows for early detection and timely intervention. (1) Fall in premembrane pressure indicates decreased venous return. (2) Rise in postmembrane pressure indicates malfunction of membrane oxygenator or heat exchanger. g. Other devices have been recently introduced to regulate venous flow, including a thin-walled section of tubing encased in rigid housing that is mounted vertically in the circuit, and does not have the clotting issues associated with the conventional silicone bladder (Hansell, 2005). h. Advantages. (1) Can be operated manually in case of power failure. (2) Lower cost. (3) Less initial volume of blood needed to prime circuit. (4) Flow not dependent on patient’s afterload status. (5) Used for more than 20 years and can equate to greater experience and comfort level of ECMO providers. i. Disadvantages. (2) Requires use of servo-regulated bladder box or pressure servo-regulator. (3) Total pressure delivered by the rollers to the circuit is critical in delivery calculations. (4) Limited availability of circuits due to transition of many ECMO centers to the centrifugal pump. 2. Centrifugal/constrained vortex/nonocclusive pump: works on the principle of a constrained vortex in which an object spins in a fluid environment. The spinning results in an area of low pressure at the nucleus of the vortex, and an area of high pressure results at the periphery. The centrifugal pump contains a plastic housing composed of a single inlet located in the center low-pressure region and outlet positioned along the high-pressure outer border (Harris et al., 2010). (2) If the patient’s volume fluctuates, the RPMs remain steady, resulting in less negative (volume decrease) or positive pressure (volume increase) on the atrium. (3) Can be operated by hand or short-term battery backup. b. Disadvantages. (1) Inaccuracy of flow measurements at low RPMs. C. Gas-exchange devices. a. Maximizes gas exchange across the membrane. b. Only oxygenator currently approved by the U.S. Food and Drug Administration for long-term use. c. No direct blood-to-gas interface. 2. Polypropylene (microporous) hollow-fiber oxygenator—contains small tubes with tiny pores through which gas exchange takes place (Harris et al., 2010). a. Potential advantage during priming; can have bioactive coating. b. Direct blood-to-gas interface. c. Designed for short-term use (~ 2 hours). d. Good for rapid response scenarios. 3. Polymethylpentene (PMP) hollow-fiber oxygenator—contains microporous tubes with tiny pores through which gas exchange takes place (Harris et al., 2010). a. Potential advantage during priming; can have bioactive coating. b. Direct blood-to-gas interface. c. Can be used for up to 6 hours. d. Good for rapid response scenarios. D. Heat exchanger. 1. Located after the oxygenator, or incorporated into the oxygenator. 2. Can add or subtract heat from the blood before returning it to the infant’s circulation. 3. Heat loss occurs from cooling effect of ventilating gases inside the oxygenator and circuit exposure to ambient air temperature. 4. Important for maintenance of homeostasis of clotting functions and physiologic functions. E. ECMO console. 1. Monitors circuit pressure on both the venous and arterial sides of the circuit. 2. Provides bubble detection. 3. Thorough automated alarm system, including servo-regulation of the pump when aberrant conditions are discovered. F. Bubble detector. 2. In some systems, when air is detected, the roller head pump is shut off and flow to the patient ceases. 3. Critical importance in VA ECMO. G. Activated clotting time (ACT) monitoring. 1. Because heparin is used to prevent clotting, ACT is the most common bedside method to measure anticoagulation in the ECMO patient (Harris et al., 2010). 2. Use to titrate heparin infusion based on ECMO center’s desired ACT ranges and required effects. 3. Initial bolus of heparin during cannulation (usually 100 units/kg). 4. Infusion pump for continuous infusion of heparin solution at 25 to 100 units/kg/hr into ECMO circuit. 5. Titration of heparin solution to keep ACT within the desired range (usually 180 to 220 seconds, but acceptable at 160 to 250 seconds). 6. For control of heparin administration, no heparin added to any other medications or fluids (an exception may be the fluids being infused into umbilical or peripheral arterial lines). 7. Factors that influence heparin requirements: thrombocytopenia, abnormal clotting studies, urinary output, and infusions of blood products that contain clotting factors. H. Blood gas monitoring. 2. Arterial blood gas measurements obtained beyond the membrane oxygenator in the ECMO circuit reflect the function of the membrane lung. 3. Patient blood gas values and noninvasive oxygen saturation monitoring are used to assess the recovery of lung function and the infant’s acid–base balance. I. Cannulas. 2. Before insertion of the cannulas, the infant is paralyzed and given opiates to prevent respiratory movement and air embolism, and systemic heparin is given to prevent clotting of the cannulas and the circuit; this is because when circulating blood comes into contact with artificial surfaces, coagulation is activated (Annich and Miskulin, 2005). 3. In VA ECMO, the venous cannula tip is positioned in the right atrium to drain blood flow from the inferior vena cava and the superior vena cava. The arterial cannula tip reaches just to the aortic arch. 4. In VV ECMO, the double-lumen cannula is commonly placed in the internal jugular vein, and is positioned in the right atrium, with the arterial side directed toward the tricuspid valve. 5. Double-lumen cannulas are now available that could potentially avoid cannulation of two vessels in order to avoid the carotid artery (Harris et al., 2010). The primary goal behind ECMO is to sufficiently supply the body with oxygen so that metabolic requirements are met while removing the body of subsequent waste by-products (Rees and Waldvogle, 2010). The body naturally exchanges gases at the alveolar and tissue levels. Oxygenation of the blood is achieved during ECMO through the use of a membrane oxygenator. The arterial partial pressure of oxygen (PaO2) and carbon dioxide (CO2) are the primary forces in diffusion of gases across the membrane. CO2 exchange is independent of blood flow, and dependent on gas diffusion gradient, sweep gas flow rate, and membrane surface area. Conversely, PaO2 is independent of sweep gas flow rate, yet dependent on blood flow rate, blood path thickness, membrane diffusion thickness, PaO2 concentrations, and membrane surface area. ECMO improves PaO2 delivery by improving PaO2 content through stabilization of the saturation of hemoglobin, taking 60% of the cardiac output away from native lung, and providing a stable source of delivery via the membrane oxygenator (Rees and Waldvogle, 2010). 2. As bypass flow increases, flow through the pulmonary artery decreases faster than bypass flow and reduces total flow in the systemic circulation, causing peripheral and pulmonary hypotension. 3. Blood volume replacement is required for optimal tissue perfusion. 4. ECMO perfusion is nonpulsatile (pulse contour decreases as flow rate increases); kidneys interpret this as inadequate flow and promote the release of renin and aldosterone, which causes sodium retention, extracellular fluid expansion, and a decreased total body potassium concentration. 5. Total patient flow is the sum of ECMO flow and pulmonary blood flow; adequate flow is reached when oxygen delivery and tissue perfusion result in normoxia, normal pH, normal SvO2, and normal organ function. 6. Total gas exchange and support are achieved at a flow rate of 120 to 150 mL/kg/min. B. Gas exchange. 2. Carbon dioxide diffuses from the blood compartment to the gas compartment as a result of a pressure gradient between venous carbon dioxide pressure and the ventilating gas. The carbon dioxide transfer rate is 6 times greater than that of oxygen transfer. The ventilating gas mixture is usually enriched with carbon dioxide to prevent hypocapnia. C. Blood–surface interface. 2. Clot formation is prevented by systemic heparinization; platelet destruction is minimized by preexposure of the circuit to albumin. 3. Platelets show the greatest effect of exposure to a foreign surface, as evidenced by decreased platelet count (thrombocytopenia) and function. 4. Hemolysis is monitored regularly by measuring plasma free hemoglobin levels. The level is usually not significantly altered by ECMO flow, although increases may indicate problems with red blood cell destruction in the membrane oxygenator or small-lumen cannula. 5. All types of white blood cells decrease in concentration, and phagocytic activity is significantly decreased. 6. After cessation of ECMO, platelets and white blood cell counts return to normal. The neonate requiring ECMO requires a collection of highly trained personnel each with unique skills and training. The team composition minimally consists of the pediatric surgical team, neonatologist specializing in the management of ECMO, and an ECMO specialist (physician [MD], registered nurse [RN], registered respiratory therapist [RRT], or perfusionist) to operate and manage the circuit, as well as the bedside nurse (Williams and Short, 2010). The following is an outline of the responsibilities of the ECMO specialist and the bedside nurse in the care of the infant during ECMO (Nugent and Matranga, 1997; Rais-Bahrami and Powell, 2010; Remenapp et al., 2005; Sheehan, 1999). 2. See Table 27-1 for nursing responsibilities and interventions. TABLE 27-1 Nursing Responsibilities and Interventions for ECMO
Extracorporeal Membrane Oxygenation
ECMO: A HISTORICAL PERSPECTIVE
COMMON NEONATAL ECMO PATHOPHYSIOLOGY
CRITERIA FOR USE OF ECMO
ECMO PERFUSION TECHNIQUES
Venoarterial Perfusion
Venovenous Perfusion
CIRCUIT COMPONENTS AND ADDITIONAL DEVICES
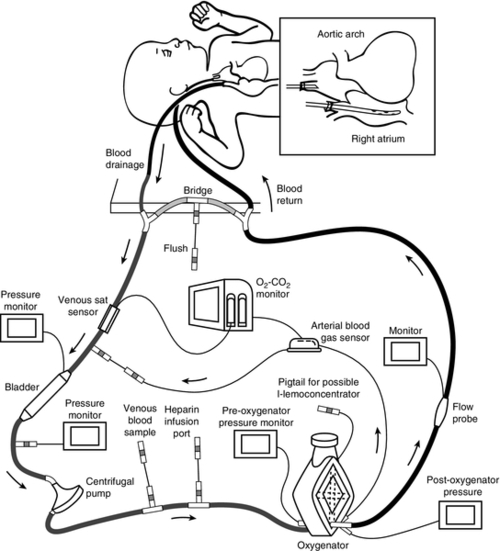
PHYSIOLOGY OF EXTRACORPOREAL CIRCULATION
CARE OF THE INFANT REQUIRING ECMO
Responsibility
Intervention
BEFORE CANNULATION
Obtain and document baseline physiologic data.
Record weight, length, and head circumference.
Draw blood samples for CBC, electrolytes, calcium, glucose, BUN, creatinine, PT/PTT, platelet count and function, ABGs, ACT.
Record vital signs: heart rate; respiratory rate; systolic, diastolic, and mean blood pressure; and temperature.
Ensure adequate supply of blood products for replacement.
Draw, type, and cross-match samples for 2 units of packed red blood cells and fresh frozen plasma.
Keep 1 unit of packed cells and fresh frozen plasma always available in the blood bank and a separate set at the bedside.
Diagnostic exams.
Pre-ECMO CXR.
Pre-ECMO HUS.
Pre-ECMO ECHO.
Maintain prescribed pulmonary support.
Maintain ventilator parameters.
Administer muscle relaxants if indicated.
Assemble and prepare equipment.
Prepare infusion pumps to maintain arterial lines and infusion of parenteral fluids and medications into the ECMO circuit.
Place the infant on a radiant warmer with the head positioned at the foot of the bed to provide thermoregulation and access for cannulation.
Attach infant to physiologic monitoring devices to monitor heart rate, intra-arterial blood pressure, transcutaneous oxygen, and other parameters.
Circuit priming meds and fluids.
To be added to circuit for priming: 2 units PRBCs; Plasmalyte A, 1000 mL; albumin 25%, 50 mL; heparin, 200 units; sodium bicarbonate, 20 mEq; calcium gluconate, 200 mg.
Labs drawn from prime.
ABGs, whole blood sodium, whole blood potassium, whole blood ionized calcium, whole blood glucose, hemoglobin, and hematocrit.
Insert urinary catheter and NG tube; place to gravity drainage.
Remove IV lines just prior to heparinization (optional).
Prepare loading dose of heparin (50 to 100 units/kg).
Prepare heparin solution for continuous infusion (100 units in 30-mL syringe to be infused into circuit at 25 units/kg/hr).
Prepare and infuse pain medication: morphine sulfate 0.03 mg/kg/hr or fentanyl 1 mcg/kg/hr.
Prepare paralyzing drug (pancuronium bromide, 0.1 mg/kg, or succinylcholine, 1 to 4 mg/kg).
Assist in insertion of arterial line (umbilical or peripheral).
Administer prophylactic antibiotics.
Additional meds/fluids to have on hand.
D10W, 250-mL bag; D5W, 250-mL bag; NS, 250-mL bag.
Optional to be administered by surgeon—cryoprecipitate, 10 mL; calcium chloride 10%, 5 mL; bovine thrombin, 5000 units/5 mL.
Be prepared to administer cardiopulmonary support.
Have medications and blood products available to correct hypovolemia, bradycardia, acidosis, and cardiac arrest.
DURING CANNULATION
Monitor cardiopulmonary status during procedure.
Monitor heart rate and intra-arterial blood pressure continuously.
Obtain blood gas values after paralysis and during cannulation, as indicated by the infant’s response to the procedure.
Administer medications.
Give loading dose of heparin systemically when vessels are dissected free and are ready to be cannulated.
Give paralyzing drug systemically just before cannulation of internal jugular vein if infant has not been previously paralyzed. Give analgesia for anesthetic effect.
Reduce ventilator parameters to minimal settings.
Once adequate bypass is achieved, reduce PIP to 16 to 20 cm H2O, PEEP to 4 cm H2O, ventilator rate to 10 to 20 breaths per minute, and FiO2 to 21% to 30%. Patients undergoing VV bypass may require greater respiratory support.
DURING ECMO RUN
Monitor and document physiologic parameters.
Record hourly and PRN: heart rate, blood pressure (systolic, diastolic, mean), respirations, temperature, oxygen saturation, ECMO flow.
Measure hourly accurate intake and output of all body fluids (urine, gastric contents, blood); test all stools for occult blood.
Assess hourly: color, breath sounds, heart tones, murmurs, cardiac rhythm, arterial pressure waveform, peripheral perfusion.
Assess hourly: level of consciousness, reflexes, tone, and movement of extremities.
Assess every 8 to 12 hours: neurologic examination, including fontanelle tension, pupil size, and reaction.
Assess weight and head circumference daily.
Circuit check per ECMO perfusion flow sheet.
Routine labs.
All other blood specimens are drawn from ECMO circuit by ECMO specialist: hourly ABGs until stable; ACT every 2 hours, platelets every 6 hours, aPTT every 6 hours × 48 hours, then daily.
Daily: plasma-free hemoglobin, heparin assay, fibrinogen, D-dimer; electrolytes, calcium, platelets, Chemstrip, creatinine, total and direct bilirubin.
Record ventilator parameters hourly.
Administer medications.
Remove air bubbles and double-check dosages before infusion.Place all medications and fluids (except heparin drip) into the venous side of the ECMO circuit or central venous line.
Prepare and administer the arterial line (umbilical or peripheral) infusion.
NPO, OG to gravity, and administer parenteral alimentation.
Provide pulmonary support.
Perform gentle endotracheal suctioning according to individual assessment and need.
Maintain patent airway; be alert to extubation or plugging.
Obtain daily chest films, head ultrasounds, and tracheal aspirate cultures as indicated.
Maintain ventilator parameters.
Heparin (bleeding) precautions.
Avoid all of the following: rectal probes, injections, venipunctures, heel sticks, NG tubes (oral only), nasal suctioning, administration of medications intramuscularly or by venipuncture.
Avoid invasive procedures. Do not change OG tube, urinary catheters, or endotracheal tube unless absolutely necessary; use gentle premeasured endotracheal tube suction technique.
Observe for blood in urine, stools, and endotracheal OG tubes.
Maintain excellent infection control.
Change all fluids and tubing daily.
Change dressings daily and as needed.
Maintain closed system for urinary catheter drainage.
Maintain strict aseptic and handwashing techniques.
Use universal barrier precautions.
Provide physical care.
Keep skin dry, clean, and free of pressure points.
Monitor blood loss from cannula site.
Give mouth care as needed.
Provide ROM every 8 hours or as indicated.
Turn side to side every 1 to 2 hours.
Nonnutritive sucking every 4 hours.
Provide pain management, sedation, stress reduction.
Minimize noise level.
Cluster patient care to maximize sleep period.
Administer analgesia: fentanyl or morphine as continuous IV drip.
Manage iatrogenic physical dependency by following dose reduction regimen.
Be alert to complications and emergencies.
See text.
Social services consult.
AFTER ECMO RUN
Head CT and MRI prior to discharge. ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


27: Extracorporeal Membrane Oxygenation
FIGURE 27-1 ■ Configuration of neonatal centrifugal ECMO circuit. (Adapted with permission from Short, B., and Williams, L. (Eds.): ECMO specialist training manual (3rd ed.). Ann Arbor, Mich., 2010, Extracorporeal Life Support Organization.)