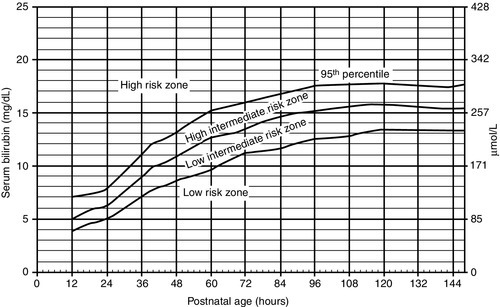
CHAPTER 23 Barbara Elizabeth Pappas; Deanna Lynn Robey 1. Review fetal development for the last 6 weeks of gestation. 2. Discuss the health and developmental risks for late preterm infants. 3. Describe emerging standards of care for the late preterm infant. 4. Identify short- and long-term outcome concerns for late preterm infants. Neonates born between 340/7 weeks and 366/7 weeks of gestation continue to account for 8.28% of all deliveries and more than 70% of the preterm population (Hamilton et al., 2012). Outcome data continue to be collected, but mortality and morbidity risks have been found to be increased. The late preterm infant is at greater risk for medical problems; has a seven-fold increased risk of morbidity, and consumes more health care dollars than full-term infants (Shapiro-Mendoza et al., 2008). A standard of care that is evidence-based has been developed for these infants (Medoff-Cooper et al., 2010). Often not ill enough to justify care in the neonatal intensive care unit, the late preterm neonate is often cared for in the normal newborn nursery where policies and care models are focused toward the needs of the full-term infant. Thorough assessment of risk and health status, interventions, and parent education for these neonates should be individualized (Campbell, 2006). These infants will have a size, weight, and overall appearance similar to that of a full-term infant, and because of this, parents and professional caregivers may tend to treat them similarly to full-term infants. These late preterm infants may present with factors that increase their risk for morbidity (Mohan and Jain, 2011) (Box 23-1). Communication among staff members is essential to provide optimal, high-quality care for these infants. There are a number of complications that can arise after birth while in the hospital and/or after discharge that require close observation (Medoff-Cooper et al., 2012) (Box 23-2). a. Decreased brown and subcutaneous fat. b. Decreased glycogen stores and ability to convert stored glycogen. c. Neuromuscular immaturity with decreased ability to flex extremities and decrease surface area. d. Increased susceptibility to large temperature gradient between neonate and environment, especially during transition. e. Immature epidermal barrier, which leads to increased transepidermal water loss. f. High metabolic rate with decreased ability to generate heat. 2. Risk factors: b. Increased susceptibility to heat loss. c. Increased comorbidities with hypothermia, such as hypoglycemia, respiratory distress, and respiratory and/or metabolic acidosis. 3. Clinical management (Medoff-Cooper et al., 2010): (2) Evaluate temperature every 4 hours for the first 24 hours of life, then if stable, once per shift until discharge. b. Warm and humidify oxygen and keep infant away from drafts to decrease convective losses. c. Encourage skin-to-skin holding, breastfeeding, and rooming-in. d. Provide additional layers of clothing, prewarmed hats, and blankets. e. May need additional support from incubator or radiant warmer—use servo-control to maintain normal temperature. f. Utilize institutional protocol for weaning to open crib. g. Delay first bath until transition is complete. Bath should be given under warming lights and last less than 10 minutes. h. Observe for signs of cold stress: tachypnea, color change (pallor, mottling, cyanosis), and lethargy. B. Respiratory distress (see Chapter 24). a. Respiratory system is one of the last systems to mature. b. Normal lung fluid production and absorption for gestational age suggest difficulty with clearance of fluid, increased fluid retention, and delayed completion of transition. c. Surfactant production incomplete. d. Increased chest compliance, decreased muscle mass, and muscle immaturity. e. Central nervous system immaturity and increased risk for apnea and bradycardia. f. Decreased airway stability due to larger head size and decreased neck stability. g. Higher percentage of fetal hemoglobin and risk for tissue and end-organ hypoxia. 2. Risk factors: b. More likely to require mechanical ventilation if delivered by cesarean section without labor (Jain and Dudell, 2006; Gyamfi-Bannerman, 2012). 3. Common conditions: a. Transient tachypnea of newborn. b. Respiratory distress syndrome. c. Pneumonia. d. Pulmonary hypertension of the newborn. e. Apnea and bradycardia of prematurity. 4. Clinical management (Medoff-Cooper et al., 2010): b. Evaluate the cardiac system: heart rate and perfusion every 4 hours in the first 24 hours, and then once per shift until discharge. c. Maintain a neutral thermal environment to prevent excessive energy consumption and increased oxygen requirements. d. Pulse oximeter: monitor oxygenation. e. Arterial blood gases: monitor ventilation and acid–base balance. f. Supplemental respiratory support as needed. C. Sepsis (see Chapter 32). a. Immature immune system despite normal cell counts. b. Decreased transference of maternal antibodies. c. Impaired skin integrity. d. Increased exposure to microorganisms due to prolonged hospitalization and invasive tests and procedures. 2. Risk factors: a. Maternal group β-hemolytic streptococcus (GBS) status positive or unknown. b. Previous delivery of a baby with GBS infection. c. Prolonged rupture of membranes (> 18 hours). d. Inadequate antenatal antibiotic prophylaxis. e. Maternal temperature greater than 100.4° F and/or diagnosed chorioamnionitis. 3. Clinical management: (1) Change in feeding behaviors, poor feeding. (2) Lethargy, hypotonia, irritability, jitteriness. (3) Temperature instability. (4) Respiratory distress. (5) Apnea and bradycardia. (6) Hypotension, poor perfusion. (7) Vomiting, diarrhea, gastric distention. (8) Hypoglycemia. (9) Jaundice. (10) Rashes, pustules, or other skin lesions. b. If signs of infection are observed, obtain cultures and treat with antibiotics. (2) Vital signs monitored every 4 hours or more frequently as needed. c. Identify if criteria for respiratory syncytial virus prophylaxis are met (Medoff-Cooper et al., 2010): (1) Household contains more than five people. (2) Small for gestational age. (3) Presence of environmental pollutants, such as tobacco smoke. (4) Preschool-age siblings. (5) Child care attendance. (6) Male gender. D. Hyperbilirubinemia (see Chapter 29). b. Decreased gastric motility. c. Increased normal breakdown of red blood cells. d. Bilirubin levels generally peak by 5 to 7 days of life; increased risk for kernicterus (Askin et al., 2007). e. Late preterm infants have an 8 times higher risk for total bilirubin level ≥ 20 (Medoff- Cooper et al., 2012). 2. Risk factors for hyperbilirubinemia requiring treatment or kernicterus (Medoff-Cooper et al., 2010): (1) Jaundice appearing in first 24 hours. (2) Presence of cephalohematoma or extensive ecchymosis. (3) Exclusive breastfeeding, suboptimal feeding, and risk of dehydration. (4) Blood group incompatibility. (5) Previous sibling who required phototherapy. (6) Predischarge total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) in the high-risk zone on the bilirubin nomogram (Fig. 23-1).
Care of the Late Preterm Infant

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


23: Care of the Late Preterm Infant
FIGURE 23-1 ■ Nomogram for designation of risk. (From American Academy of Pediatrics: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics, 114[1]:297-316, 2004.)
