Regulations, Microscope Setup, and Quality Control
Objectives
After completing this chapter you should be able to:
Laboratory Regulations
Microscopic Procedure
Quality Assurance, Quality Control, and Risk Management
1. Discuss the 10 areas of Good Laboratory Practices.
2. List and describe the three analytical phases of laboratory testing requiring quality assessment.
3. Explain the difference between quality assurance and quality control.
4. Define accuracy, precision, and reliability when observing the results of standard controls.
5. Identify trends, shifts, random error, out-of-control, and patient panic values.
6. Discuss current risk management and HIPAA issues as they apply to the physician’s office laboratory.
Key Terms
accuracy (correctness) when controls consistently fall within two standard deviations of the mean
calibration the process of setting an instrument to accurately respond to the test reagents or devices
certificate of waiver (CoW) CLIA document that allows a facility to perform only waived tests
CLIA-waived tests tests that provide simple, unvarying results and require a minimum amount of judgment and interpretation
control sample a manufactured specimen that has a known value of the analyte being tested
external controls liquid positive and negative controls that are tested before the patient specimen to check the reliability of the instrument and the testing technique
internal control built-in positive control used in qualitative tests to prove the device or test kit is working
kit all components of a test packaged together
Levy–Jennings chart a graph used to plot and visualize the results of control samples over time
mean the average test result of a series of control tests
medical office risk management overseeing the physical and procedural risks that may bring about an injury or legal action against the practice
optics check confirming that the light source and light sensor in optical analyzers are working properly
precision (reproducibility) ability to produce the same test result each time a test is performed
proficiency testing proving laboratory competency by testing a sample specimen from an outside accreditation agency and obtaining the correct result
protected health information (PHI) any health information in any form (written, electronic, or oral) that contains patient-identifiable information (e.g., name, Social Security number, telephone number) that must be kept confidential
qualitative test test that simply looks for the presence or absence of a substance
quality assurance (QA) overall process to aid in improving the reliability, efficiency, and quality of laboratory testing in general
quality control (QC) process in which known samples (controls) are routinely tested to establish the reliability, accuracy, and precision of a specific test system
quantitative test test that produces a numerical value indicating the amount of a substance present
reagent substance or ingredient used in a laboratory test to detect, measure, examine, or produce a reaction
reliability when both accuracy and precision are accomplished
semiquantitative test determines the approximate quantity of an analyte
standard deviation (SD) statistical term describing the amount of variation from the mean in a data set
work practice controls policies that are recorded, monitored, and evaluated with a view to protecting employees from exposure to the pathogens in blood or body fluids
Abbreviations
CLIA Clinical Laboratory Improvement Amendments
CMS Centers for Medicare and Medicaid Services
FDA Food and Drug Administration
HIPAA Health Insurance Portability and Accountability Act
PPM provider-performed microscopy
PPMP Certificate provider-performed microscopic procedures certification
This chapter presents additional government regulations, microscope setup, and quality assurance procedures as they apply to the medical laboratory.
 CLIA: GOVERNMENT REGULATIONS
CLIA: GOVERNMENT REGULATIONS
Whenever a specimen is removed from a human body and an analysis occurs that translates to a result, that activity is considered a medical laboratory test. As stated earlier, physicians use test results for the following reasons: (1) to screen and detect possible diseases, (2) to confirm a clinical diagnosis and make treatment decisions, and (3) to monitor the progress of diseases and treatments. If testing is not performed properly, the incorrect results can pose a threat to the patient’s overall care and treatment. For example, if a clinical laboratory misreads or misreports a patient’s blood sample as having a normal cholesterol level when in fact the patient’s cholesterol is abnormally high, that patient may not receive the necessary treatment to prevent a heart attack.
To protect patients from inaccurate test results, Congress passed the Clinical Laboratory Improvement Amendments (CLIA) in 1988. CLIA 1988 required that all laboratories examining materials derived from the human body for diagnosis, prevention, or treatment purposes be certified by the Secretary of Health and Human Services (HHS). Centers for Medicare and Medicaid Services (CMS) administers the certification process by requiring all medical laboratories to register and pay a certification fee based on the level of complexity of the tests they perform.
CLIA Levels of Complexity and Their Certification Requirements
There are four complexity levels: high complexity, moderate complexity, provider-performed microscopy (PPM; a subset of moderate complexity), and waived testing. The Food and Drug Administration (FDA) is involved in determining the level of complexity for all commercial medical laboratory tests on the market.
CLIA Certificate of Waiver
The CLIA Certificate of Waiver (CoW) is used predominantly by physician’s office laboratories (POLs), ambulatory settings, and institutions with point-of-care testing (POCT).
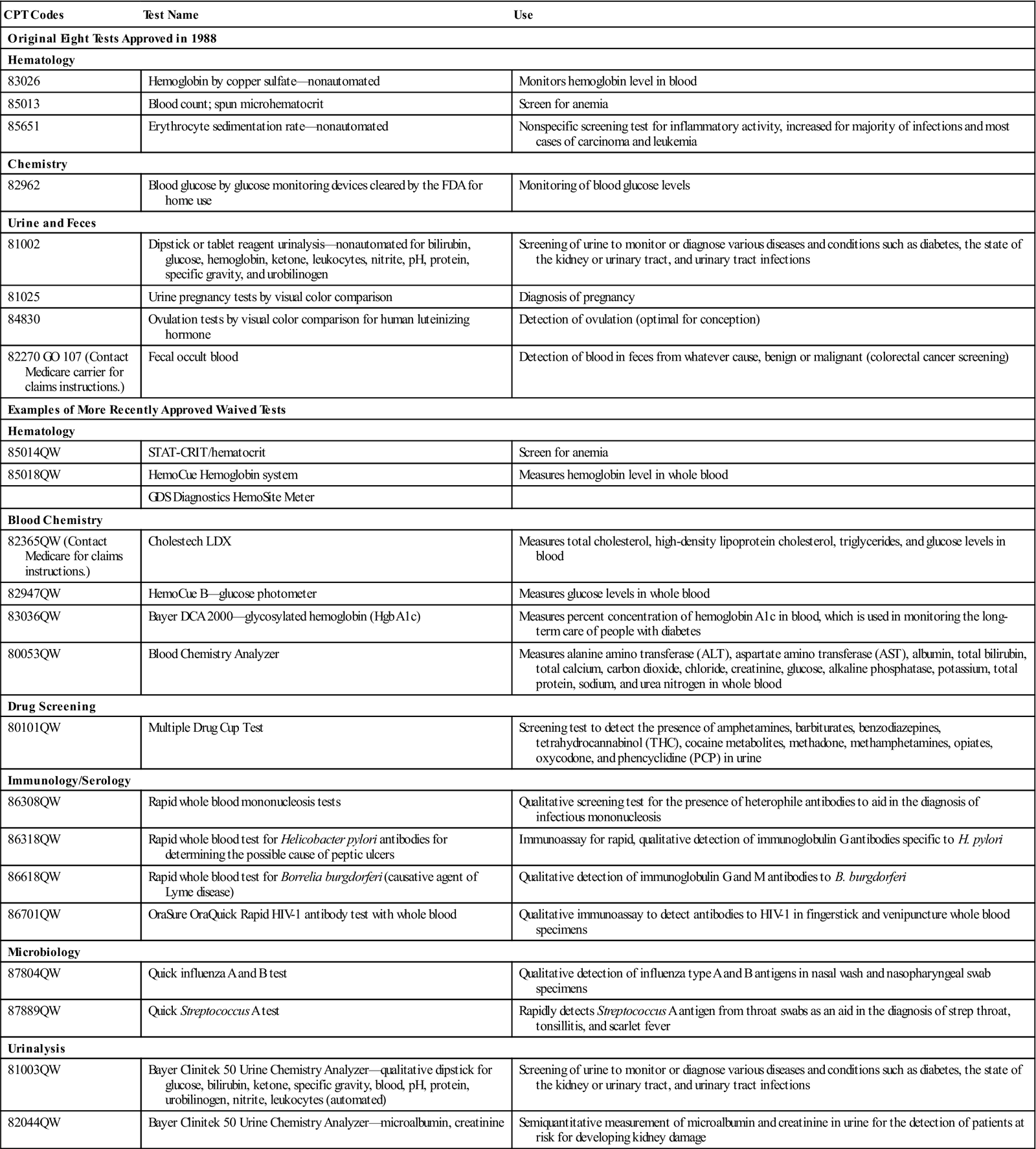
According to CLIA, CoW laboratories can perform only tests that are determined by the FDA to be so simple that there is little risk of error. The CLIA-waived tests with their appropriate procedure codes are listed in Table 2-1.
TABLE 2-1
Tests Granted Waived Status Under CLIA
| CPT Codes | Test Name | Use |
| Original Eight Tests Approved in 1988 | ||
| Hematology | ||
| 83026 | Hemoglobin by copper sulfate—nonautomated | Monitors hemoglobin level in blood |
| 85013 | Blood count; spun microhematocrit | Screen for anemia |
| 85651 | Erythrocyte sedimentation rate—nonautomated | Nonspecific screening test for inflammatory activity, increased for majority of infections and most cases of carcinoma and leukemia |
| Chemistry | ||
| 82962 | Blood glucose by glucose monitoring devices cleared by the FDA for home use | Monitoring of blood glucose levels |
| Urine and Feces | ||
| 81002 | Dipstick or tablet reagent urinalysis—nonautomated for bilirubin, glucose, hemoglobin, ketone, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen | Screening of urine to monitor or diagnose various diseases and conditions such as diabetes, the state of the kidney or urinary tract, and urinary tract infections |
| 81025 | Urine pregnancy tests by visual color comparison | Diagnosis of pregnancy |
| 84830 | Ovulation tests by visual color comparison for human luteinizing hormone | Detection of ovulation (optimal for conception) |
| 82270 GO 107 (Contact Medicare carrier for claims instructions.) | Fecal occult blood | Detection of blood in feces from whatever cause, benign or malignant (colorectal cancer screening) |
| Examples of More Recently Approved Waived Tests | ||
| Hematology | ||
| 85014QW | STAT-CRIT/hematocrit | Screen for anemia |
| 85018QW | HemoCue Hemoglobin system | Measures hemoglobin level in whole blood |
| GDS Diagnostics HemoSite Meter | ||
| Blood Chemistry | ||
| 82365QW (Contact Medicare for claims instructions.) | Cholestech LDX | Measures total cholesterol, high-density lipoprotein cholesterol, triglycerides, and glucose levels in blood |
| 82947QW | HemoCue B—glucose photometer | Measures glucose levels in whole blood |
| 83036QW | Bayer DCA 2000—glycosylated hemoglobin (Hgb A1c) | Measures percent concentration of hemoglobin A1c in blood, which is used in monitoring the long-term care of people with diabetes |
| 80053QW | Blood Chemistry Analyzer | Measures alanine amino transferase (ALT), aspartate amino transferase (AST), albumin, total bilirubin, total calcium, carbon dioxide, chloride, creatinine, glucose, alkaline phosphatase, potassium, total protein, sodium, and urea nitrogen in whole blood |
| Drug Screening | ||
| 80101QW | Multiple Drug Cup Test | Screening test to detect the presence of amphetamines, barbiturates, benzodiazepines, tetrahydrocannabinol (THC), cocaine metabolites, methadone, methamphetamines, opiates, oxycodone, and phencyclidine (PCP) in urine |
| Immunology/Serology | ||
| 86308QW | Rapid whole blood mononucleosis tests | Qualitative screening test for the presence of heterophile antibodies to aid in the diagnosis of infectious mononucleosis |
| 86318QW | Rapid whole blood test for Helicobacter pylori antibodies for determining the possible cause of peptic ulcers | Immunoassay for rapid, qualitative detection of immunoglobulin G antibodies specific to H. pylori |
| 86618QW | Rapid whole blood test for Borrelia burgdorferi (causative agent of Lyme disease) | Qualitative detection of immunoglobulin G and M antibodies to B. burgdorferi |
| 86701QW | OraSure OraQuick Rapid HIV-1 antibody test with whole blood | Qualitative immunoassay to detect antibodies to HIV-1 in fingerstick and venipuncture whole blood specimens |
| Microbiology | ||
| 87804QW | Quick influenza A and B test | Qualitative detection of influenza type A and B antigens in nasal wash and nasopharyngeal swab specimens |
| 87889QW | Quick Streptococcus A test | Rapidly detects Streptococcus A antigen from throat swabs as an aid in the diagnosis of strep throat, tonsillitis, and scarlet fever |
| Urinalysis | ||
| 81003QW | Bayer Clinitek 50 Urine Chemistry Analyzer—qualitative dipstick for glucose, bilirubin, ketone, specific gravity, blood, pH, protein, urobilinogen, nitrite, leukocytes (automated) | Screening of urine to monitor or diagnose various diseases and conditions such as diabetes, the state of the kidney or urinary tract, and urinary tract infections |
| 82044QW | Bayer Clinitek 50 Urine Chemistry Analyzer—microalbumin, creatinine | Semiquantitative measurement of microalbumin and creatinine in urine for the detection of patients at risk for developing kidney damage |
The number and types of tests waived under CLIA have increased from the eight originally approved tests in 1992 to more than 40. The number of waived laboratories has also grown exponentially, from 20% to more than 55% of the total laboratories enrolled in CLIA.
To become a CLIA-waived laboratory, the physician or facility simply needs to enroll in the CLIA-waived program through CMS, pay an applicable certification fee of $150 every 2 years, and follow the manufacturer’s instructions for each FDA-approved waived test.
CLIA High and Moderately Complex Laboratories
Hospital and reference laboratories are staffed with highly educated laboratory professionals who have been trained to perform the complex tests in the various laboratory departments, such as hematology, chemistry, microbiology, and blood bank. To be registered and certified by CLIA, laboratory professionals must obtain a Certificate of Registration, then be surveyed to receive a Certificate of Compliance and then a Certificate of Accreditation. They must maintain their accreditation status by being surveyed periodically to validate that they are performing only tests within their level of complexity and that their personnel are qualified and sufficiently trained in the test procedures.
All testing procedures are continuously monitored by the following systems:
Further details in high and moderate complexity testing may be found at the government websites for HHS, CMS, CDC, and FDA. The purpose of this text, however, is to explore the lesser-regulated CLIA certificates most commonly used in physicians’ offices, ambulatory care, and point-of-care settings, specifically the CLIA-waived tests and the PPM tests.
CLIA: Provider-Performed Microscopy Procedures Certificate
The PPM Procedures (PPMP) Certificate allows qualified health care providers to do waived testing and basic microscopic examinations during the patient’s visit. The microscopic tests are performed on specimens that are not easily transportable. To receive PPMP certification, the physician or medical facility must enroll in the CMS program for PPMP certification, pay applicable certificate fees of $200 every 2 years, and maintain certain quality assurance (QA) and administrative requirements such as the following:
• Proficiency testing by an outside agency to evaluate microscopic accuracy twice a year
• Documentation of microscope/centrifuge main-tenance
• Confirmation that competent personnel are interpreting the microscopic findings
Some of the microscopic tests that require proper preparation before being interpreted are presented in this text. The preparations for these microscopic tests include the following:
• Urine sediment preparation as part of a complete urinalysis
• Potassium hydroxide preparations for identifying fungi
The actual reading and reporting of the microscopic findings must be done by the physician or a laboratory professional who is trained and certified to interpret the particular test. Table 2-2 lists the approved PPM tests and their numerical Current Procedural Terminology (CPT) codes.
TABLE 2-2
CLIA Approved Provider-Performed Microscopy Tests
| Q0111 | Wet mounts, including preparations of vaginal, cervical, or skin specimens |
| Q0112 | All potassium hydroxide preparations |
| Q0113 | Pinworm examinations |
| Q0114 | Fern test |
| Q0115 | Postcoital direct, qualitative examinations of vaginal or cervical mucus |
| 81015 | Urinalysis, microscopic only |
| 81000 | Urinalysis, by dipstick or tablet reagent for bilirubin, glucose, hemoglobin, ketones, leukocytes, nitrite, pH, protein, specific gravity, urobilinogen, and any number of these constituents; nonautomated, with microscopy |
| 81001 | Urinalysis, by dipstick or tablet reagent for bilirubin, glucose, hemoglobin, ketones, leukocytes, nitrite, pH, protein, specific gravity, urobilinogen, and any number of these constituents; automated, with microscopy (NOTE: This may be used only when the laboratory is using an automated dipstick urinalysis instrument approved as waived.) |
| 81020 | Urinalysis; two- or three-glass test |
| 89055 | Fecal leukocyte examination |
| G0027 | Semen analysis; presence and motility of sperm, excluding Huhner test |
| 89190 | Nasal smears for eosinophils |
MICROSCOPE PROCEDURE
If the physician chooses to perform basic microscopic examinations of specimens, his or her employees will need to learn how to set up a microscope slide for observation and how to focus and maintain the microscope for optimal performance. The procedure for using a clinical microscope is described in the following section. (A microscope procedure skill checklist can also be found in the workbook at the end of Chapter 2.)
Preparation: Identifying the Parts and Functions of a Microscope
The microscope can be divided into the following three basic functional areas:
1. The foundational structures—consisting of the base, arm, and stage
2. The illuminating structures—consisting of the light source and condenser
3. The magnifying structures—consisting of the objectives lenses, the ocular lenses, and the focus adjustment knobs. As you look at the picture of the microscope in Fig. 2-1, note the name of each lettered structure in the text as it is described in the three subsequent categories.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


