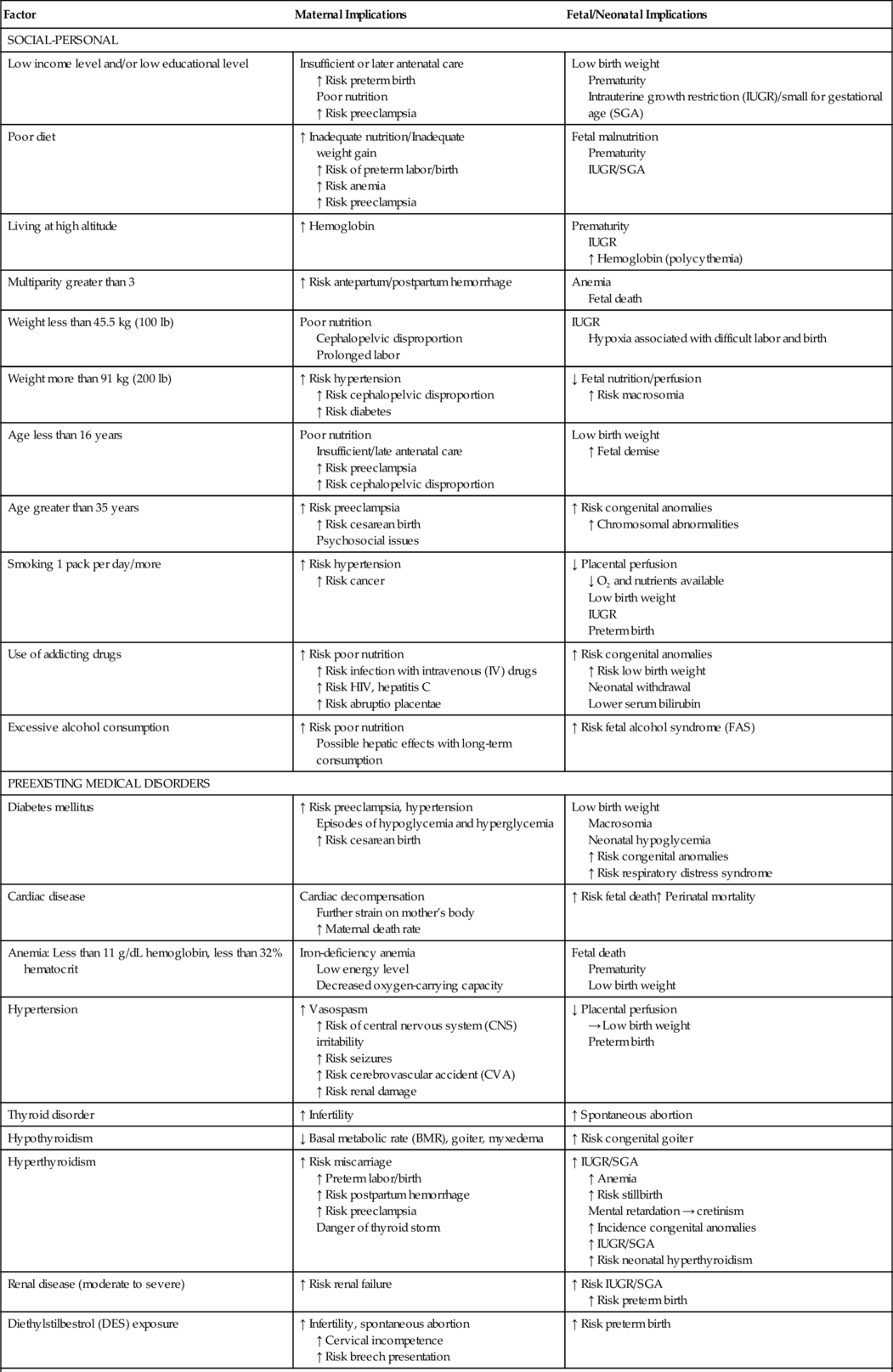
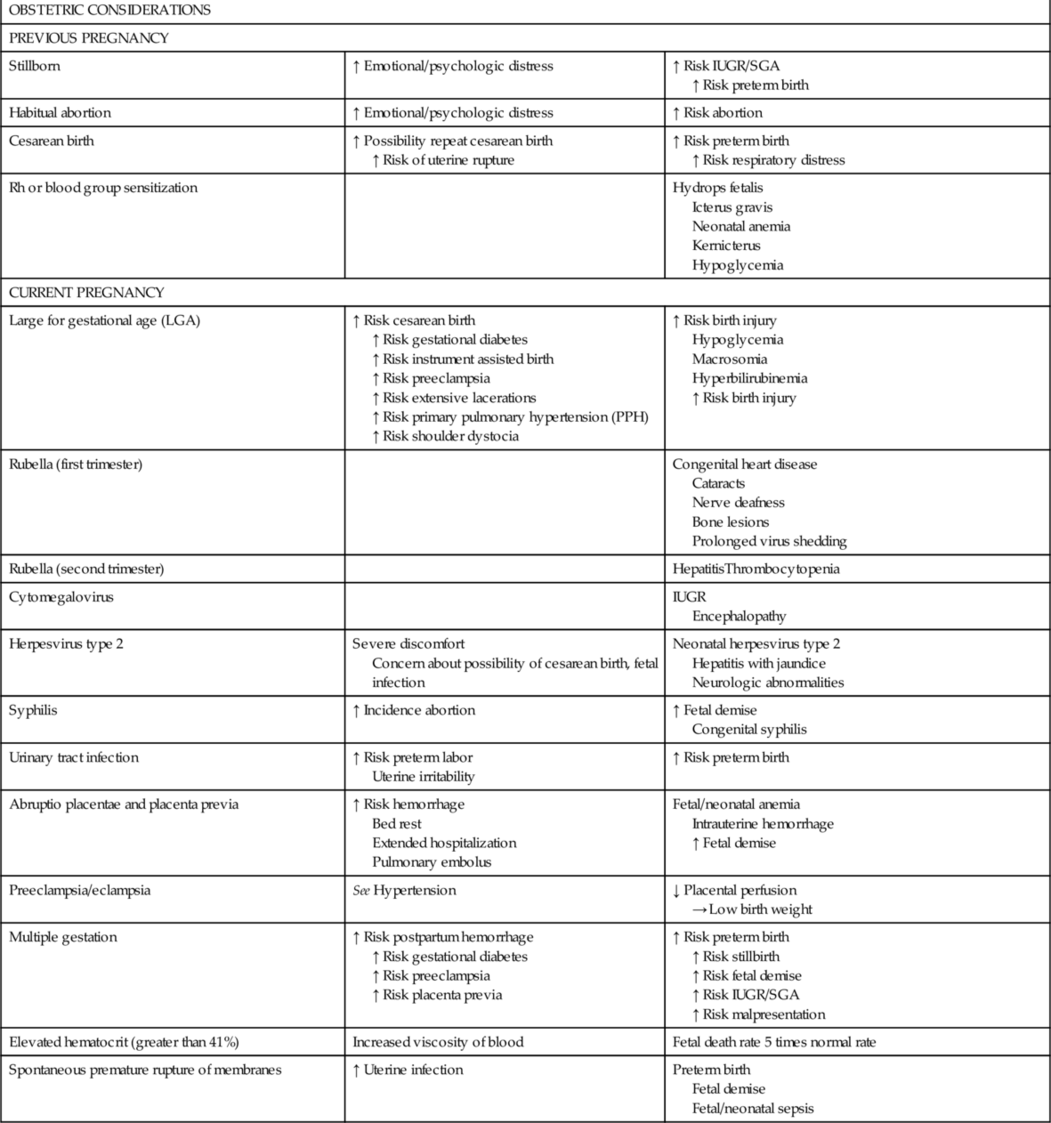
CHAPTER 2 1. List maternal risk factors that may exist before pregnancy. 2. Discuss the effects of hypertension and diabetes on the maternal–placental–fetal complex. 3. Categorize intrapartum conditions that may result in complications for the newborn infant. 4. Assess the fetus/neonate for effects of tocolytic drugs. 5. Describe the effect on the fetus/neonate of select intrapartum crises: placental abruption, placenta previa, cord prolapse, and shoulder dystocia. 6. List neonatal complications associated with breech delivery. 7. Examine the effect of obstetric analgesia/anesthesia and cesarean birth on the newborn infant. An understanding of maternal complications enhances the ability of the nurse to anticipate and recognize neonatal complications and intervene appropriately. The purpose of this chapter is to provide a comprehensive review of possible neonatal complications resulting from maternal risk factors. These risk factors may exist before the pregnancy or develop during the antepartum and intrapartum periods (Table 2-1). A. The fetus. The fetus is a part of the maternal–placental–fetal complex. B. Conditions and substances that affect the pregnant woman. These have the potential to affect placental functions of respiration, nutrition, excretion, and hormone production. Decreased placental function can in turn adversely affect the fetus. C. The placenta. The placenta is the connection between the maternal and embryonic circulatory systems, facilitating metabolic and nutrient exchange. Functions of the placenta include fetal nutrition, respiration and excretion. Placental development begins at implantation and the placenta becomes a discrete organ by 14 weeks of gestation (London et al., 2011). D. Placental transport mechanisms. These mechanisms, including passive and facilitated diffusion, are affected by a number of factors (Baschat, 2011; Burton et al., 2012; Ross et al., 2012). b. A placenta that is not keeping pace with fetal growth or that has decreased functional area as a result of infarct or separation does not allow optimal transport of materials between the fetus and the mother. c. The outcome of decreased functional placental area can include a decrease in fetal growth, fetal or neonatal distress, and even fetal or neonatal death. 2. Concentration gradient. b. The greater the concentration gradient, the faster will be the rate of diffusion. c. Concentration gradients are maintained when dissolved substances are removed from the plasma by metabolism, cellular uptake, or excretion. For example, the excretion of CO2 from the maternal lungs maintains the concentration gradient for CO2, permitting fetal plasma CO2 to cross from fetal plasma to maternal plasma. Inefficient maternal excretion of CO2 may lead to maternal respiratory acidosis and fetal acidosis. 3. Diffusing distance. b. Any edema that develops in the placental villi increases the distance between the fetal capillaries within the villi and the maternal arterial blood in the intervillous spaces, thus slowing the diffusion rate of substances between the maternal and fetal circulations. c. Edema of villi may occur in: (2) Transplacental infections. (3) Erythroblastosis fetalis. (4) Twin-to-twin transfusion syndrome (donor twin). (5) Fetal congestive heart failure. d. Thinning of the placental membrane in the second half of pregnancy decreases diffusing distance, thus increasing the functional efficiency of the placenta. However, this change also facilitates the passage of drugs in pregnancy and the intrapartum period. 4. Uteroplacental blood flow. b. Decreased blood flow to the uterus or within the intervillous spaces will decrease the transport of substances to and from the fetus. c. Causes of decreased uteroplacental blood flow include: (1) Maternal vasoconstriction in hypertension, cocaine abuse, diabetic vasculopathy, and smoking. (2) Maternal vasodilatation caused by vasodilators, antihypertensives, and regional anesthetics with sympathetic blockade actions. (3) Decreased maternal cardiac output in supine hypotension. (4) Decreased maternal blood flow in intervillous spaces resulting from edema of the placental villi. (5) Tachysystole (>5 contractions in 10 minutes, averaged over 30 minutes) (American College of Obstetricians and Gynecologists [ACOG], 2010). (6) Increased uterine resting tone (7) Severe maternal physical stress. (8) Degenerative placental changes near term. 5. Fetal factors. b. Conversely, fetal bradycardia resulting from hypoxia or anoxia leads to an increased CO2 level. Fetal bradycardia, in the absence of congenital heart disease, represents an acute decrease in oxygen. c. Umbilical cord compression leads to CO2 accumulation and acidosis. d. Fetal pH during labor is usually 0.1 to 0.15 unit less than the maternal pH; this difference increases the transport of acidophilic substances from the mother to the fetus and reduces albumin binding of drugs, resulting in a more free drug in the fetal bloodstream. Hypertensive disorders in pregnancy, including gestational hypertension, preeclampsia and eclampsia, chronic hypertension, and chronic hypertension with superimposed preeclampsia, are a major cause of maternal–fetal morbidity and death in the United States. The main pathophysiologic events in preeclampsia are vasospasm, hematologic changes, and endothelial damage, leading to tissue hypoxia and multiple organ involvement. B. Etiology/predisposing factors (Sibai, 2012; Dekker, 2011). 2. Preeclampsia and eclampsia are associated with nulliparity, extremes of maternal age (teenagers and age >40 years), family history of preeclampsia, preeclampsia in a previous pregnancy, obesity, maternal low birth weight, chronic inflammatory conditions (lupus, rheumatologic disease), a history of gestational diabetes or type 1 diabetes mellitus, chronic hypertensive or renal disease, thrombophilias (factor V Leiden mutation, antiphospholipid syndrome), multifetal gestation, or large fetus. Other predisposing factors include poor outcomes in a previous pregnancy such as placental abruption, fetal death, and intrauterine growth restriction (IUGR) in previous pregnancies. C. Clinical presentation. 2. Proteinuria (≥300 mg/dL in a 24-hour urine collection) due to decreased renal perfusion resulting in the development of glomerular capillary endotheliosis. 3. Edema is no longer considered to be a relevant factor in the diagnosis of preeclampsia, as one third of preeclamptic patients may not exhibit edema. However, if a pregnant woman demonstrates a rapid increase in generalized edema, she should be screened for preeclampsia (Dekker, 2011). 4. Other signs and symptoms: headache, hyperreflexia with clonus, visual and retinal changes, irritability, nausea and vomiting, epigastric pain, dyspnea, and oliguria. D. Potential complications. a. Eclampsia (grand mal seizure). b. Cardiopulmonary failure and pulmonary edema. c. Hepatic failure, hemorrhage or rupture. d. Cerebrovascular accident. e. Renal cortical necrosis. f. Disseminated intravascular coagulation. g. HELLP syndrome (i.e., hemolysis, elevated liver function test results, and low platelet count). h. Retinal detachment. i. Stroke or death (rare). j. Long-term cardiovascular morbidity. 2. Placental/fetal. a. Premature placental aging, placental infarction, and decrease in amniotic fluid. b. Abruptio placentae in 1% to 4% of cases, depending upon the severity of the disease (Dekker, 2011). c. Fetal growth restriction (10% to 25%). d. Fetal hypoxia and neurologic injury (<1%). e. Prematurity (15% to 67%). E. Assessment and management (Dekker, 2011; Sibai, 2012). b. Seizure precautions. c. Placental–fetal function tests: continuous electronic fetal monitoring; fetal movement; ultrasonography to determine fetal age and detect IUGR; serial nonstress tests, biophysical profile, and/or umbilical artery Doppler studies; and amniocentesis to determine fetal lung maturity. d. Medications. (2) Use of antihypertensives is indicated when systolic BPs are greater than 160 mm Hg or diastolic BPs are greater than 110 mm Hg: (b) Hydralazine (Apresoline). Should no longer be considered a first-line drug. Although effective in lowering BP, this drug has many side effects and alternative medications are preferred. Observe fetal heart rate for signs of hypoxia (tachycardia, bradycardia, or late decelerations), which can occur with a sudden decrease in maternal BP. (3) Use of corticosteroids to increase fetal lung maturity when birth can be delayed for 24 to 48 hours and the woman is at less than 34 weeks of gestation. Infants born to mothers who are treated with antenatal steroid therapy are at decreased risk for respiratory distress syndrome, cerebrovascular hemorrhage, and death. e. Delivery. (2) Severe preeclampsia is not an indication for cesarean section, and the vaginal route is preferred. 2. Eclampsia (Dekker, 2011; Sibai, 2012). a. Immediate notification of physician or midwife. b. Control of current seizure and prevention of recurrent seizures with administration of IV magnesium sulfate. c. Control of severe hypertension with an antihypertensive (labetalol) to maintain the systolic BP between 140 and 160 mm Hg and the diastolic BP between 90 and 105 mm Hg. d. Safety measures for woman during and after seizures to prevent injury. e. Support of respirations with airway, oxygen, and suctioning, and the correction of hypoxemia and/or acidemia. f. Observe fetal heart rate for transient bradycardia, rebound tachycardia, decreased variability, and late decelerations. g. Continuous maternal assessment, including assessment for uterine contractions and signs of placental abruption. h. Laboratory work: complete blood count (CBC), clot observation, serum creatinine, liver function tests, fibrinogen, arterial blood gases, and electrolytes. i. Initiate the process of delivery according to the status of the maternal–placental–fetal complex. The mother must be stabilized before a cesarean section is performed. j. Maternal emotional support. 3. Assessment of newborn infant for the following: b. Preterm gestational age. c. Hypoxia and acidosis. d. Possible adverse drug effects on neonate: (1) When maternal administration of high doses of magnesium sulfate occurs near the time of birth, the newborn must be observed for 24 to 48 hours for signs of magnesium toxicity (respiratory depression and neuromuscular depression, as evidenced by weakness, lethargy, hypotonia, flaccidity, and poor suck) (Davidson et al., 2012). (2) Hypotension, bradycardia, hypoglycemia, respiratory depression, and transient tachypnea, with maternal administration of labetalol. Women with insulin-dependent diabetes who become pregnant, and pregnant women in whom gestational diabetes mellitus (GDM) or type 1 diabetes develops, are at risk during the antepartum period due to altered carbohydrate metabolism. Therefore, the fetus/neonate is also at risk. Optimal control of maternal blood glucose concentration and anticipatory management of the newborn infant are important elements of perinatal care. A. Incidence: 3% to 5% of all pregnancies are complicated by diabetes mellitus and 7% by GDM. B. Etiology and predisposing factors in gestational diabetes. 2. Risk factors for GDM include maternal obesity, previous history of gestational diabetes, a family history of diabetes, age greater than 25 years, member of an ethnic group at risk for diabetes (Native North American, Hispanic, African American, Pacific Islanders, and South or East Asian Americans), and prior obstetric history (infant weighing >4500 g, congenital anomaly, stillbirth, hydramnios). C. Clinical presentation and screening for gestational diabetes. 2. Women at high risk for GDM (severe obesity, strong family history of type 2 diabetes mellitus, previous history of gestational diabetes, impaired glucose metabolism, or glycosuria) should be screened at the first prenatal visit, because GDM may be asymptomatic or evidenced only by subtle changes. Screening should be repeated at 24 to 28 weeks of gestation, or when hyperglycemia is evident. 3. Women with average risk for GDM should be screened with a 1-hour glucose challenge test at 24 to 28 weeks of gestation, with further testing if values are abnormal. 4. Women who are low risk for GDM (ethnic group with a low prevalence of GDM, no known primary relative with diabetes mellitus, age <25 years, normal weight prior to pregnancy, no history of poor obstetric outcomes, no history of abnormal glucose metabolism) may not require glucose screening. D. Potential complications. a. Hypoglycemic reactions in the first trimester. b. Ketoacidosis in the second and third trimesters. c. Progression of vasculopathy, nephropathy, and retinopathy with preexisting diabetes. d. Hydramnios. e. Gestational hypertension and preeclampsia. f. Anemia. g. Infections such as monilial vaginitis and urinary tract infections. h. Increased rates of episiotomy, perineal tears, and cesarean section (Perry et al., 2010). 2. Fetal/neonatal: Outcomes can be improved via careful attention to prepregnancy and pregnancy glycemic control. a. Macrosomia (weight >4000 g) with possible traumatic vaginal birth, such as with shoulder dystocia, and subsequent birth trauma. IUGR when the mother has vascular disease (Davidson et al., 2012). b. Fetal death. c. Respiratory distress syndrome. d. Hypoglycemia, hypocalcemia, and hypomagnesemia. e. Polycythemia, hyperviscosity, and hyperbilirubinemia. f. Cardiomyopathy with congestive heart failure. g. Congenital anomalies as a consequence of poorly controlled preexisting diabetes may include anencephaly, open spina bifida, holoprosencephaly, ventricular septal defects, transposition of the great vessels, sacral agenesis, or caudal dysplasia. Currently, 30% to 50% of perinatal mortality results from congenital anomalies in pregnancies in which the mother has type 1 or type 2 diabetes. E. Assessment and management. b. Glycosylated hemoglobin tests may be performed before conception and during the pregnancy to assess glucose control during the previous 1 to 2 months, with an acceptable hemoglobin A1c goal of 5% to 6%. c. Home blood glucose monitoring, diet, exercise, and either insulin pump therapy or several daily injections of insulin are prescribed to maintain euglycemia (60 to 120 mg/dL). Optimal control is associated with a decreased risk of macrosomia, respiratory distress syndrome, congenital anomalies, and perinatal death, as well as maternal urinary tract infection and preterm labor. d. Women are evaluated early in pregnancy for evidence of diabetic retinopathy and nephropathy. e. In the first trimester, screening for nuchal translucency, free β-human chorionic gonadotropin (β-hCG) and pregnancy-associated plasma protein A (PAPP-A) should be offered (Fraser and Farrell, 2011). f. At 16 weeks of gestation, the woman should be offered maternal serum α-fetoprotein (MSAFP) testing, accompanied by a comprehensive ultrasound at 18 to 21 weeks to assess for the presence of neural tube defects or other anomalies. An alternative choice of testing may be the triple screen (MSAFP, maternal serum unconjugated estriol, and β-hCG) or the quad test, which includes inhibin A in addition to the three elements of the triple screen (Fraser and Farrell, 2011). Ultrasounds for fetal growth should be performed monthly between 28 and 36 weeks of gestation. Fetal and Doppler umbilical artery velocimetry is also recommended. g. Weekly prenatal visits are made after 28 weeks, with fetal assessment by means of nonstress tests, biophysical profiles, and daily fetal movement counting. h. Timing and mode of delivery are based upon the clinical picture of both the woman and the fetus, and some evidence suggests delivery at 38 weeks of gestation. Birth should take place in a facility with a neonatal intensive care unit (NICU). i. Insulin may be given intravenously during labor to maintain blood glucose between 70 and 90 mg/dL. Glucose levels are monitored hourly to ensure optimum titration of insulin in order to decrease the risk of neonatal rebound hypoglycemia. j. Before any decision is made about induction of labor, amniocentesis is performed to determine the lecithin/sphingomyelin ratio and the presence of phosphatidylglycerol. Delivery is accomplished before term if maternal or fetal complications develop. 2. In gestational diabetes: b. Self-monitoring of blood glucose is encouraged, with fasting and 2-hour postprandial glucose levels checked regularly; and c. Nonstress testing begins at 28 to 32 weeks of gestation. 3. In the neonate: a. Assess for gestational age and size (large for gestational age or IUGR). b. Assess for: (2) Hypoglycemia, hypocalcemia, and hypomagnesemia; (3) Polycythemia and hyperviscosity; (4) Complications resulting from decreased blood flow, erythrocyte hemolysis, and thrombosis; (5) Congenital malformations; and (6) Birth injuries: fractured clavicles, intracranial bleeding, facial nerve paralysis, brachial palsy, and skull fractures. Preterm labor is labor occurring at greater than 20 and before 37 completed weeks of gestation. Preterm labor is defined as the presence of uterine contractions and documented cervical change. Threatened preterm labor is defined as the presence of uterine contractions without cervical change. The prognosis for the fetus improves with each week of pregnancy gained. A. Incidence: In 2012, 11.7% of live births in the United States were preterm, and complications from prematurity are the leading cause of perinatal death (March of Dimes, 2012b). B. Etiology/predisposing factors. 2. A number of maternal factors have been associated with an increased incidence of preterm labor: maternal age (<15 or >35 years), socioeconomic effects (lower socioeconomic status or educational level, African American race, poor nutrition, inadequate prenatal care), medical/obstetric history (use of assisted reproductive technologies, anemia, preexisting or gestational hypertension or diabetes, previous preterm birth, prior stillbirth, grand multiparity, one or more midtrimester pregnancy losses, pregnancy termination, short interpregnancy interval, uterine anomalies and cervical insufficiency, systemic and genitourinary tract infections, hydramnios, immunologic factors, placental abruption, and placenta previa), and lifestyle factors (use of alcohol, cigarettes, and illicit drugs such as cocaine, and domestic violence or other stressors) (Perry et al., 2010). 3. Fetal factors contributing to the development of preterm labor may include fetal congenital anomalies and complications from multifetal gestation (Perry et al., 2010). 4. Risk scoring systems, designed to screen women during pregnancy, have a predictive value of only 17% to 34%. Many women who give birth before term do not have any known risk factors. C. Clinical presentation. 1. Painless or painful persistent uterine contractions. 2. Low, dull, intermittent, or constant backache. 3. Intermittent or constant menstrual-like cramping. 4. Pelvic pressure. 5. Abdominal cramps, which may be accompanied by diarrhea. 6. Increased vaginal discharge, which may be mucoid, watery, or slightly bloody. 7. Spontaneous premature rupture of membranes. 8. A generalized feeling that something is wrong. 9. Progressive cervical effacement and dilatation. a. Cervix >3 cm dilatation/≥80% effaced: Preterm labor diagnosis is confirmed. b. Cervix 2 to 3 cm dilatation/<80% effaced: Preterm labor likely but not established. c. Cervix <2 cm dilatation/<80% effaced: Diagnosis uncertain. D. Potential complications. a. Side effects from tocolytic agents. b. Emotional stress and financial issues. c. Complications from the mode of delivery, especially when a cesarean section is performed at less than 30 to 32 weeks of gestation as the lower uterine segment is poorly developed; there is a greater chance of infection, hemorrhage, and subsequent poor uterine function. 2. Fetal/neonatal. a. Preterm birth with an increase in neonatal morbidity and death. b. Risks associated with prematurity, such as respiratory distress syndrome, necrotizing enterocolitis, intracranial hemorrhage, seizures, septicemia, and sequelae of IUGR. c. Side effects from pharmacotherapeutics (tocolytic agents, antibiotics, corticosteroids). E. Assessment and management. 2. Patient education should be provided to all pregnant women, including the symptoms of preterm labor and the actions to take if they occur (lie down on the left side and drink several glasses of fluid; report to the physician or midwife if contractions are still occurring after 1 hour). 3. Helping high-risk women modify their risk factors and take measures to prevent preterm labor (e.g., stop smoking, improve nutrition and hydration, treat infections, decrease work hours and stress, increase rest, and avoid nipple preparation or sexual activity that may initiate signs of preterm labor). 4. Although regular cervical examinations and ultrasonographic evaluation of the cervix are being performed by some providers, these methods have not been validated as predictors of preterm birth. 5. The use of the fetal fibronectin test, performed on vaginal secretions in symptomatic women, may help prevent a false-positive diagnosis of preterm labor and prevent unnecessary and potentially harmful pharmacologic treatment. 6. ACOG and the Society for Maternal Fetal Medicine recommend that women with a singleton pregnancy, who have had a previous episode of a singleton spontaneous preterm labor or birth, or premature rupture of membranes, should receive progesterone supplementation (17α-hydroxyprogesterone caproate) (ACOG, 2008). Studies indicate a 40% decrease in the preterm birth rate of women who had a previous preterm birth, when given 17α-hydroxyprogesterone caproate. 7. Although episodes of suspected preterm labor are widely treated with bed rest, hydration, and pelvic rest, there is little evidence that these interventions are effective (Davidson et al., 2012). Antenatal corticosteroid therapy is recommended for women at risk for preterm birth who are between 24 and 34 weeks of gestation, to reduce the incidence of neonatal morbidity and death from respiratory distress syndrome and intraventricular hemorrhage. 8. When appropriate and not contraindicated, tocolytics should be used to allow enough time for antenatal corticosteroid therapy to benefit the fetus and/or for transfer of the mother to a hospital with a level III nursery. b. Magnesium sulfate, given intravenously before 32 weeks of gestation, appears to have a neuroprotective effect and may decrease the incidence of cerebral palsy. It is recommended that its use be limited to between 24 and 32 weeks of gestation. Data do not support the use of this drug as a tocolytic, and research has not demonstrated that its use prolongs pregnancy. When maternal administration of high doses of magnesium sulfate occurs near the time of birth, the neonate should be monitored for respiratory depression, and neuromuscular depression, as evidenced by weakness, lethargy, hypotonia, flaccidity, and poor suck. c. Prostaglandins (indomethacin) mediate contractions and are effective as tocolytics. Indomethacin is given prior to 32 weeks, orally, and use is restricted to 2 to 3 days. Maternal side effects include nausea, vomiting and dyspepsia. Contraindications to its use include fetal renal anomalies, oligohydramnios, IUGR, chorioamnionitis, ductal-dependent cardiac defects, and twin-to-twin transfusion syndrome. Fetal side effects include oligohydramnios, constriction of the ductus arteriosus, and neonatal pulmonary hypertension. d. Terbutaline (Brethine), a β-mimetic, has commonly been used as a tocolytic, but due to potential side effects its use should be limited to a single 0.25-mg dose subcutaneously. This may be used while a therapy with a slower onset of action is being started, or to stop contractions during the initial evaluation of the patient to assist in the diagnosis of preterm labor. Data indicate that there are few side effects for a single dose. 9. Antibiotic therapy should be instituted to prevent neonatal group B streptococcal infection, or to treat specific conditions such as urinary tract infections. 10. If the measures noted above are not successful, and the cervix continues to efface and dilate, the following measures are important: b. The head is delivered in a slow, controlled fashion. c. Cesarean delivery is often suggested for the preterm fetus with a breech presentation because of the risk of cord prolapse and the potential risk of difficult birth of the head. There is no justification for elective cesarean delivery of all preterm infants. In abruptio placentae, the placenta separates suddenly, prematurely, and in varying degrees from the uterine wall during pregnancy or labor. It is a common cause of bleeding in the second half of pregnancy. A. Incidence: Placental abruption occurs in 1 in 100 pregnancies (March of Dimes, 2013). One third of all bleeding in pregnancy results from placental abruption (Francois and Foley, 2012). B. Etiology/predisposing factors. 1. Although the cause of placental abruption has not been definitively established, there is a high correlation with hypertensive disorders during pregnancy, history of previous abruption, cocaine use, trauma, and placental abnormalities (circumvallate). Additional risk factors include uterine fibroids or malformations, rapid uterine decompression associated with polyhydramnios and multifetal pregnancy, increased parity, chorioamnionitis and intrauterine infections, inherited or acquired thrombophilias, preterm premature rupture of membranes, and maternal cigarette smoking (Francois and Foley, 2012). C. Clinical presentation (Francois and Foley, 2012; Murray and McKinney, 2010). 1. Maternal signs and symptoms. a. Dark or bright red vaginal bleeding, ranging from spotting to frank hemorrhage. In 20% to 30% of patients with abruption, there is no visible evidence of bleeding (Navti and Konje, 2011). b. Abdominal or lower back pain. c. Persistent cramping or sharp, continuous abdominal pain. d. Board-like and tender abdomen. e. Uterine irritability. f. Elevated uterine resting tone. g. Tachysystole. h. Enlargement of the uterus as blood accumulates, with increasing abdominal girth. i. Signs of hypovolemic shock as bleeding increases. 2. Evidence of fetal compromise. a. Loss of fetal heart tones or movement. b. Tachycardia. c. Late or variable decelerations. d. Decreased fetal heart rate variability. B. Potential complications. 1. Maternal (Francois and Foley, 2012; Navti and Konje, 2011). b. Hypovolemic shock. c. Couvelaire uterus (blood forced between the muscle fibers of the uterus). d. Disseminated intravascular coagulation. e. Postpartum hemorrhage. f. Fetomaternal hemorrhage. g. End-organ damage. h. Death. 2. Fetal/neonatal. b. Preterm birth and sequelae associated with prematurity. c. Hypoxemia. d. Hypovolemia. e. Greater risk for long-term neurobehavioral problems. f. Risk for sudden infant death syndrome and ventricular leukomalacia. g. IUGR. h. Perinatal death (10-fold increase). C. Assessment and management. 1. Any episode of bleeding during pregnancy in an Rh-negative woman requires a Kleihauer–Betke test and the administration of Rh immunoglobulin (Murray and McKinney, 2011). 2. Management decisions are based on the severity of the abruption, complications, gestational age, and maternal–fetal status. 3. Ongoing assessment of: a. Amount and nature of bleeding. b. Pain. c. Maternal vital signs. d. Fetal condition. e. Uterine contractions and tone. 4. Management if fetus is stable and maternal hematologic status can be maintained: b. Bed rest in left lateral position, close assessment of abdomen for rigidity and pain, and close assessment of vaginal bleeding. c. Monitoring of maternal vital signs and continuous monitoring of fetal heart for tachycardia, late decelerations, and decreasing baseline variability. d. Insertion of large-gauge IV catheters (16- to 18-gauge) for possible administration of fluids and blood products. e. CBC, coagulation studies, and type and cross-match for blood. f. Possible collection of urine for drug screening if abuse is suspected. g. Possible induction of labor and/or vaginal birth. h. Notify the NICU and the neonatologist/pediatrician. i. Emotional support of woman and family. 5. Preparation for cesarean birth if evidence of fetal compromise or severe hemorrhage occurs: a. Inform and support parents and ensure that surgical consent is obtained. b. Notification of anesthesia department and NICU. c. Laboratory tests as above. d. Preparation of the abdomen for surgery (clipping of hair around incision site) and insertion of an indwelling urinary catheter. Placenta previa is a placenta that is implanted in the lower part of the uterus near the cervix (marginal) or in varying degrees (partial or total) over the cervix. Cervical dilatation at or near term is accompanied by bleeding from the placenta. Placenta previa is a common cause of bleeding in the second half of pregnancy, when the lower uterine segment stretches and thins. A. Incidence: The incidence is 1 in 200 births in the United States (March of Dimes, 2012a. B. Etiology/predisposing factors (Francois and Foley, 2012). 2. Other associated and predisposing factors include previous placenta previa, increasing parity or maternal age, prior cesarean birth, living in higher altitudes, cigarette smoking, maternal race (Asian women have the highest incidence), multifetal gestation, and prior curettage. C. Clinical presentation (Navti and Konje, 2011). 2. Uterine contractions occur in 10% to 20% of cases, but otherwise the uterus is usually soft and nontender. 3. If an ultrasound is performed at less than 20 weeks of gestation, a low-lying placenta may be noted. However, at this gestational age, the lower uterine segment is not yet fully developed and this is not diagnostic of a placenta previa. 4. Failure of presenting part of fetus to become engaged. Fetus may lie transversely or be in a breech position. D. Potential complications. b. Hypovolemic shock. c. Endometritis. d. Decreased contractile strength of the lower uterine segment, which can lead to postpartum hemorrhage and need for hysterectomy. e. Abnormal placental implantation (placenta accreta, percreta, and increta). f. Air embolism. 2. Fetal/neonatal. b. Fetal anemia. c. Malpresentation. d. IUGR. e. Prematurity and subsequent sequelae. E. Assessment and management. 1. Treatment and delivery decisions are based on amount of bleeding, gestational age, cervical status, grade of previa, and condition and presentation of fetus (Navti and Konje, 2011). Any episode of bleeding during pregnancy in an Rh-negative woman requires a Kleihauer–Betke test and the administration of Rh immunoglobulin to Rh-negative, unsensitized women. 2. Marginal or partial placenta previa with minimal bleeding is managed conservatively: a. Serial ultrasounds to confirm diagnosis, rule out IUGR, and monitor fetal growth. b. No vaginal examinations. c. Activity restrictions at home or in the hospital determined by clinical presentation. d. Nutritional supplements and dietary management to prevent anemia. e. Antenatal corticosteroid therapy may be considered. f. Avoidance of intercourse and orgasm, which can cause uterine contractions. g. Time of delivery is based on the clinical picture but generally recommended at 37 weeks when fetal lung maturity is documented. h. Vaginal birth can be planned if the placenta is greater than 2 to 3 cm from the cervical os (Francois and Foley, 2012). 3. Partial or total placenta previa with greater amounts of bleeding is handled as noted above, except that vaginal birth may not be possible. In addition: a. Frequent assessment of vaginal bleeding, with pad counts and/or weighing of pads. b. Frequent assessment of maternal vital signs and fetal heart tones, and palpation of abdomen. c. Laboratory work: CBC, type and cross-match for possible blood transfusion. d. With significant bleeding, placement of IV lines with 16- to 18-gauge catheters for blood administration. e. Cesarean section is the preferred mode of delivery for a partial or complete placenta previa. Timing is dependent on the clinical picture. Umbilical cord prolapse is an event that is life threatening to the fetus and requires immediate and effective management by the nurse. It occurs when the cord falls below the presenting part or is compressed between the presenting part and the pelvis or cervix. A. Incidence: Varies from 1 in 265 to 426 births, with an incidence in vertex presentations of 3% and in breech presentations of 3.7% (Davidson et al., 2012; Steer and Danielian, 2011). B. Etiology/predisposing factors. 2. Predisposing factors include fetal malpresentations such as breech and transverse lie, obstetric manipulations (e.g., amniotomy and forceps), abnormally long cord, preterm labor, low birth weight fetus, multiple gestation, polyhydramnios, lack of engagement before the onset of labor, multiparity, and abnormal placentation (Davidson et al., 2012; Steer and Danielian, 2011). C. Clinical presentation. 1. Cord is protruding from vagina or is palpable on vaginal examination. 2. In an occult prolapse, cord is not visible or palpable but is located between the presenting part and the pelvis or cervix. 3. More commonly occurs with a high station of presenting part, and membranes are often ruptured. 4. Fetal heart rate changes observed may include an abrupt occurrence of persistent, severe variable decelerations or bradycardia. D. Potential complications. a. Trauma to the birth canal from rapid forceps delivery. b. If general anesthesia is used for surgery, may result in uterine atony with subsequent postpartum bleeding. c. Blood loss from cesarean birth. d. Emotional distress. 2. Fetal/neonatal. b. Fetal anoxia leading to long-range neurologic complications. c. Fetal death. E. Assessment and management. 1. Assessments on admission to labor and delivery. a. Presenting part and its station. b. Dilation of cervix. c. Status of membranes. d. Estimation of fetal weight and fetal heart rate. 2. Assessment for presence of polyhydramnios or lack of engagement of presenting part. Ambulation during labor and artificial rupture of membranes may be contraindicated if either of the above is present. 3. Assessment after artificial or spontaneous rupture of membranes. a. Monitor fetal heart rate for changes as indicated above. b. Perform vaginal examination to detect prolapse if indicated. 4. If prolapse has occurred (Steer and Danielian, 2011): b. Monitor fetal heart rate continuously and palpate cord lightly for continued pulsation. Administration of oxygen, insertion of IV lines if not already present, and notification of the anesthesia and neonatology departments. c. An alternative measure is to insert an indwelling catheter to fill the mother’s bladder with sterile saline solution in order to elevate the fetal presenting part so that it is off the cord. d. Help woman into knee–chest or steep Trendelenburg’s position, with hips elevated and head down (Davidson et al., 2012. e. If the cervix is fully dilated and the fetal station is below the ischial spines, vaginal birth may be expedited. However, emergency cesarean delivery may be preferable, especially if the cervix is not fully dilated and the fetus exhibits signs of potential compromise. Shoulder dystocia is an acute emergency in which the physician or midwife is unable to deliver the shoulders of the infant by the usual maneuvers (downward traction) after birth of the head. A. Incidence: Incidence is 0.2% to 3% of all births. As this is a major obstetric emergency that must be acted upon quickly and occurs infrequently, multidisciplinary simulation drills should be instituted in the facility (Gherman, 2011). B. Etiology/predisposing factors. 1. The fetal shoulders are too broad to be delivered between the symphysis pubis and the sacrum. 2. Factors associated with shoulder dystocia include maternal obesity, macrosomia, a history of previous shoulder dystocia, prolonged second stage of labor, diabetes mellitus or impaired glucose metabolism, previous birth of a macrosomic infant, and instrumented midpelvic delivery. However, up to 50% of cases occur with fetuses less than 4000 g (Gherman, 2011; Lanni and Seeds, 2012). C. Clinical presentation. 1. Prolonged transitional phase of labor (8 to 10 cm). 2. Prolonged second stage of labor (>2 hours). 3. After birth of the head, it recoils against the perineum and restitution does not occur (“turtling”). The usual traction from below is not successful in delivering the neonate. D. Complications. a. Third- or fourth-degree lacerations. b. Postpartum hemorrhage. c. Vaginal and cervical lacerations. d. Ruptured uterus. e. Bladder atony. 2. Fetal/neonatal. b. Hypoxia. c. Permanent brain injury. d. Intrapartum or neonatal death. E. Assessment and management (Gherman, 2011; Lanni and Seeds, 2012). 2. Anticipate shoulder dystocia if descent of the head is slow and estimated weight is large. Make sure the woman’s bladder is empty before birth occurs. 3. If shoulder dystocia occurs, the physician or midwife will: a. Use the McRoberts maneuver (maternal hip flexion; an exaggerated lithotomy position). b. Apply suprapubic pressure to attempt to release the anterior shoulder; the pressure may be directly downward or lateral. c. Turn the woman onto her side or pull the hips off the bed to free the sacrum. d. Turn the woman on “all fours” facing downward to widen the pelvic outlet if this can be easily accomplished (Gaskin’s maneuver). e. Manually rotate the shoulders from the anteroposterior to the oblique diameter. f. Use the Woods’ corkscrew maneuver, in which both hands are inserted internally to rotate the posterior shoulder to the anterior position for delivery under the pubic bone, with the maneuver repeated for the other side. g. Deliver the posterior arm. h. The expulsive efforts of the mother, as opposed to traction by the provider, are of the utmost importance. A. Incidence: Incidence is dependent on gestational age, and is 14% at 29 to 32 weeks, 2.2% to 3.7% at term, and overall 3% to 4% of all labors (Penn, 2011). B. Etiology/predisposing factors. a. Polyhydramnios or oligohydramnios. b. Uterine abnormalities (e.g., bicornuate uterus). c. Contracted pelvis. d. Use of anticonvulsant medications or alcohol abuse. 2. Placental/fetal. a. Placenta previa or cornual placenta. b. Multifetal gestation. c. IUGR or fetal anomalies, especially those related to CNS problems. d. Short cord. e. Preterm fetus. C. Clinical presentation. 1. Woman feels fetus kicking in the lower abdomen. 2. Fetal heart sounds are heard loudest above the umbilicus. 3. Use of Leopold’s maneuvers indicates head is in the fundal area and the breech is in the pelvis. 4. On vaginal examination, it is found that the presenting part is soft, no fontanelles are felt, and the genitalia may be identified. D. Assessment and management. 1. Postural exercise, in which the woman assumes either the knee–chest or an elevated-hip posture several times a day to help the fetus turn from breech to cephalic presentation, has been suggested, but is not supported in the literature (Penn, 2011). 2. The physician may attempt external cephalic version (after 36 weeks in nulliparas; after 37 weeks in parous women) with or without the use of a uterine relaxant and if the fetus remains in a cephalic presentation, vaginal birth. However, in many cases the fetus reverts to breech (Penn, 2011). 3. Assessments on admission to labor and delivery: a. Perform Leopold’s maneuvers and vaginal examination to determine presentation. b. Report clinical findings immediately to the physician or midwife. 4. Ultrasonography may be ordered to confirm breech presentation, determine degree of flexion of fetal head, evaluate size of fetal head, estimate fetal weight, diagnose fetal anomalies, and locate placenta. 5. ACOG recommends that the mode of delivery be based on the experience level of the provider, and the majority will choose elective cesarean delivery. If the provider is experienced in breech delivery, he or she may plan a delivery as long as specific guidelines are followed and the woman is provided with informed consent regarding maternal and neonatal risks (ACOG, 2012). 6. Assessment of the neonate who was in the breech presentation may reveal: a. Edema of the external genitalia. b. A continuation of the frank breech position for a period of time after the birth. E. Complications. 1. Fetal/neonatal complications resulting from vaginal birth (Davidson et al., 2012; Penn, 2011). b. Asphyxia from slow birth of fetal head or from compression of umbilical cord between pelvis and head during birth. c. Aspiration of amniotic fluid with potential for meconium aspiration syndrome. d. Genital damage in the male infant. e. CNS injuries such as intracranial hemorrhage, brachial plexus injury, and severed spinal cord, especially if fetal head is hyperextended. f. Damage to the nose and pharynx. There is no method of pharmacologic pain relief that is completely safe for all laboring women. In addition, side effects or adverse reactions in the woman affect the fetus to some degree. For this reason, nonpharmacologic methods of pain management (e.g., labor support, freedom of movement, hypnosis, acupressure and acupuncture, application of heat or cold, listening to music, breathing techniques, massage, hydrotherapy, and transcutaneous electrical nerve stimulation) can be important and useful for the laboring woman (Tsen, 2011). Obstetric analgesia is given by either the intramuscular or the IV route and in as small a dose as possible. Any use of analgesia in the laboring woman should take into account the potential effects on the mother/fetus, effects on contractions and the progress of labor, and the medical condition of the mother (Davidson et al., 2012). Narcotic analgesics such as butorphanol tartrate (Stadol) and nalbuphine hydrochloride (Nubain) are commonly used for pain relief. A. Potential side effects or complications (Davidson et al., 2012). b. Nausea and vomiting. c. Hypotension. d. Drowsiness and dizziness. e. Clammy skin and sweating. 2. Fetal/neonatal. a. Decreased variability of the fetal heart rate, sinusoidal pattern (Stadol). b. Neonatal respiratory depression. B. Assessment and management. 1. Avoid administration of analgesics close to birth if possible. 2. Administer IV analgesics slowly; give during a uterine contraction to minimize amount of drug the fetus receives. 3. Observe the woman for side effects and monitor the fetal heart rate with the electronic fetal monitor or via intermittent auscultation. 4. With maternal hypotension, turn the woman onto her left side, increase IV infusion of fluids, and closely monitor the fetal heart rate and maternal BP. 5. Have naloxone (Narcan), oxygen, and ventilatory equipment available to manage potential newborn respiratory depression. 6. Document use of analgesic and transmit this information to the nursery nurse. 7. Observe the neonate for side effects of maternal analgesia. Several types of anesthesia are used with women in labor and delivery. General anesthesia is used primarily for emergency cesarean and complicated vaginal births when it is not possible to have immediate and effective regional anesthesia. Regional anesthesia includes continuous lumbar epidural, spinal, and pudendal block. Local anesthesia involves perineal infiltration prior to episiotomy, birth, and/or perineal repair. A. Potential complications with general anesthesia. 1. Maternal (Davidson et al., 2012). b. Respiratory depression. c. Hypotension or hypertension. d. Tachycardia. e. Laryngospasm. f. Uterine atony. 2. Fetal/neonatal. a. Neonatal respiratory depression and hypotonicity. b. Fetal depression in proportion to the amount of anesthesia. B. Assessment and management with general anesthesia. 2. Note the time of her last meal. 3. Physician may order 30 mL of clear antacid to be administered before general anesthesia to increase the pH of the stomach contents in case of aspiration. 4. Endotracheal tube and cricoid pressure are techniques used by the anesthesiologist to prevent aspiration. 5. Place a wedge under the right hip to cause displacement of the uterus from the aorta and vena cava and to prevent supine hypotensive syndrome during surgery. 6. Monitor the woman’s cardiorespiratory status during and after surgery, and uterine bleeding postoperatively. 7. Monitor the newborn infant after surgery for complications. C. Potential complications with regional anesthetics. 1. Maternal (Davison et al., 2012; Tsen, 2011). a. With spinal and epidural anesthesia: (1) Hypotension due to sympathetic blockade. (2) Allergic reaction to the injected anesthetic. (3) Toxic reaction to overdose or intravascular injection, with seizure activity. (4) Respiratory paralysis from inadvertent high spinal anesthesia. (5) Postdural puncture headache. (6) Failure of anesthetic to be effective. (7) Urinary retention during labor and in the postpartum period. (8) Formation of a hematoma that compresses the spinal cord, with potential for permanent damage. (9) Paralysis (rare). b. With epidural anesthesia: (2) Bladder distention. (3) Prolonged second stage and subsequent increase in instrumented vaginal delivery (forceps and vacuum-assisted). (4) Increase in the use of oxytocin (Hawkins and Bucklin, 2012). (5) Back pain and postdural puncture headache. (6) “Epidural shakes” and “epidural fever” (involuntary shivering that leads to an elevated temperature). Fever may also be caused by a decrease in hyperventilation and dissipation of heat (Murray and McKinney, 2011). c. With pudendal block (Hawkins and Bucklin, 2012; Murray and McKinney, 2011): (2) Perforated rectum. (3) Anesthetic toxicity. (4) Broad-ligament hematoma. 2. Fetal/neonatal. a. Toxic reaction from overdose or intravascular injection. b. Fetal compromise with prolonged maternal hypotension, as evidenced by late decelerations, bradycardia, and either increased or decreased variability. D. Assessment and management with regional anesthetics. 1. Note history of allergies to local anesthetics. 2. Prehydrate with 500 to 1000 mL IV fluid before spinal or epidural anesthesia to minimize hypotensive effects from sympathetic blockade. 3. Position and reassure woman during administration of anesthetic. To prevent supine hypotension, a small roll may be placed under the right hip. 4. Monitor the woman’s BP after administration of spinal or epidural anesthetic; monitor fetal heart rate after any type of regional anesthesia. 5. Monitor bladder distention and catheterize if necessary. 6. Complications and their management. (b) Dizziness or affected vision. (c) Nausea and vomiting. (2) Management. (b) Displace uterus from aorta and vena cava. (c) Administer oxygen and IV ephedrine as ordered. (d) Monitor the fetal heart rate for evidence of compromise, and the fetus/newborn for side effects of ephedrine (tachycardia, jitteriness, and increased muscular activity). b. High spinal. (a) Breast numbness, indicating a rising level of anesthesia. (b) Sensation of inability to breathe. (c) Respiratory arrest. (2) Management. (a) Notify anesthesia immediately. (b) Maintain airway and ventilation. (c) Observe fetal heart rate for signs of compromise. c. Toxic reaction. (b) Ringing in ears. (c) Slurring of speech. (d) Numbness of tongue and mouth. (e) Seizures. (f) Cardiovascular and respiratory depression. (2) Management. (a) Cardiorespiratory support. (b) Drugs to control seizures. (c) Monitor the newborn infant for seizures, bradycardia, apnea, and hypotonia. d. Allergic reaction. (b) Laryngeal edema. (c) Urticaria. (2) Management: use of IV antihistamine such as diphenhydramine (Benadryl). A. Incidence: The cesarean birth rate in the United States was 32.8% in 2010. The primary cesarean section rate was 23.6% and the vaginal birth after cesarean section (VBAC) rate was 9.2% (Martin et al., 2012). Possible reasons for the increasing cesarean rate include an increase in women who have had a previous cesarean section, the use of continuous electronic fetal monitoring, increased number of labor inductions with failure of induction, decline in vaginal breech birth and VBACs, decreased operative vaginal deliveries, repeat cesareans, increased multifetal pregnancies, changes in obstetric training, medical–legal issues, parental–societal expectations of the outcome of the pregnancy, and some evidence that women may be requesting elective cesarean (Berghella and Landon, 2012; Dickinson, 2011). B. Indications. a. Previous cesarean delivery. b. Cephalopelvic disproportion. c. Dystocia (inadequate progress in labor). d. Maternal medical conditions. 2. Placental. b. Placenta previa. c. Placental insufficiency. 3. Fetal. a. Suspected fetal compromise. b. Breech or other malpresentation. c. Multifetal gestation. d. Congenital anomalies such as myelomeningocele and anterior abdominal wall defects. C. Potential complications. b. Anemia. c. Hemorrhage. d. Morbidity and death from anesthesia. e. Inadvertent operative injuries. f. Pulmonary embolus and atelectasis. g. Thrombophlebitis. 2. Fetal/neonatal. b. Iatrogenic preterm birth. c. Respiratory distress syndrome caused by retained fluid in the lungs. d. Persistent pulmonary hypertension (Murray and McKinney, 2011). e. Anemia from blood loss caused by incision of placenta and lack of full placental transfusion. D. Assessment and management. 1. Perform usual interventions to prepare the woman for operative delivery. 2. Notify neonatology and pediatrician. 3. Give an antacid if ordered. 4. Remove fetal scalp electrode before surgery if present. 5. Place wedge under woman’s right hip to displace the uterus to the left to avoid supine hypotension and fetal hypoxia. 6. Follow Neonatal Resuscitation Program protocols for neonatal care following birth.
Antepartum–Intrapartum Complications
ANATOMY AND PHYSIOLOGY
CONDITIONS RELATED TO THE ANTEPARTUM PERIOD
Preeclampsia and Eclampsia (Sibai, 2012; Dekker, 2011)
Diabetes Mellitus (ACOG, 2011; Landon et al., 2012)
CONDITIONS RELATED TO THE INTRAPARTUM PERIOD
Preterm Labor (Svigos et al., 2011; Simhan et al., 2012)
Abruptio Placentae
Placenta Previa
Umbilical Cord Prolapse
Shoulder Dystocia
Breech Presentation
OBSTETRIC ANALGESIA AND ANESTHESIA
Obstetric Analgesia
Obstetric Anesthesia
Cesarean Delivery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree