CHAPTER 13. Parenteral Fluids
Lynn Phillips, MSN, RN, CRNI®∗
Physiology Related to Delivery of Parenteral Solutions, 229
Assessment of Fluid Requirements, 230
Types and Characteristics of Intravenous Solutions, 232
Summary, 241
Fluids and electrolytes play an important role in maintaining homeostasis. When imbalances occur, parenteral fluids are the most common intravenous (IV) agents used for correction. IV solutions are also used to provide nutrients or act as a vehicle for medication administration.
To provide infusion therapy safely, the infusion nurse should be aware of the patient’s physical status and clinical picture, and understand the legal implications of treatment. Determination of the type of fluid needed is based on the nursing assessment, laboratory findings, and the purpose for which it is being prescribed. To act properly on the authorized prescriber’s order, the nurse must be familiar with the various IV solutions, including their uses, components, and potential complications. This information should be incorporated into a plan of care or clinical pathway. This care plan should also include applicable nursing diagnoses with measurable outcomes. With careful organization and a strong database, the delivery of infusion therapy should be a safe and effective treatment modality.
PHYSIOLOGY RELATED TO DELIVERY OF PARENTERAL SOLUTIONS
The human body is a contained fluid environment of water and electrolytes. The human body is approximately 60% water by weight; of this, 40% of body weight is water contained in cells (intracellular water) and 20% is water outside cells (extracellular water). The usual blood volume of the human body is 7% to 8% of the total body weight (Beck, 2003).
WATER
The cell wall separates the intracellular compartment from the extracellular compartment. The capillary endothelium and the walls of arteries and veins divide the extracellular compartment into the intravascular and interstitial compartments. Water moves freely through cell and vessel walls and is distributed through all these compartments (Grocott, Mythen, and Gan, 2005).
Holliday and Segar (l957) established that regardless of age, to dissolve and eliminate metabolic wastes all healthy persons require approximately 100 milliliters (mL) of water per 100 calories metabolized. This means that a person who expends 1800 calories of energy requires approximately 1800 mL of water for metabolic purposes. The metabolic rate increases with fever; it rises approximately 12% for every 1° C (7% for every 1°F) increase in body temperature. Fever also increases the respiratory rate, resulting in additional loss of water vapor through the lungs (Porth, 2007). Water and solute depletion may occur because of either decreased intake (e.g., fasting before surgery, anorexia, or altered consciousness level) or increased losses (e.g., diarrhea, vomiting, or pyrexia). There are two main physiological mechanisms that assist in regulating body water: thirst and antidiuretic hormone (ADH). Thirst is primarily a regulator of water intake and ADH a regulator of water output. Both mechanisms respond to changes in extracellular osmolality and volume (Porth, 2007).
OSMOSIS AND OSMOTIC PRESSURE
Osmosis is a process by which a solvent, usually water, moves through a semipermeable membrane from a solution of lower concentration to a solution of higher concentration. The osmotic pressure exerted by particles in a solution is determined by the number of particles per volume of fluid versus the mass or size of the particles (Phillips, 2005). The osmotic pressure at the cell membrane differs from that at the capillary membrane. At the capillary membrane, the pressure is referred to as oncotic pressure, while at the cell membrane the pressure is referred to as osmotic pressure (Hankins, 2006). Because proteins are the only dissolved substances in the plasma and interstitial fluid that do not diffuse readily through the capillary membrane, the colloid osmotic pressure is influenced by proteins. The concentration of proteins in plasma is two to three times greater than the concentration of proteins found in the interstitial fluid. Only those substances that do not pass through the semipermeable membrane exert osmotic pressure, and proteins are the only substances that do not readily penetrate the pores of the capillary membrane. Therefore proteins in the extracellular fluid spaces are responsible for the osmotic pressure at the capillary membrane.
In a healthy person, the net intracapillary pressures are more than the interstitial pressures, and this results in a pressure gradient that produces a slow continuous flow of fluid from capillary lumen to interstitium. This tissue space, or interstitial fluid, drains via the lymphatic system back into the systemic circulation. In disease, all these factors can be altered, often resulting in an increase in the loss of fluid from the circulation (Grocott et al, 2005). Starling (1896) formulated the Law of Capillaries, which states that equilibrium exists at the capillary membrane when the fluid leaving the circulation and the amount of fluid returning to circulation are exactly equal.
Capillary pressure tends to force fluid and dissolved substances through the capillary pore into the interstitial spaces. Colloid osmotic pressure tends to cause fluid to move via osmosis from the interstitial spaces into the blood, thus preventing a significant loss of fluid volume (Hankins, 2006). The volume of distribution of infused fluids is dictated by their solute content. In turn, the plasma volume expansion effect is directly related to the volume of distribution:
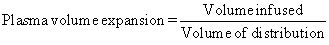
Infusion of isotonic crystalloid (e.g., 0.9% sodium chloride or lactated Ringer’s solution) will expand all the components of the extravascular volume, and 20% of the volume infused will remain in the intravascular space. Infusion of an “ideal colloid,” containing large molecules that do not escape from the circulation, will expand the intravascular volume by 100% of the volume infused (Grocott et al, 2005). To rationally prescribe fluid replacement, it is important to identify which compartment is depleted.
The osmotic activity of a solution may be expressed in terms of either its osmolarity or its osmolality. Osmolarity refers to the osmolar concentration in 1 liter (L) of solution (mOsm/L) and osmolality is the osmolar concentration in 1 kilogram (kg) of water (mOsm/kg of H 2O). Osmolarity is usually used when referring to fluids outside the body and osmolality for describing fluids inside the body. Because 1 L of water weighs 1 kg, the terms osmolarity and osmolality are often used interchangeably (Porth, 2007). The term osmolality will be used in this chapter. Extracellular osmolality is primarily determined by the sodium level because it is the main solute found in extracellular fluid. A rough estimation of extracellular osmolality can be made by multiplying the plasma sodium concentration by 2 (Porth, 2007).
TONICITY
A change in water content causes cells to swell or shrink. The term tonicity refers to the tension or effect that the osmotic pressure of a solution, with impermeable solutes, exerts on cell size because of water movement across the cell membrane. Tonicity is determined solely by effective solutes such as glucose that cannot penetrate the cell membrane, thereby producing an osmotic force that pulls water into or out of the cell, causing it to change size.
Solutions to which body cells are exposed can be classified as isotonic, hypotonic, or hypertonic depending on whether they cause cells to swell or shrink. Cells placed in an isotonic solution, which has the same effective osmolality as intracellular fluids (280 to 295 mOsm/L), neither shrink nor swell. When cells are placed in hypotonic solution, which has a lower effective osmolality than intracellular fluids, they swell as water moves into the cell, and when they are placed in a hypertonic solution, which has a greater effective osmolality than intracellular fluid, they shrink as water is pushed out of the cell.
ASSESSMENT OF FLUID REQUIREMENTS
To understand the use of parenteral solutions the nurse must understand two important concepts: (1) the rationale for the authorized prescriber’s order of infusion therapy, and (2) the type of solution ordered, together with the composition and clinical use of that solution (Phillips, 2005). Objectives or rationales for administration of IV solutions fall into three broad categories:
1. Maintenance therapy for daily body fluid requirements
2. Replacement therapy for existing losses
3. Replacement therapy for ongoing losses
These three objectives differ with regard to the time necessary to complete the IV solution administration. The following factors affect the choice of objective in prescribing a parenteral solution and the rate of administration determined by the authorized prescriber: patient’s renal function, daily maintenance requirements, existing fluid and electrolyte imbalance, clinical status, and disturbances in homeostasis as a result of parenteral therapy (Metheny, 2000).
MAINTENANCE THERAPY
Water has the priority in maintenance therapy. The body needs water to replace insensible loss, which can occur as perspiration from the skin and moisture from respirations. The average adult loses 500 to 1000 mL of water over 24 hours through insensible loss. Water is also an important dilutor for waste products excreted by the kidneys. In addition, an individual’s fluid requirements are based on age, height, weight, and amount of body fat.
Maintenance therapy provides nutrients that meet the daily needs of a patient for water, vitamins, electrolytes, glucose, and protein, with water having priority. The typical patient profile for maintenance therapy is an individual who is allowed nothing by mouth (NPO) or whose oral intake is restricted for any reason. Remember that insensible loss is approximately 500 to 1000 mL every 24 hours. Maintenance therapy should be 1500 mL per square meter (m 2) of body surface area over 24 hours (Metheny, 2000). For example, a man weighing 85 kg (187 lb) has a body surface area of 2 m 2; 1500 times 2 equals 3000; therefore he needs 3000 mL of fluids for maintenance therapy. Balanced solutions for maintenance therapy include water, daily needs of sodium and potassium, and glucose.
REPLACEMENT THERAPY FOR EXISTING LOSSES
Replacement therapy is necessary to take care of the fluid, electrolyte, or blood product deficits of patients in acute distress; this type of therapy is supplied over a 48-hour period. The following are examples of conditions of patients needing replacement infusion therapy (and their replacement requirements):
• Hemorrhage (for replacement of cells and plasma)
• Low platelet count (for replacement of clotting factors)
• Vomiting and diarrhea (for replacement of losses of electrolytes and water)
• Starvation (for replacement of losses of water and electrolytes) (Phillips, 2005)
Replacement therapy has a twofold rationale. The first rationale is to restore fluid loss when the previous output exceeded intake. After kidney status has been considered, a hydrating solution (e.g., 5% dextrose in 0.2% sodium chloride) is administered. This restores an adequate output of urine and then allows electrolytes to be replaced. The other rationale for replacement therapy is to restore present fluid and electrolyte losses, such as loss of intestinal fluid through continuing diarrhea. A solution such as lactated Ringer’s injection can be used to replace this type of loss. Replacing continuing losses prevents acidosis and alkalosis.
When the maintenance of body requirements cannot be met, the authorized prescriber should institute replacement therapy. The authorized prescriber must determine the losses and calculate replacement over a 48-hour period. Assessment of kidney function is the first step in determining appropriate replacement therapy. Patients requiring replacement therapy, except those in shock, require potassium. Patients under stress from tissue injury, wound infection, or gastric or bowel surgery also require potassium. Adequate replacement is achieved using 20 mEq/L of potassium (Metheny, 2000). A key nursing assessment before beginning replacement therapy is validation of kidney function before administering potassium in replacement therapy.
REPLACEMENT THERAPY FOR ONGOING LOSSES
Therapy for concurrent losses is achieved on an ongoing daily basis. Critical evaluation of concurrent losses of fluids and electrolytes is done at least every 24 hours. Accurate documentation of intake and output is extremely important in this type of fluid and electrolyte management. Restoration of homeostasis depends on the nursing assessment of intake of IV solutions, as well as on the documentation of all body fluid losses. The types of clinical patients who require 24-hour evaluation are those with draining fistulas, abscesses, nasogastric tubes, burns, and abdominal wounds. The fluid and electrolyte management of these patients cannot be completed in 48 hours. Therefore maintenance therapy and replacement therapy do not meet these patients’ needs. A day-by-day restoration of vital fluids and electrolytes is necessary. With these types of patients, you will see frequent changes in the types of solutions ordered, in the amounts of electrolytes ordered based on laboratory test results, and in the rate of infusion. Restoration of electrolyte imbalance is imperative for proper homeostatic management therapy. The type of restoration fluid ordered depends on the type of fluid that is being lost. For example, excessive loss of gastric fluid must be replaced by solutions that include sodium, potassium, and chloride.
NURSING FOCUSED ASSESSMENT
Assessment of a patient for delivery of parenteral solutions includes collecting a nursing history, performing a focused physical assessment, monitoring pertinent laboratory tests, and evaluating intake and output. The purpose of this assessment is to identify patients at risk for or already experiencing alterations in fluid and electrolyte balance.
Nursing history related to fluid and electrolyte balance includes questions about the patient’s medical history, current health concerns, food and fluid intake, fluid elimination, medications, and lifestyle. Physical assessment correlates data with the nursing history and laboratory studies. Focused assessment would include, but not be limited to, the following:
• Skin: Assess for color, temperature, moisture content, continuity, turgor, and edema.
• Mucous membranes: Inspect the tongue and buccal mucosa; assess the color, moisture, and continuity of mucous membranes.
• Cardiovascular system: Pulse, blood pressure, and respiration are all affected by fluid and electrolyte status. Assess for orthostatic hypotension, capillary refill, and venous filling.
• Respiratory system: Assess respiratory rate, depth, and pattern, as well as breath sounds.
• Neurological system: Level of consciousness provides cues to fluid and electrolyte as well as acid-base balance.
• Vital signs: All of the vital signs are affected by fluid, electrolyte, and acid-base balance.
In addition to the systems’ assessment listed, the nurse will need to track vital sign changes, monitor daily weights, and record intake and output. The intake and output record should be a standard nursing order on all patients receiving intravenous solutions. Accuracy is of utmost importance; therefore all liquids (intake or output) should be measured carefully and should be estimated when they cannot be measured. Monitoring for signs and symptoms of fluid volume overload includes assessment of lungs, symptoms of fluid volume excess, vital signs, and laboratory values. These are independent nursing actions, and they do not require an authorized prescriber’s order (Heitz and Horne, 2005).
The next important aspect of determining fluid needs is the review and interpretation of a patient’s laboratory findings. The two systems that have the most direct impact on fluid and electrolyte balance are the renal and cardiovascular systems. Therefore tests that reflect the proper functioning of the kidneys and heart require consistent and close scrutiny. Box 13-1 summarizes laboratory findings for monitoring effective parenteral fluid therapy.
Box 13-1
PATIENT OUTCOMES
SUMMARY OF LABORATORY VALUES NEEDING EVALUATION DURING PARENTERAL FLUID THERAPY
| Test | Clinical considerations |
|---|---|
| Blood urea nitrogen (BUN) | Renal function assessment during replacement therapy |
| Creatinine | Renal function assessment during replacement therapy |
| Specific gravity | Urine concentration reflects fluid volume concentrations |
| Urine osmolarity | Reflects fluid volume changes |
| Serum electrolytes | Reflect deviations from normal |
| Complete blood count (CBC) | CBC screening for hemoglobin, hematocrit, red blood cells, white blood cells, and platelets before replacement of these components or when expanding ECF |
| Blood gases | Evaluation of acid-base status |
| Coagulation studies | Evaluation needed before use of plasma volume expanders |
| Serum glucose | Monitor osmotic diuresis |
| Lactate dehydrogenase | Monitor cellular enzyme associated with carbohydrate metabolism |
PATIENT OUTCOMES
Expected outcomes with the infusion of parenteral solutions include:
• The patient’s intake and output remains adequate for effective fluid and electrolyte replacement.
• The patient’s hemodynamic measurements remains stable or returning to stability.
• The patient’s physical signs and symptoms of imbalance returns to normal for patient.
• The patient’s laboratory measurements remains within normal limits or returns to normal.
• The patient remains free of complications associated with colloid administration (e.g., allergic reaction, anaphylactoid reaction, electrolyte imbalances).
• The patient’s hemodynamic parameters improve.
The Infusion Nurses Society (INS, 2006) identifies standards of practice for infusion nursing while the Centers for Disease Control and Prevention (CDC, 2002) provides guidelines for the implementation of care related to infusion therapy. The Infusion Nursing Standards of Practice provides measurable components that assist the clinician when establishing nursing competencies and evaluation of patient outcomes related to delivery of parenteral therapy (INS, 2006).
TYPES AND CHARACTERISTICS OF INTRAVENOUS SOLUTIONS
CRYSTALLOID SOLUTIONS
Crystalloids are materials capable of crystallization (i.e., have the ability to form crystals). Crystalloids are solutes that, when placed in a solution, mix with and dissolve into the solution and cannot be distinguished from the resultant solution. Because of this, crystalloid solutions are considered true solutions that are capable of diffusing through membranes. Crystalloid solutions contain electrolytes as dissolved particles in solution that diffuse readily between compartments. To expand the vascular space to a degree equal to that resulting from colloid solutions, crystalloids must be given in three to four times their volume. Types of crystalloid solutions include dextrose solutions, sodium chloride solutions, balanced electrolyte solutions, and alkalizing and acidifying solutions (Phillips, 2005).
IV crystalloid solutions will be distributed to the compartment that contains similar sodium concentration and fluid volume. Crystalloid solutions are rated, before infusion, according to their comparative tonicity to plasma. Their composition dictates fluid movement within and between compartments. Crystalloid solutions are further divided into isotonic, hypotonic, and hypertonic categories.
Initial and therapeutic responses
Crystalloid administration can be divided into two phases: the initial response and the therapeutic response. The initial response is the immediate reaction that occurs when the IV solution is introduced into the circulation. As the solution enters the bloodstream, it immediately contacts red blood cells (RBCs) and the cells of the vein intima. Hypotonic and hypertonic solutions change the immediate surroundings of the red blood cells (Humes, 2000). Extremely hypertonic solution may cause shrinkage of the RBC. As red blood cells move toward a more isotonic environment, they regain their original shape (Hill and Petrucci, 2004).
Hypotonic solutions will cause absorption of water into the intravascular cells (Humes, 2000 and Metheny, 2000). The cells will return to their normal shape as they move into a more isotonic environment, as do the cells bathed in hypertonic solutions.
Compared to the initial response of RBCs, the initial response of crystalloid therapy to the vein intima may be as dramatic but is not life-threatening. The intima, at the point of fluid injection, will be repeatedly subjected to the fluid. Hypotonic fluids will cause the endothelial cells to swell while hypertonic solutions will draw fluid from the endothelial cells, causing them to shrink. The risk of cellular dehydration increases as the tonicity of fluid increases. The administration of hypertonic saline or dextrose preparations greater than 10% through small veins is associated with phlebitis, as a result of this cellular dehydration (Cook, 2003).
The therapeutic response of crystalloid administration occurs as the fluid disperses through the extracellular fluid (ECF) and intracellular fluid (ICF). The therapeutic response is predictable and is the reason one fluid is chosen over another.
The therapeutic response to isotonic solutions when administered by the intravenous route results in the tonicity of the plasma remaining unchanged. The solutions 0.9% sodium chloride and lactated Ringer’s remain isotonic even after they disperse into the interstitial spaces; therefore the tonicity of the interstitial space is unchanged. The interstitial space is three times as large as the intravascular space; 75% of the fluid will be dispersed interstitially and 25% will remain in the plasma (Jordan, 2000).
The solution of 5% dextrose in water is considered isotonic in the initial response but the mechanics are different than with isotonic electrolyte solutions. Dextrose in water is an electrolyte-free solution. As the fluid disperses through the ECF, the dextrose is absorbed into the cells to be used for energy. What is left is free water that dilutes the osmolality of the ECF (Porth, 2007). The cells are suddenly suspended in a hypotonic environment and osmosis will occur, with the cells absorbing the fluid until the two compartments are isotonic. The intracellular compartment is two thirds the size of the ECF compartment; 67% of the water will enter the cells and 33% will remain in the ECF. The dispersion of 1 L of 5% dextrose in water will divide the 1000 mL into 667 mL intracellularly, approximately 250 mL into the interstitial space, and 83 mL into plasma. Hypertonic dextrose solutions alter the initial response because the solutions are irritating to the vein intima; however, the therapeutic response is the same in dextrose solution with higher dextrose concentrations (Humes, 2000).
Many crystalloid solutions are made up of a combination of dextrose and electrolyte solutions, most of which are hypertonic initially. The therapeutic response to these fluids can be predicted based on the tonicity of the solution. Once the cells use the dextrose, the remaining sodium chloride and electrolytes will be dispersed as isotonic electrolyte solution, hydrating only the ECF compartment.
Isotonic crystalloid solutions
Isotonic solutions have an osmolality of 250 to 375 mOsm/L. Blood and normal body fluids have an osmolarity of 280 to 295 mOsm/L. These fluids are used to expand the extracellular fluid (ECF) compartment. Infused isotonic solution is distributed between the intravascular space and interstitial space, with very little moving into the cell. Approximately 20% of the isotonic crystalloid solution volume remains in the intravascular compartment and 80% moves into the interstitial space. The concentration of sodium within the isotonic solution determines the distribution time in which the solution remains in the intravascular space. This influences the choice of isotonic solution selected for replacement or maintenance therapy (Cooper and Moore, 2000).
Many isotonic solutions are available. Examples include 0.9% sodium chloride, lactated Ringer’s solution, and 5% dextrose in water. Dextrose 5% solution becomes hypotonic when dextrose is metabolized. The solution should be used cautiously in patients with renal and cardiac disease because of the increased risk of fluid overload (Kraft, 2000). Isotonic solutions are commonly used to treat fluid loss, dehydration, and hypernatremia (sodium excess).
Hypotonic crystalloid solutions
Hypotonic solutions have fewer electrolytes and a lower osmolarity than isotonic solutions. Hypotonic fluids have an osmolarity lower than 250 mOsm/L. By lowering serum osmolarity, the crystalloid solutions shift out of blood vessels into cells and interstitial spaces. These solutions only increase the intravascular volume by approximately 8% with the remaining 92% distributing evenly between the interstitial space and intracellular space (Cooper and Moore, 2000). The resulting osmotic pressure gradient draws water into the cells from the ECF, causing the cells to swell. Hypotonic solutions hydrate cells and can deplete the circulatory system. Water moves from the vascular space to the intracellular space when hypotonic fluids are infused.
Hypotonic solutions are used for patients who have hypertonic dehydration, require water replacement, and have diabetic ketoacidosis after initial sodium chloride replacement (Kraft, 2000). Examples of hypotonic solutions include 0.45% sodium chloride (half-strength saline), 0.33% sodium chloride, and 2.5% dextrose in water. The use of hypotonic solutions for patients with low blood pressure will further a hypotensive state (Phillips, 2005).
Hypertonic crystalloid solutions
Hypertonic solutions have a higher osmolality than isotonic solutions as a result of a greater number of electrolytes pulling water from the intracellular space and interstitial space into blood vessels, increasing intravascular volume. Hypertonic fluids have an osmolarity of 375 mOsm/L or higher. The resulting osmotic pressure gradient draws water from the intracellular space, increasing extracellular fluid volume. These fluids are used to replace electrolytes, to treat hypotonic dehydration, and for temporary treatment of circulatory insufficiency and shock. Examples of hypertonic fluids include 5% dextrose in 0.45% sodium chloride, 5% dextrose in 0.9% sodium chloride, 5% dextrose in lactated Ringer’s, and 10% dextrose in water.
Hypertonic solutions are irritating to the vein walls and should be given slowly to prevent circulatory overload. Some hypertonic solutions are contraindicated in patients with cardiac or renal disease because of the increased risk of congestive heart failure and pulmonary edema.
pH
The pH of a crystalloid solution reflects the degree of acidity or alkalinity of a solution. Blood pH is not a significant problem for routine parenteral therapy. Normal kidneys can achieve an acid-base balance as long as enough water is supplied. The United States Pharmacopeia (USP) standards require that solution pH must be slightly acidic (a pH of between 3.5 and 6.2). Many solutions have a pH of 5.0. The acidity of solutions allows them to have a longer shelf life.
DEXTROSE SOLUTIONS
Dextrose solutions contain carbohydrates and can be administered by the parenteral route as dextrose, fructose, or invert sugar. Dextrose is the most commonly administered carbohydrate. The percentage solutions express the number of grams of solute per 100 g of solvent. Thus 5% dextrose in water (D 5W) solution contains 5 g of dextrose in 100 mL of water.
Dextrose is a non-electrolyte, and the total number of particles in a dextrose solution does not depend on ionization. Dextrose is thought to be closest to the ideal carbohydrate available because it is well metabolized by all tissues. The tonicity of dextrose solutions depends on the particles of sugar in the solution. Dextrose 5% in water is rapidly metabolized and has no osmotically active particles after it is in the plasma. The USP pH requirements for dextrose fall between 3.5 and 6.5 (Metheny, 2000).
Dextrose in water is available in various concentrations including 2.5%, 5%, 10%, 20%, 30%, 40%, 50%, and 70%. Dextrose is also available in combination with other types of solutions. The 5% and 10% concentrations can be given peripherally. Concentrations higher than 10% are given through central veins. A general exception is the administration of limited amounts of 50% dextrose given slowly through a peripheral vein for emergency treatment of hypoglycemia (usually 3 mL/min) (Phillips and Kuhn, 1999).
More concentrated dextrose solutions, 20% to 70%, are hypertonic, and range from 1010 to 3530 mOsm/L. When these solutions are being administered, consideration should be given to the possibility that tolerance to glucose may be compromised by sepsis, stress, hepatic and renal failure, and by some medications, such as steroids or diuretics. Refer to Table 13-1 for a comparison of dextrose solutions.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


