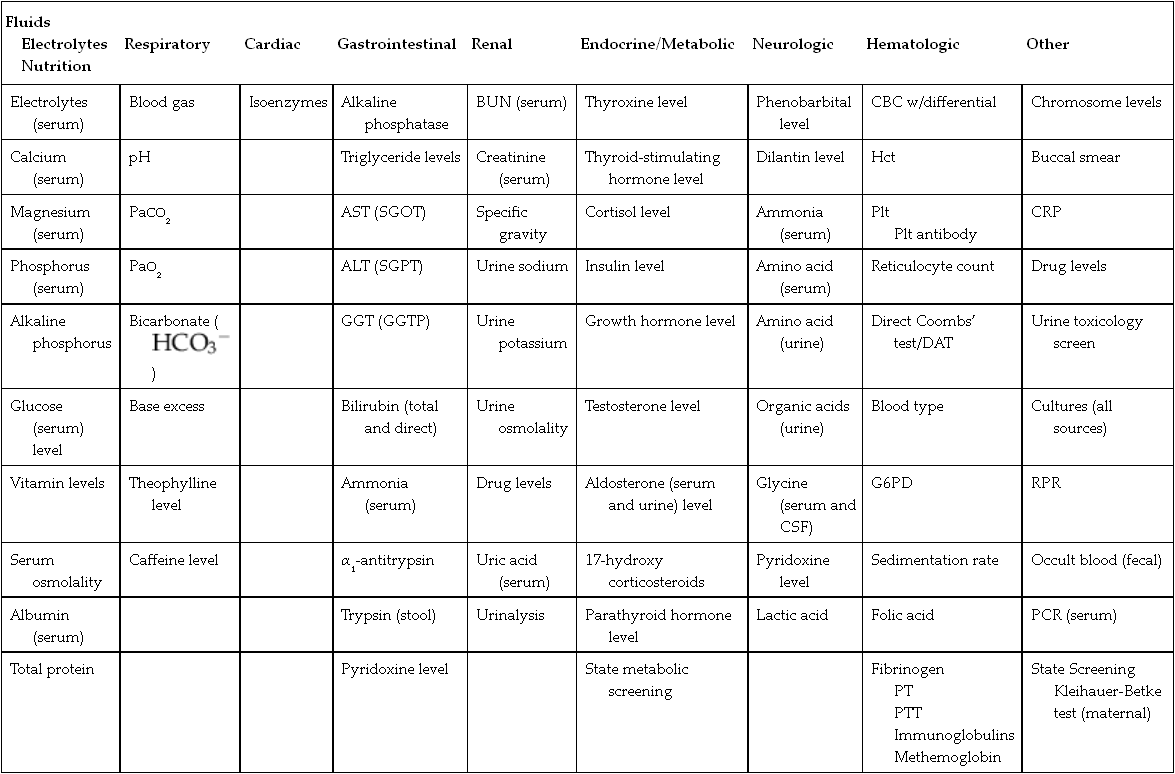
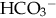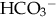CHAPTER 13 1. Identify the types of laboratory testing in the neonatal intensive care unit. 2. Discuss the purpose of laboratory testing and diagnostics. 3. Review the process of specimen collection and the principles of test utilization. 4. Review the principles of laboratory interpretation. 5. Describe iatrogenic sequelae associated with laboratory testing and diagnostic procedures. 6. Discuss strategies to minimize iatrogenic sequelae associated with laboratory testing and diagnostic procedures. 7. Discuss the decision-making process of ordering and interpreting laboratory values—a decision tree. Newborns delivered in the United States receive an average of one or two laboratory tests, typically in the form of screening for jaundice, genetic disorders, and birth defects. In addition, all infants admitted to the neonatal intensive care unit (NICU) require laboratory testing to assess their clinical status. Given the U.S. birth rate of 3.95 million infants (Centers for Disease Control and Prevention [CDC], 2013) and average NICU admission rates of 7% of live births (CDC, 2011), this translates to a minimum of 7.9 million screening/laboratory tests per year. It has been estimated that approximately 3% (Rusckowski, 2012) of the U.S. national health budget is spent on laboratory testing, constituting 15% to 20% of each patient’s hospital bill (Sacher et al., 2000d). The impact of laboratory testing on the patient, health care, and its cost is significant. Because all health care workers in the NICU are in some way involved in obtaining laboratory samples, understanding the principles of laboratory testing and the contribution of laboratory tests to patient care and cost is essential. Developing skills in laboratory interpretation at the NICU bedside provides a key element in comprehensive patient care. This chapter features a review of the types and purposes of laboratory testing in the NICU, describes principles of laboratory testing and interpretation, and discusses potential iatrogenic sequelae. Included is a discussion of strategies to avoid sequelae, when to obtain laboratory testing, and a strategy to interpret results for patient care. A decision-making process for laboratory testing and interpretation is presented. A. Laboratory testing occurs daily in the NICU. Why and when laboratory testing is performed is driven by patient history and presenting symptoms. By one estimate, 70% of all medical decisions are based on laboratory results (Kurec and Lifshitz, 2011; Rusckowski, 2012). Data obtained from laboratory analyses assist clinicians in determining diagnosis, measuring success of treatment, and monitoring trends in an infant’s clinical course. The common laboratory tests performed in the NICU include the following (Table 13-1): 1. Chemistry analysis: serum tests that measure the chemical activity or state of the body. Serum is the by-product of clotted blood that is centrifuged to remove the clot and any other cells (Laposata, 2002). Chemical substances reflect metabolic processes and disease states in the body. Measuring changes in chemical concentrations (chemical substances) is useful in diagnosis, planning care, monitoring of therapy, screening, and determining the severity of disease and response to treatment (Sacher et al., 2000a). a. Four categories of chemical analyses include: (2) Metabolites: nonfunctioning waste products in process of being cleared. Laboratory examples: bilirubin, ammonia, blood urea nitrogen (BUN), creatinine, and uric acid. (3) Substances released from cells as a result of cell damage and abnormal permeability or abnormal cellular proliferation. Laboratory examples: alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine kinase. (4) Drug and toxic substances. Laboratory examples: antibiotics, theophylline, caffeine, digoxin, phenobarbital, and substances of abuse. 2. Hematologic tests: study of the blood and blood-forming tissues of the body such as the bone marrow and reticuloendothelial system (Sacher et al., 2000b). This area of testing also includes the study of hemoglobin (Hgb) structure, red cell membrane, and red cell enzyme activity. Whole blood is composed of blood cells suspended in plasma fluid (see also Chapter 31). Plasma is unclotted blood that has been centrifuged to remove any cells (Laposata, 2002). Plasma contains the protein fibrinogen, which is converted to the substance composing the fibrin clot (Kee, 2010). b. Plasma: plasma proteins, coagulation factors I through XIII, immunoglobulins. Laboratory examples: total protein, albumin, fibrinogen (factor I), prothrombin (factor II), thromboplastin (factor III), factors IV through XIII assays, immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM). 3. Microbiology tests: identification of infectious microorganisms causing disease. Tests include diagnostic bacteriology, mycology, virology, parasitology, and serology. Laboratory examples: culture of any body fluid, bacterial stain, and bacteria antigen detection. 4. Microscopy tests: examination of body fluids and tissues under a microscope. Laboratory examples: cell counts, fecal blood, fecal fat, Apt test, and urinalysis. 5. Blood bank tests (transfusion medicine): area of blood component preparation, blood donor screening and testing, blood compatibility testing, and blood and stem cell banking. (1) Blood typing and cross-match. (2) Direct antiglobulin test (DAT)/Coombs’ test. (3) Indirect antiglobulin test (IAT)/indirect Coombs’ test. (4) Erythrocyte rosette test, Kleihauer-Betke test for fetomaternal hemorrhage. 6. Immunoassays: laboratory method based on antigen–antibody reactions employed in therapeutic drug monitoring, toxicology screening, detection of plasma proteins, and certain endocrine testing. Laboratory examples: urine and meconium toxicology test for “street drugs,” latex agglutination test, and drug levels. 7. Cytogenetic tests: testing used to determine genetic composition by chromosome analysis. Laboratory examples: simple karyotype (blood, amniotic fluid, tissue, bone marrow, buccal swab), chromosome-specific probes, and fluorescent in-situ hybridization (FISH). 8. Immunology tests: laboratory evaluation measuring immune system activity (Sacher et al., 2000c). This consists of complement activity and its cascade of activation, humoral, and cell-mediated immunity. Tests are used to diagnose inflammatory responses, immunodeficiency, and autoimmune disorders. Laboratory examples: C-reactive protein, cytokine measurement, complement C3 and C4, IgG, IgM, and IgA. 1. Determine the health state of the patient. 2. Monitor the clinical status, trends, and disease severity. 3. Assist in a differential diagnosis. 4. Confirm a diagnosis or cure. 5. Screen for common disorders and disease prevention. 6. Measure the effect of therapy. 7. Assist in the management of disease. 8. Establish a prognosis. 9. Assist in genetic counseling. 10. Evaluate specific events—example: medication errors, sudden clinical decompensation, medical–legal problems, and postmortem evaluation. A. Collection of laboratory specimens requires meticulous technique to ensure the best possible results. Proper technique, source of laboratory sample, use of collection tubes, labeling, and laboratory processing combine to play a key role in patient treatment, in minimizing blood loss and painful stimuli, and in reducing the costs of NICU care. Anticipatory pain management is needed prior to heel sticks, venipuncture, and arterial puncture (American Academy of Pediatrics [AAP] and Canadian Paediatric Society [CPS], 2007; Folk, 2007; see also Chapter 16) as well as other specimen collection procedures such as lumbar puncture and suprapubic bladder tap. 1. Types of laboratory collection (see also Chapter 15). a. Capillary blood sampling: admixture of arterial, venous, and capillary blood and tissue fluid obtained from a well-perfused heel. Heel sticks should not be performed on infants whose feet are edematous, injured, bruised, infected, or anomalous (Folk, 2007). NOTE: Finger-stick sampling is contraindicated in infants, because distance from skin surface to bone is less than 1.5 mm (Olsowka and Garg, 2001). NOTE: The Cochrane Database of Systematic Reviews concludes that venipuncture, performed by skilled phlebotomists, appears to be the method of choice for blood sampling in term neonates (Shah and Ohlsson, 2011). (1) Most common route for obtaining small blood samples in the infant. (2) Heel stick less than 2 mm (Garza and Becan-McBride, 2010c, 2010d) and with heel-stick devices ranging from 0.65 to 1 mm depending on infant size (Vedder and Sawyer, 2011). (3) Avoid squeezing or milking site to obtain blood (Folk, 2007; Garza and Becan-McBride, 2010c). NOTE: Excessive scooping of blood from nearby skin with the lip of collection tube can interfere with the laboratory result (Vedder and Sawyer, 2011). (4) Cell counts such as WBC, platelet count, or blood gas PO2 may not be accurate. (5) Anticipatory pain management should be considered (see also Chapter 16). b. Venipuncture: venous blood typically obtained from the hand, arm, foot, leg, or scalp. Venous blood can also be obtained from an umbilical vein catheter, tunneled central venous catheter, or peripherally inserted central catheter (PICC) (Folk, 2007). In term infants, venipuncture has been shown to be a less painful procedure than heel stick when performed by skilled phlebotomists (Shah and Ohlsson, 2011). (1) May need use of tourniquet to distend peripheral veins. Recommended time for tourniquet application is ≤ 1 minute. Tourniquet application ≥ 3 minutes may alter laboratory test results (Garza and Becan-McBride, 2010b, 2010f; Kee, 2010). (2) Venipuncture may be difficult to obtain in small infants. (3) May wish to ration available veins for future intravenous (IV) sites versus use for laboratory drawing (Folk, 2007; Vedder and Sawyer, 2011). (4) Anticipatory pain management should be considered (see also Chapter 16). c. Arterial puncture (see also Chapter 15): arterial blood typically obtained from an artery stick of the radial, tibial, or temporal arteries. Brachial artery stick is performed less often due to potential for arteriospasm of brachial artery supplying the lower arm. Femoral artery stick is rarely performed in the NICU population. Arterial blood can also be obtained from an umbilical artery catheter or percutaneous arterial line. (1) Allen test (modified) should be done prior to radial artery stick (Garza and Becan-McBride, 2010a). (2) Anticipatory pain management should be considered (see also Chapter 16). d. Point-of-care testing (POCT), alternate-site testing, near-patient testing, patient-focused testing: tests done in a variety of settings, often at the bedside (Threatte and Schexneider, 2011). (1) Uses whole blood obtained from capillary stick, arterial, or venous sources. (2) Provides “real-time,” rapid testing. (3) Uses small blood volumes (< 0.5 mL). (4) Common POCT assays obtained in the NICU include levels of electrolytes, ionized calcium, BUN, creatinine, Hct, Hgb, blood gases, and glucose. (5) Anticipatory pain management should be considered (see also Chapter 16). e. Lumbar puncture (see Chapter 15): procedure done to remove cerebrospinal fluid (CSF) from the spinal canal. (1) CSF evaluated in the laboratory for: (b) Hemorrhage. (c) Demyelinating diseases. (d) Malignancy. f. Urine sampling (see also Chapter 15): urine obtained for chemical analysis, bacterial cultures, or microscopic examination. (1) Techniques for obtaining samples include: (b) Straight catheterization. g. Thoracentesis (see also Chapter 15): procedure using a needle tap or chest tube to remove abnormal collection of fluid (effusion) from the thoracic cavity. (1) Thoracic cavity fluid evaluated in laboratory for: (a) Microorganisms: cultures, Gram stains. (b) Chemistries: levels of electrolytes, total protein, albumin, glucose, and triglycerides. (c) Hematology: WBC count and WBC differential count. h. Peritoneal tap: procedure using a needle tap to remove an abnormal collection of fluid (ascites) from the abdomen. (1) Peritoneal cavity fluid evaluated in laboratory for: (a) Microorganisms: cultures, Gram stains. (b) Chemistries: levels of electrolytes, total protein, albumin, glucose, and triglycerides. (c) Hematology: WBC count and WBC differential count. 2. Process of laboratory collection. b. Check for appropriateness of order. c. Cluster laboratory drawing as much as possible. d. Check patient identification prior to laboratory sampling per hospital protocol. e. Prepare infant for laboratory test, for example: (1) Wrap foot with a heel warming device for capillary specimen—a warmed foot arterializes specimen and helps promote blood flow (Garza and Becan-McBride, 2010c; Kaplan and Tange, 1998; Olsowka and Garg, 2001). Some studies suggest that heel warming has not resulted in greater blood volume yield (Folk, 2007). (2) Provide pain management. f. Observe strict aseptic technique per institution guidelines. g. Observe Standard Precautions per institution guidelines. h. Obtain laboratory test using an appropriate route, for example: (1) Capillary versus arterial versus venous. i. Discard the initial few drops of blood after lancing the heel—along with prewarming the heel, this reduces the magnitude of capillary and venous laboratory value differences (Blackburn, 2013; Kee, 2010). j. Obtain blood cultures first, hematology specimens next to minimize platelet clumping, then chemistry and blood bank samples (Folk, 2007; Garza and Becan-McBride, 2010e; Sanford and McPherson, 2011) or per institution protocol. Note: Blood cultures cannot be obtained via capillary source. k. Insert proper amount of specimen into proper specimen container. l. Label specimen. m. Place specimen in biohazard container or bag per institutional guidelines. n. Promptly send specimen to laboratory. 3. Specimen tubes: standardized, color-coded containers indicating whether they contain whole blood, plasma (which contains fibrinogen), or serum (Kee, 2010). Specimen tubes can be glass or plastic, with many labs converting to plastic collection tubes to increase occupational safety (Sanford and McPherson, 2011). Tube colors in the NICU typically come in red, blue, green, lavender, and yellow. Plastic microtainers are the most common types of specimen collection tubes in the NICU. Microtainers allow use of smaller blood volumes to obtain laboratory results. Blood culture tubes, sterile swabs, and glass slides are also used in the NICU for specimen collection. Tubes with anticoagulant coating should be gently inverted, end over end, 7 to 10 times for proper anticoagulant–blood mixing (Fischbach, 2009; Garza and Becan-McBride, 2010a). Tubes with anticoagulant must be filled to the indicated fill line to properly dilute anticoagulant and avoid potential erroneous results. b. Blue tube (light): contains sodium citrate, an anticoagulant that removes calcium to prevent clotting (Fischbach, 2009). Use for unclotted blood–plasma specimens. Laboratory specimens: prothrombin time (PT), partial thromboplastin time (PTT), factor assays. Another light-blue tube containing thrombin and soybean trypsin inhibitor is useful for measurement of fibrin degradation products (Sanford and McPherson, 2011). c. Green tube: contains anticoagulant heparin (sodium, lithium, ammonium) that inhibits thrombin activation to prevent clotting. Used for unclotted blood–plasma specimens. Laboratory examples: chromosome analysis (use sodium heparin tube), ammonia levels, and hormone levels. d. Lavender tube: contains ethylenediaminetetraacetic acid (EDTA) that removes calcium to prevent clotting. Tube used for whole blood and plasma specimens. Laboratory examples: CBC, Retic count, platelet count, Hct. e. Yellow tube/bottle cap (SPS)—sterile, contains sodium polyanethol sulfonate (SPS) that aids in bacterial recovery by inhibiting complement, phagocytes, and certain antibiotics (Sanford and McPherson, 2011). Whole blood used for blood cultures. These cultures should be processed quickly to minimize the potential for decreased yield owing to storage or prolonged exposure to SPS (Olsowka and Garg, 2001). A yellow, acid citrate dextrose (ACD) tube is not typically used in the NICU; however, it is used for human leukocyte antigen (HLA) phenotyping and paternity testing (Sanford and McPherson, 2011). A. It is important to be familiar with the limitations and applications of the laboratory data as they apply to patient care. Many factors influence blood values and their interpretation in the newborn, including timing, site and amount of blood sample, placental transfusion, and infant growth rate (Blackburn, 2013; Letterio et al., 2013). A laboratory test has certain characteristics that influence how it is interpreted and used in the clinical setting. The following concepts are integral in the process of laboratory interpretation: 1. Accuracy: synonymous with “correctness,” this term refers to how close a test result is to the true value (Laposata, 2002; Oxley et al., 2001). a. Point-to-point variability in test results exists. b. Variation in value may be more reflective of an analytic variation of automated chemistry systems than actual patient status. 2. Precision: synonymous with “reproducibility,” this term describes the distribution of results when a sample is analyzed repeatedly. a. Imprecision is known as random error. b. Test precision is a more desirable test characteristic than accuracy in measuring treatment response or clinical changes. 3. Sensitivity: the ability of a test to correctly identify an individual with disease and not miss anyone by falsely testing “healthy.” It refers to a test’s ability to generate more true-positive results and fewer false-negative results. a. Sensitive tests have a low threshold for abnormality. b. Sensitive tests usually have a low specificity. c. Certain testing requires sensitivity over specificity—example: blood bank donors screened for infectious diseases, when it is better to err in excluding donors who are falsely positive than include donors who are falsely negative. 4. Specificity: the ability of a test to identify only those individuals with disease as opposed to individuals testing positive when there is no disease. It refers to a test’s ability to generate more true-negative results and fewer false-positive results. a. Specific test has a high threshold for “normal” or negative test results. b. Specific test usually has low sensitivity. c. Certain testing requires specificity over sensitivity—examples: urine toxicology screen to detect presence of cocaine, cardiac isoenzymes to rule out a myocardial infarct. d. Positive predictive value (yield): the probability that an individual with a positive screening test really has the disease. It is dependent on the prevalence of the disease in the population being tested (LaMorte, 2013). (1) Probability ratio is a percentage based on the population screened. (2) Computation based on a 2 × 2 contingency table. (b) Certain testing, such as state screening programs, considers this probability. e. Negative predictive value: the probability that an individual with a negative screening test really does not have the disease. (1) Probability ratio is a percentage based on the population screened. (2) Computation based on a 2 × 2 contingency table. 5. Reference range: established upper and lower boundary levels of a laboratory value by which a patient’s result will be measured for presence of disease. The range of normality (mathematical) is dependent on population subsets such as age, gender, pregnancy, and other patient attributes. a. Often referred to as “normal” range. b. Term normal is misleading—reference range determined based on specific attributes of a population subset, not due to “normalcy” (Oxley et al., 2001). c. Approximately 5% of “healthy” laboratory results fall outside the reference range. d. Reference range can vary from laboratory to laboratory. A. Patient management depends on good clinical skills, judicious use of laboratory testing, and careful interpretation of laboratory data. Once a laboratory value is determined and the patient’s clinical status evaluated, the combined information is used to direct treatment. In an effort to optimize care, minimize patient discomfort, and contain health care costs, Wallach (2007) identifies key principles of test utilization: 1. Even under the best of circumstances, no test is perfect. b. Specificity or sensitivity of a test is never 100%. 2. Choice of tests should be based on the prior probability of the diagnosis being sought, which affects the predictive value of the test. b. Patient history and examination should precede choice of laboratory tests. 3. The combination of short-term physiologic variation and analytic error is sufficient to render the interpretation of single determinations difficult when the concentrations are in the borderline range. a. Despite the high quality of a laboratory, any laboratory result may be incorrect. b. Laboratory tests may need to be rechecked or redrawn, at times in another laboratory. 4. Reference ranges vary from one laboratory to another. a. Age, gender, race, size, and physiologic status must be considered. b. 5% of test results will be outside the reference range in the absence of disease. 5. Tables of reference values represent statistical data for 95% of the population; values outside these ranges do not necessarily represent disease. b. Certain conditions warrant serial testing. 6. An individual’s test values when performed in a good laboratory tend to remain fairly constant over a period of years. 7. Multiple test abnormalities are more likely to be significant than single test abnormalities. a. Two or more positive tests for a given disease reinforce diagnosis. 8. The greater the degree of abnormality of a test result, the more likely that a confirmed value is clinically significant or represents a real disorder. 9. Characteristic laboratory test profiles representing full-blown or advanced disease may all be present in only one third of patients with said condition. 10. Excessive repetition of tests is wasteful, and the excess burden increases the possibility of laboratory errors. a. Patient’s acuity should dictate testing interval. 11. Tests should be performed only if they will alter the patient’s diagnosis, prognosis, treatment, or management. 12. Clerical errors are far more likely than technical errors to cause incorrect results. 13. The effect of drugs on laboratory test values must never be overlooked. 14. The effect of artifacts can cause spurious values and factitious disorders, especially in the face of discrepant laboratory results. 15. Negative laboratory tests (or any other type of tests) do not necessarily rule out a clinical disease. A. The goal of laboratory testing is to help diagnose and guide management of disease. Estimates suggest an average of 61 invasive procedures are performed on NICU patients during their stay, with the smallest of infants experiencing the largest amount of painful procedures (Walden, 2007). Inadvertently, the pain of laboratory testing can complicate patient care by creating additional illness, stress, injury, and/or cost. An understanding of the potential sequelae associated with laboratory sampling and strategies that can be used to minimize sequelae is integral to patient care. a. Adverse physical symptoms triggered by pain stimuli, sensory stimuli, and/or disease state. b. Laboratory sampling via skin puncture elicits a painful stimulus creating physiologic stress. c. Common physiologic stress symptoms include the following (AAP and CPS, 2007) (see also Chapter 11): (1) Tachycardia or bradycardia. (2) Hypertension or hypotension. (3) Apnea or crying. (4) Cyanosis or respiratory distress. (5) Changes in skin color and temperature. d. An event creating physiologic stress can potentially alter a laboratory sample result; for example: (1) Change in PCO2 and PO2 reading when infant cries (Kaplan and Tange, 1998). (2) Altered blood pH if infant becomes hypothermic. e. Strategies to minimize sampling-induced physiologic stress include the following: (1) Minimize skin punctures for laboratory sampling by minimizing or combining lab work. (2) Utilize existing indwelling catheters (when available) to obtain laboratory samples. (3) Use noninvasive pain management techniques to assist infant in coping with painful stimuli (AAP and CPS, 2007; see also Chapter 16). (4) Use spring-loaded lancets for heel-stick sampling (Folk, 2007; Walden, 2007). (5) Ensure quick, efficient execution of laboratory sampling. (6) Apply warm compress to the heel, which might promote blood flow and improve testing accuracy, thus avoiding repeat laboratory sampling. (7) Venipuncture may be preferable to the heel-stick procedure in minimizing procedure-related pain in term neonates (Shah and Ohlsson, 2011). (8) Careful specimen collection, handling, and labeling can decrease repeat laboratory draws. 2. Pain (see also Chapter 16). a. An unpleasant sensory and emotional experience associated with actual or potential tissue invasion (Walden, 2007). b. Laboratory sampling by skin puncture evokes pain. c. Pain causes adverse physiologic stress (see 1. c., this section), including potential central nervous system (CNS) alterations (AAP and CPS, 2007). d. It is difficult to differentiate between acute and chronic pain in the infant (AAP and CPS, 2007). e. Strategies to minimize sampling-induced pain include the following: (1) Strategies that minimize physiologic stress (see 1. e., this section). (2) Nonpharmacologic pain management strategies; examples: swaddling, facilitated tucking, nonnutritive sucking, skin-to-skin contact (AAP and CPS, 2007; Folk, 2007). (3) Pharmacologic pain management strategies; examples: 24% sucrose, local anesthetic, and opioid and nonopioid analgesics. (4) Usefulness of topical local anesthesia for pain control in infants can be helpful for procedures such as venipuncture, lumbar puncture, and IV catheter insertion. It is not effective for heel sticks (AAP and CPS, 2007). (5) Using venipuncture when obtaining blood samples may be less painful and have less potential sequelae (i.e., nerve damage, arterial spasm) compared with arterial puncture. (6) In term infants, venipuncture has been shown to be less painful a procedure than heel stick when performed by skilled personnel (Shah and Ohlsson, 2011). 3. Skin injury. a. Alteration in normal barrier function of skin as a result of invasive procedures, adhesives to skin, reaction to skin antiseptics, and/or disease states (Lund and Kuller, 2007). b. Arterial punctures, venipunctures, and capillary heel sticks used to obtain laboratory samples may potentially create skin injury (LeFrak and Lund, 2013). c. Potential skin injury from peripheral laboratory samples includes: (2) Hematoma. (3) Abrasion from antiseptic solutions, friction, or tape application. (4) Dermal stripping from friction or tape application. (5) Scarring from multiple punctures. (6) Calcifications. (7) Burns secondary to prewarming heel with a soak that is too hot; warm soak should not exceed 44° F. (8) Chemical burns secondary to antiseptic skin-cleansing agents. d. Strategies to minimize sampling-induced skin injury from skin puncture include: (2) Avoid excessive “squeeze” when obtaining capillary blood sample. (3) Apply adequate pressure to puncture sites to minimize bleeding and formation of hematoma. (4) Use nonadhering products to apply as a dressing to puncture site; example: (a) Loose elastic wrap (Coban™) around extremity holding gauze in place. (5) Thoroughly wash skin of antiseptic solutions. (6) Use proper skin puncture devices when obtaining blood; example: (a) Spring-loaded lancet of proper length (< 2 mm) (Garza and Beacon-McBride, 2010b) and with heel-stick devices ranging from 0.65 to 2 mm depending on infant size (Folk, 2007). (b) Butterfly needle of proper gauge and length for site. 4. Infection. a. An important skin function is to provide a barrier to infection. b. Any break in skin barrier creates potential for infection. c. Common infections associated with altered skin barrier include: (1) Bacterial/candidal skin surface infection. (2) Cellulitis. (3) Abscess formation at puncture site. (4) Septicemia. (5) Osteomyelitis. (6) Urinary tract/bladder infection. (7) Meningitis. d. Strategies to minimize sampling-induced infection include: (1) Minimize laboratory sampling. (2) Avoid sampling in area of existing skin injury. (3) Avoid repeated sampling from dedicated central lines (Folk, 2007); example: routine laboratory sampling via PICC or Broviac® catheter. (4) Meticulous aseptic technique when obtaining a laboratory sample. (5) Use of nursing strategies to maintain optimal skin integrity (see Chapter 36). 5. Organ/nerve injury. a. Needle puncture for laboratory sampling can potentially contribute to organ injury. b. Potential organ injury associated with needle puncture includes: (1) Damage to nerve and/or tissues in wrist or brachial area from arterial puncture. (2) Damage to lung and/or breast tissue from thoracentesis. (3) Damage to abdominal organs from peritoneal tap. (4) Damage to nerves or tissue of spine from lumbar puncture. (5) Damage to skin as mentioned in 3., this section. (6) Tissue ischemia from arterial vasospasm secondary to arterial puncture. c. Strategies to minimize sampling-induced organ/nerve injury include the following: (1) Prudent use of laboratory sampling. (2) Proper technique for needle-stick sampling (see also Chapter 15). (3) Ultrasound guidance as needed for pleural and peritoneal aspiration. 6. Anemia. a. Iatrogenic anemia owing to blood loss from laboratory sampling (Bagwell, 2007; Kaplan and Tange, 1998). b. Iatrogenic anemia, along with physiologic anemia, constitute the most common causes of chronic anemia in infants (Bagwell, 2007). c. Despite microsampling and conservative laboratory sampling, sick infants can lose more than 5 mL of blood per day (Letterio et al., 2013). d. Removal of 1 mL of blood from a 1-kg infant equals removal of 70 mL of blood from an adult (Blackburn, 2013). e. Iatrogenic blood loss correlates with degree of illness. f. Laboratory sample overdraws (19% ± 1.8% more than needed) are common in the NICU (Blackburn, 2013). g. Strategies to minimize sampling-induced anemia include: (1) Judicious use of laboratory sampling. (2) Microsampling when possible. (3) Accurate documentation of blood loss. (4) Avoiding overdrawing of blood samples. 7. False diagnosis. a. Even under the best circumstances, no test is perfect. b. Excess repetition of a test increases the possibility of laboratory error. c. The more laboratory samples drawn, the more likely one or more results will be outside the reference range. d. Clinical decision making should be based on trends or multiple laboratory values pointing to disease versus spurious results. e. Strategies to minimize sampling-related false diagnosis include: (2) Verify spurious laboratory values. (3) Draw laboratory samples using proper technique and conditions. 8. Cost factor. a. 2.3% to 3% of a patient’s hospital bill results from laboratory sampling (Rusckowski, 2012). b. If the average daily cost of a NICU stay is $3000 (Kornhauser and Schneiderman, 2010), a patient minimally spends $90 per day for laboratory testing. c. Cost of hospitalization in the NICU is inversely related to gestational age. The average cost of healthy, term newborn is $2830, while premature infant costs range from $41,610 to greater than $250,000 (Kornhauser and Schneiderman, 2010). d. Rising cost of medical care is complicating delivery of care. e. Strategies to minimize sampling-related laboratory costs include: (1) Minimize laboratory sampling. (2) Prudent medical and nursing decision making regarding laboratory sampling. (3) Minimizing laboratory errors precipitating repeat sampling. A. Judicious use of laboratory testing is critical in any setting, particularly in the NICU, where acuity is high. Below are questions to consider prior to ordering and/or obtaining a laboratory sample. Asking oneself these questions will assist in refining critical thinking skills and aid in selective use of lab work (Box 13-1). To simplify the discussion process, one presenting symptom—jittery infant—will be used and applied to all phases of critical thinking. 1. Does the patient require the laboratory test? a. Are the patient examination results abnormal, whereby a laboratory test will help in diagnosis? (a) Abnormal finding: model–jittery infant. (b) Possible laboratory tests: glucose, calcium, and urine toxicology screen. b. Is the medical history helpful in directing which laboratory test to order? (1) Example: model–jittery infant. (b) Infant with delayed drying of skin after birth and axilla/skin temperature of 96° F? Might choose to place infant under heat source and not obtain lab work. (c) Infant 1800 g, IDM-A1, and mother with minimal prenatal care? Might choose to obtain all possible laboratory tests. 2. Will the laboratory test requested answer the “so what” question? Model–jittery infant. a. Is the laboratory result integral to the immediate clinical management of the infant? (1) Hypoglycemia in an IDM-A1 will mandate increasing the carbohydrate intake to treat the problem. (2) Mild hypothermia in an otherwise healthy infant will not require a change in management directed by lab work. b. Is the laboratory result contributory to the infant’s diagnosis? (1) Serum glucose test result will help diagnose hypoglycemia as a cause of jitteriness. (2) Serum glucose test result will not help diagnose respiratory distress syndrome. 3. Is the laboratory test requested still applicable to current clinical status? a. Example: model–jittery infant. (1) Has the infant’s clinical status changed? (b) An hour-old IDM-A1 who breastfed for 20 minutes and is no longer jittery may not require further testing, particularly if the original serum glucose level was normal. (c) The hypothermic infant who continues to remain hypothermic after 2 hours under heat source may need serum glucose test and sepsis evaluation. b. Example: model–jittery infant. (a) Infant with multiple normal glucose test results may no longer require frequent glucose testing. (b) Infant no longer jittery after acquiring a normal body temperature—may need to cancel lab work ordered to evaluate jitteriness. 4. Is the timing of the laboratory test appropriate? a. Example: model–jittery infant. (1) When should the lab work be obtained? (b) Jittery infant with normal serum glucose and calcium levels—might obtain first voided specimen for a urine toxicology screen. 5. Is the laboratory test ordered the “best” test to answer the clinical question? a. Example: model–jittery infant. (2) Should the jittery infant with polycythemia have a POCT whole-blood glucose test or should a serum glucose sample be sent to the laboratory? A POCT glucose result may not be accurate in the face of polycythemia. 6. Does the laboratory test require too much blood volume? a. Example: model–jittery infant. (2) A 5-mL sample of blood is required for metabolic screening in a 500-g infant—10% blood volume loss may not be tolerated; need to prioritize lab work and draw in stages based on most common to least common disorders. 7. Does the potential benefit of the laboratory test outweigh the risk of sequelae in the patient? a. Example: model–jittery infant. (2) A 5-mL blood sample for metabolic screening in a 500-g, symptomatic infant—potential harm of an acute 10% blood loss outweighs the need to simultaneously obtain all the ordered metabolic screening laboratories. 8. If the laboratory sample is inadequate, faulty, or “lost,” is it necessary to redraw? a. Example: model–jittery infant. (1) Has the clinical status changed? (2) Has the infant recovered? (See 3., this section.) If the status improves or the infant recovers while waiting for sample, repeat sampling may not be indicated.
Laboratory and Diagnostic Test Interpretation
LABORATORY TESTS IN THE NICU
PURPOSE OF LABORATORY TESTING
PROCESS OF LABORATORY COLLECTION
CONCEPTS OF LABORATORY TEST INTERPRETATION
PRINCIPLES OF TEST UTILIZATION
IATROGENIC SEQUELAE OF LABORATORY TESTING—PREVENTIVE STRATEGIES
DECISION—QUESTIONS TO ASK PRIOR TO OBTAINING A LABORATORY TEST
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree