Health Problems Complicating Pregnancy
Objectives
2. Discuss three causes of spontaneous abortion.
3. Describe ectopic pregnancy.
4. Describe placenta previa, and state the characteristic symptom.
5. Explain five nursing measures for the care of a woman who is hemorrhaging.
6. Compare two types of abruptio placentae.
7. Review the cause of coagulation defects in pregnancy.
8. List five causes of high-risk pregnancies and three leading causes of maternal death.
9. Recognize four factors that increase the risk for gestational hypertension.
10. Discuss three signs that a pregnant hypertensive woman should report immediately to her physician.
12. Compare the effects of the physiologic changes in pregnancy related to thromboembolic disease.
13. Discuss heart disease in pregnancy.
14. Explain hyperemesis gravidarum.
15. Explain three ways diabetes mellitus affects pregnancy.
16. Review four aspects of self-care for the diabetic woman.
17. Describe rubella and its consequences in pregnancy.
18. Identify the changes that occur in pregnancy that predispose the woman to urinary tract infections.
19. Discuss the cause and prevention of toxoplasmosis.
20. Describe three self-care measures for a pregnant woman with a urinary tract infection.
21. Describe how the use of nicotine, alcohol, and recreational drugs can affect the fetus.
22. Discuss the effects of substance abuse on women’s health.
24. Recognize the effects of drugs used to treat bioterrorist infections on the developing fetus.
25. Identify signs of fetal demise.
Key Terms
abortion (ă-BŎR-shŭn, p. 250)
abruptio placentae (ă b-RŬP-shē-ō plă -SĔN-tē, p. 256)
cervical cerclage (sĕr-KLĂZH, p. 252)
disseminated intravascular coagulation (DIC) (dĭ-SĔM-ĭ-NĀT-ĕd ĭn-tră -VĂS-cū-lă r kō-ă g-ū-LĀ-shŭn, p. 257)
eclampsia (ĕ-KLĂMP-sē-ă, p. 259)
ectopic pregnancy (ĕk-TŎ P-ĭk, p. 253)
gestational diabetes mellitus (GDM) (jĕs-TĀ-shŭn-ă l dī-ă -BĒ-tēz MĔL-ī-tŭs, p. 271)
gestational hypertension (GH) (p. 259)
gestational trophoblastic disease (trōf-ō-BLĂS-tĭk, p. 254)
HELLP syndrome (p. 259)
hydatidiform mole (hī-dă -TĬD-ĭ-fŏrm mōl, p. 254)
hyperemesis gravidarum (hī-pĕr-ĕm-Ē-sĭs gră v-ĭ-DĂR-ŭm, p. 268)
incompetent cervix (ĭn-KŎ M-pĕ-tĕnt SĔR-vĭks, p. 252)
isoimmunization (p. 258)
macrosomia (mă k-rō-SŌ-mē-ă, p. 269)
placenta previa (plă -SĔN-tă PRĒ-vē-ă, p. 254)
preeclampsia (prē-ĕ-KLĂMP-sē-ă, p. 259)
pregestational diabetic (prē-jĕs-TĀ-shŭn-ă l dī-ă -BĔT-ĭk, p. 269)
RhoGAM (p. 258)
TORCH infections (p. 272)
 http://evolve.elsevier.com/Leifer/maternity
http://evolve.elsevier.com/Leifer/maternity
Health problems during pregnancy can significantly affect pregnancy outcome. Some of these conditions develop as a result of the pregnancy state, whereas others are health problems that were present before pregnancy. This chapter discusses a wide variety of disorders, all of which have at least one thing in common: their occurrence during pregnancy puts the woman and her fetus at risk. For women whose pregnancies are at risk, focused prenatal care provides necessary health teaching for the promotion of health and prevention of illness. Prompt identification, assessment, and management of the problems are essential to the successful outcome of the pregnancy and the well-being of the newborn. The leading disorders of pregnancy that result in maternal death are hypertension, embolism, hemorrhage, and infection.
Effects of a High-Risk Pregnancy on the Family
Normal pregnancy can be a crisis because it is a time of significant change and growth. The woman with a complicated pregnancy has stressors beyond those of the normal pregnancy. Her family is also affected by the pregnancy and impending birth. Many new noninvasive technologies are on the horizon to detect problem pregnancies and enable early diagnosis and treatment, which may help prevent perinatal loss. The woman should be instructed to return to the health care provider if she notices any of the danger signs listed in Box 13-1.
Disruption of Usual Roles
The woman who has a difficult pregnancy often must remain on bed rest at home or in the hospital, sometimes for several weeks. Others must assume her usual roles in the family, in addition to fulfilling their own obligations. Finding caregivers for young children in the family may be difficult if extended family lives far away. Placing the children in day care may not be an option if financial problems exist. Nurses can help families adjust to these disruptions by identifying sources of support to help maintain reasonably normal household functions.
Financial Difficulties
Many women work outside of the home, and their salary may stop if they cannot work for an extended period. At the same time, their medical costs are rising. Social service referrals may help the family cope with their expenses.
Delayed Attachment to the Infant
Pregnancy normally involves gradual acceptance of and emotional attachment to the fetus, especially after the woman feels fetal movement. Fathers feel a similar attachment, although at a slower pace. The woman who has a high-risk pregnancy often halts planning for the child and may withdraw emotionally to protect herself from pain and loss if the outcome is poor.
Bleeding Disorders
Bleeding during pregnancy is abnormal, and its cause should be investigated. Maternal blood loss decreases oxygen-carrying capacity, which predisposes the woman and fetus to risks. Approximately 15% of the maternal cardiac output (1 L/min) flows through the placental bed at term; unchecked bleeding can therefore be fatal.
Bleeding occurs in about 25% of first-trimester pregnancies, and the woman is at increased risk of preterm labor or abruption (Lykke, Dideriksen, Lidegaard, & Langhoff-Roos, 2010). Nosebleeds during pregnancy can be a predictor of postpartum hemorrhage and should be reported (Dugan-Kim, Connell, Sitka, Wong, & Gossett, 2009). Early in pregnancy, the most common causes of bleeding are spontaneous abortion, cervical polyps, uterine fibroids, ectopic pregnancy, and hydatidiform mole. Frequent causes of bleeding late in pregnancy include placenta previa and abruptio placentae. Disseminated intravascular coagulation (DIC) can occur as the result of a coagulation defect. Postpartum hemorrhage is discussed in Chapter 17.
Bleeding in Early Pregnancy
Abortion
Abortion is the intentional or unintentional ending of a pregnancy before 20 weeks’ gestation, the point of viability. Miscarriage is a lay term applied to a spontaneous abortion. Abortion can be induced as a result of artificial or mechanical means for therapeutic or elective reasons.
Classification and Management of Abortions
Spontaneous abortions typically occur because of some maternal illness or genetic defect that has occurred in the fetus. Approximately 15% of all pregnancies terminate in spontaneous abortions. The majority of these abortions occur before the twelfth week of gestation. Specific causes may include:
Blood group dyscrasias (ABO and Rh) can also cause an abortion. Psychological and physical trauma has also been implicated as a cause. The use of an abdominal seat belt without a shoulder restraint in an automobile can cause placental separation and abortion because of the flexion of the body and displacement of the uterus in a car accident. Proper restraint of the upper and lower body in a moving automobile is very important (see Figure 5-9).
An induced abortion may be performed for the selective termination of a multifetal pregnancy. Often artificial reproductive technology (ART) or “fertility pills” result in multifetal pregnancies that, without intervention, can end in premature delivery and neonatal damage or loss.
Counseling the woman and family after an abortion is very important. Once the woman has had a spontaneous abortion, she often asks, “What are my chances of carrying another baby to term?” For the woman and her family, regardless of whether the pregnancy was planned, the loss is often accompanied by a certain degree of guilt. Many women go through the same grieving process as that caused by other types of personal loss. In some women, the grief response may be intensified if the abortion follows infertility treatments.
When symptoms of abortion such as bleeding and cramping occur, ultrasound scanning may be used to detect the presence of a gestational sac and assess if there is a developing embryo or live fetus. The woman is usually asked to abstain from sexual intercourse and placed on bed rest. If the abortion (loss of the products of conception) appears to be imminent, the woman is hospitalized. Intravenous therapy is started to replace the fluid loss. Blood transfusions usually are not necessary, but surgical intervention such as dilation and evacuation (D&E), formerly called dilation and curettage (D&C), may be necessary to remove the remainder of the products of conception to stop the bleeding. If the pregnancy is beyond 12 weeks’ gestation, an induction of labor may be done to expel a dead fetus.
After an abortion, the Rh-negative woman should receive Rho(D) immune globulin (RhoGAM) to protect her from isoimmunization. If the abortion is not after 12 weeks’ gestation, MICRhoGAM is used.
Abortions are clinically classified according to the symptoms and whether the products of conception are partially or completely retained or expelled (Figure 13-1, Table 13-1).
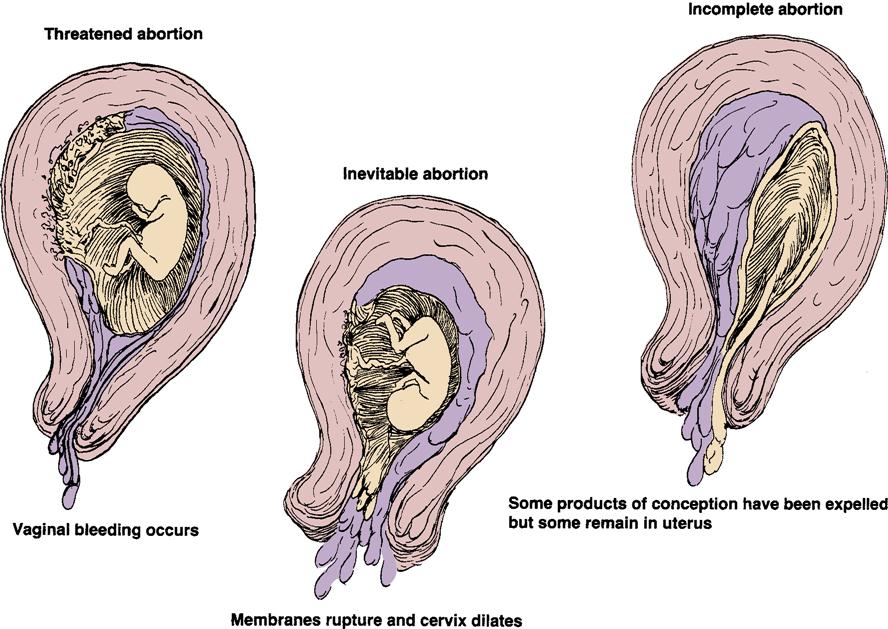
Table 13-1
Comparison of Types and Management of Spontaneous Abortion (Miscarriage)*
| Type | Cramps | Bleeding | Tissue Passed | Cervical Opening | Uterine Size | Nursing Management |
| Threatened | Slight (with or without cramps) | Slight to moderate (bleeding ceases) | None | Closed | Commensurate with date | Bed rest, sedation,† avoidance of coitus, ultrasound; observe amount of bleeding (save pads); woman to gradually increase activity; perform pregnancy tests; give Rho(D) immune globulin (RhoGAM) within 72 hours if indicated |
| Inevitable | Moderate | Moderate to severe | None | Open, with membranes or tissues bulging | Commensurate with date | Bed rest, sedation; transfusion may be indicated; observe amount of bleeding, color (save pads); give RhoGAM if indicated |
| Incomplete | Severe | Severe and continuous | Placental or fetal tissue | Open, with tissue in cervical canal or passage of tissue | Smaller than date | Bed rest, sedation; observe to determine how much tissue is passed; save all available tissue; carefully record vital signs; dilation and evacuation (D&E) as necessary; give RhoGAM if indicated |
| Complete | None | Minimal | Complete placenta and fetus | Closed, with no tissue in cervical canal | Smaller than date | Observe to determine if all tissue is passed (save pads); give RhoGAM if indicated |
| Missed | None; no life felt | Brownish discharge | None; prolonged retention of tissue | Closed | Smaller than expected | No specific treatment available; oxytocin may be used to induce labor and delivery; check for coagulation defect (DIC) |
| Recurrent (habitual) | Comprehensive and conservative care essential in early months; cerclage surgery performed if necessary for incompetent cervix |
DIC, disseminated intravascular coagulation.
*Psychologically, many patients experience a grief period and have fears of inability and inadequacy as a woman. They express anxiety over the next pregnancy or ability to conceive again.
Nursing Interventions
“Spotting” is common during pregnancy, especially after intercourse or exercise, because the highly vascular cervix is easily traumatized. However, occasional spotting does not necessarily indicate a pathologic problem. When a woman is admitted to the hospital for bleeding during pregnancy, nursing responsibilities include:
Incompetent Cervix
A woman is said to have an incompetent cervix when the cervix dilates without perceivable contractions, resulting in the products of conception being expelled early in pregnancy. In other words, the internal os of the cervix dilates because it is incapable of supporting the increasing weight and pressure of the growing fetus. For a fetus to be carried to term, the woman’s cervix may need to be reinforced surgically with a heavy ligature placed submucosally around the cervix. This technique is called the Shirodkar procedure. If a heavy silk purse-string suture is used, the technique is called a McDonald’s procedure. These procedures are known as cervical cerclage. When the pregnancy reaches term or labor begins, the suture is cut and labor is allowed to progress or a cesarean birth is accomplished. If the woman has a history of repeated late abortion in the second trimester, the cervix is dilated 3 to 4 cm, and the membranes are intact, she may be a candidate for a cerclage to help the pregnancy reach term. When cerclage is in place, the mother must be taught the importance of notifying the health care provider at the first sign of labor, such as ruptured membranes or labor pains, to avoid complications such as a ruptured uterus.
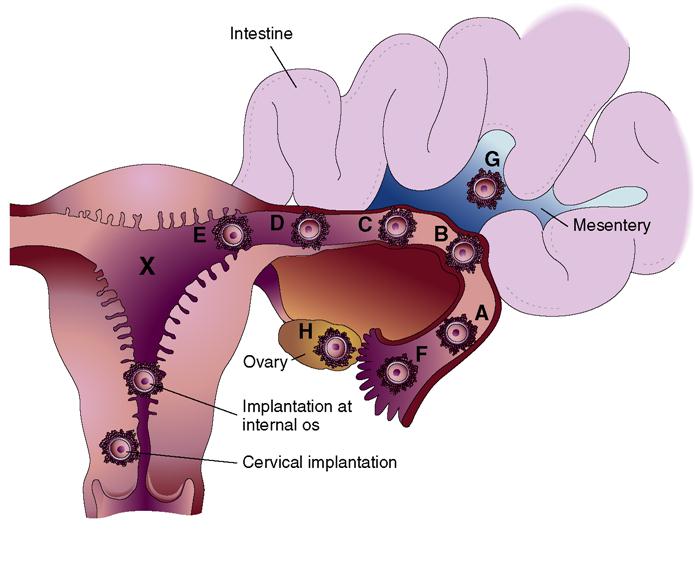
 Ectopic Pregnancy
Ectopic Pregnancy
Ectopic pregnancy refers to an abnormal implantation of the fertilized ovum outside of the uterine cavity (Figure 13-2). Implantation occurs most commonly in some portion of the fallopian tube. Other sites, although rare, include the ovary, the abdominal cavity, and the cervix. Because the fallopian tube is not anatomically suited for implantation, the trophoblast cells erode the blood vessels and weaken the tissue, resulting in tube rupture. The tubal rupture can cause a fatal hemorrhage. Predisposing conditions include any factor that affects tubal patency, ciliary action, and contractility. Two prominent causes are partial tubal occlusion from pelvic inflammatory disease (PID) and the use of a contraceptive intrauterine device (IUD). The incidence of ectopic pregnancy is increasing but mortality is decreasing due to better diagnostic methods and technology. The nurse must be alert to symptoms of hemorrhage and shock and be prepared to act quickly to minimize blood loss and hasten maternal stabilization.
Assessment and Management
A combined use of transvaginal ultrasound examination and assessment of the hormonal levels of progesterone and beta-human chorionic gonadotropin (β-hCG) is used in the early detection of an ectopic pregnancy. The vaginal probe ultrasound will show an empty uterus and may show the site of an ectopic pregnancy. Characteristically, the woman misses one or two menstrual periods and then may have stabbing pain in either lower abdominal quadrant. These signs may or may not be followed by dark red or brown vaginal spotting. Shoulder pain caused by blood irritating the diaphragm or phrenic nerve is a common symptom. The signs of shock that develop are out of proportion to the clinical blood loss because most of the blood lost is hidden within the abdominal cavity. This is one obstetric complication in which rapid surgical treatment can save the woman’s life.
Management of tubal pregnancy depends on whether the tube is intact or ruptured. If the tube is intact and hCG levels are declining, it indicates spontaneous regression of the tubal pregnancy. Methotrexate, a chemotherapeutic agent that interferes with cell reproduction, may be used to inhibit cell division in the developing embryo. The primary reason for medical management is preserving the fallopian tube to increase the chance of a future pregnancy. A woman who is receiving methotrexate therapy must avoid alcohol consumption and vitamins containing folic acid to prevent a toxic response to the drug. Photosensitivity is common with methotrexate therapy, so the woman should be counseled to protect herself from sun exposure.
Surgical management of an unruptured tubal pregnancy may involve a linear salpingostomy, which is done to salvage the tube. A laparoscopic salpingectomy (removal of the tube) is performed when the tube is ruptured to control bleeding and prevent hypovolemic shock. Future pregnancy can occur with one tube remaining. Replacement of fluid loss and maintenance of electrolytes are essential aspects of treatment. Psychological support to help the woman and family deal with emotions and to answer questions about future pregnancies should be part of the nursing care plan.
Gestational Trophoblastic Disease
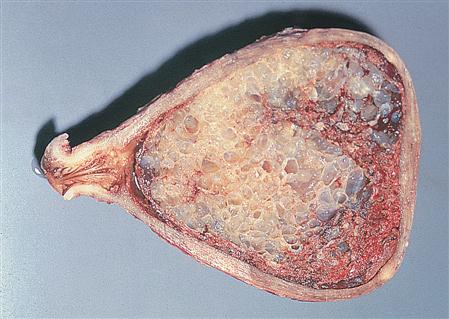
Gestational trophoblastic disease includes hydatidiform mole, invasive mole, and choriocarcinoma. Hydatidiform mole (molar pregnancy) is a condition in which the trophoblastic tissue proliferates and the chorionic villi of the placenta become swollen and fluid filled, taking on the appearance of grapelike clusters (Figure 13-3). Molar pregnancies are classified into two types: complete and partial. Chromosome banding and enzyme analysis have demonstrated that, in the complete mole, all genetic material is paternally derived. The mechanism of the loss of the genetic material from the ovum is unknown. No inner cell mass develops embryonically, and there is no fetal vascularization, which explains the avascular villi (vesicles). With partial mole, genetic material is maintained but the fetus is abnormal and usually aborts. The mole is distinguished from an abortion in that the mole shows trophoblastic proliferation. Some moles may develop into choriocarcinoma.
The cause is unknown, but the incidence increases with maternal age and parity. In the United States, this condition is fairly rare, occurring in approximately 1 in every 1000 pregnancies.
Assessment and Management
Early pregnancy appears normal. Then as gestation progresses, the uterus begins to grow rapidly because of the rapid proliferation of the trophoblastic cells. However, in 20% of the moles the uterus is found to be smaller than expected. Vaginal bleeding occurs in 84% of cases, which often is brownish (like prune juice), as well as excessive vomiting (hyperemesis gravidarum), most likely because the serum hCG levels are higher than in normal pregnancy. Symptoms of gestational hypertension (GH) occur before 24 weeks, which strongly suggests a molar pregnancy. Serial β-hCG levels and ultrasound are the primary diagnostic tools and can reveal the characteristic pattern of vesicles by the twelfth week of gestation (Goldstein, Baron, & Berkowitz, 2010).
Treatment begins with the evacuation of the mole by suction aspiration. Follow-up care is extremely critical for these women because of the great risk of choriocarcinoma (cancerous growth). Continued assessment includes hCG levels and ultrasound scans of the abdomen. Continued rising hCG levels are abnormal. If malignant cells are found, chemotherapy for choriocarcinoma may be started. If therapy is ineffective, metastasis is often rapid. Administration of RhoGAM to women who are Rh negative is necessary to prevent isoimmunization (see p. 258).
The woman is monitored for a year; if the hCG serum titers are within normal limits, the woman may be assured that she is healthy. The woman often takes oral contraceptives to prevent another pregnancy and to allow the hCG levels to return to normal. If the woman becomes pregnant immediately, it would make it impossible to monitor the decline in hCG, which is the significant part of follow-up care. Psychological support should be provided in relation to the pregnancy loss, and another pregnancy should be delayed for at least 1 year. Subsequent pregnancies should be normal because there is a low risk of recurrence.
Bleeding in Late Pregnancy
Vaginal bleeding, if slight, may be caused by the increased vascularization of the cervix, cervical polyps, or cervicitis. However, the major causes of bleeding in the second and third trimesters are placenta previa, abruptio placentae, and DIC.
 Placenta Previa
Placenta Previa
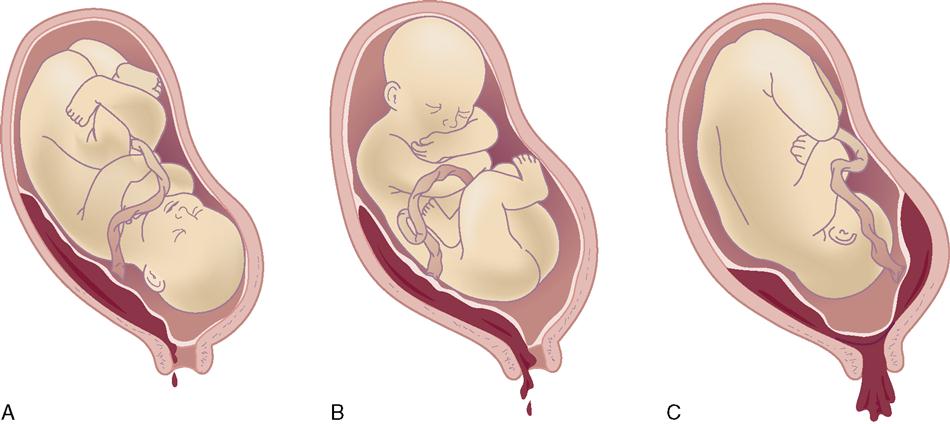
Placenta previa occurs when the placenta abnormally implants near or over the cervical os instead of in the fundus of the uterus. The degree to which the internal cervical os is covered by the placenta determines how the placenta previa is classified (Figure 13-4). Two main dangers are hemorrhage for the mother and premature delivery or fetal demise.
Placenta previa occurs in approximately 1 in 200 live births (Gabbe, Niebyl, & Simpson, 2007). Defective vascularity of the decidua or previous infection in the upper uterine segment increases the risk of placenta previa. Uterine scarring from previous cesarean births, previous placenta previa, endometritis, multifetal gestation, or multiple births increase the risk. Because the lower uterine segment is not as well vascularized as the upper segment, the placenta must cover a larger area for adequate function.
Assessment and Management
Placenta previa can be diagnosed before bleeding occurs in the third trimester because of the current routine practice of performing second-trimester ultrasound for detection of fetal anomalies. Women shown to be at risk for placenta previa at this time can have a repeat ultrasound performed at 28 and 35 weeks’ gestation. Placenta previa should be suspected with the onset of painless bleeding occurring after 24 weeks’ gestation. Painless bleeding results from the separation of the placenta from the uterus (that part of the placenta that is near, or covering, the internal cervical os). It most often occurs in the third trimester as the cervix begins to open in preparation for childbirth. Bleeding may be intermittent or in gushes. The bleeding can be extensive and can prove to be fatal. An abnormal fetal presentation may coexist in 15% to 20% of cases; therefore, a breech or transverse presentation should suggest the possibility of placenta previa.
The woman diagnosed with placenta previa must be closely monitored for the amount and character of blood loss. Vital signs, fetal heart rate, and activity are documented. Diagnosis is confirmed by ultrasound. Digital examinations (vaginal examinations) are strictly prohibited when a pregnant woman is bleeding because severe hemorrhage may result from the examination. A “double setup” vaginal examination may be performed by taking the woman to the operating room prepared for an immediate cesarean birth. However, this is required less often because of accurate ultrasound techniques available.
Management depends on the classification of previa and gestational age of the fetus. If the gestational age is less than 36 weeks and bleeding is slight, the woman is hospitalized for observation, placed on bed rest, and closely monitored. Blood count, type, and cross-match for blood and Rh factor are performed. Magnesium sulfate or other tocolytic drugs may be administered to prolong pregnancy if bleeding is not active (a tocolytic drug is a drug that stops labor contractions). The woman is told to notify the nurse immediately if she feels fluid escaping from the vagina. Vital signs are taken frequently, and external fetal monitoring is performed. Intravenous fluids may be given. Once the bleeding has subsided, the woman may be managed at home provided the following criteria are met:
• Woman understands her condition and that she must remain on bed rest and avoid coitus.
• Woman has around-the-clock transportation and communication available.
• Woman is compliant with oral tocolytic therapy.
• Woman has a hematocrit level above 30% to have some reserve in case of significant bleeding.
• Woman is able to be followed closely (e.g., ultrasound, nonstress test, and biophysical profiles).
If bleeding is heavy, an immediate cesarean birth is performed. The woman and family are kept informed during the monitoring or preparation for surgery.
Potential Complications
The main complications are hemorrhage for the woman and prematurity hypoxia or death for the fetus. Immediate postpartum hemorrhage often accompanies this condition because the surface area of attachment is greater than usual and the site of placental implantation in the lower uterine segment does not contract well after the placenta is expelled. Hemorrhage can cause hypovolemic shock and death. Postpartum infection may also occur because of the closeness of the placental site to the cervix and vagina. Therefore, the woman is closely monitored postnatally for bleeding and infection.
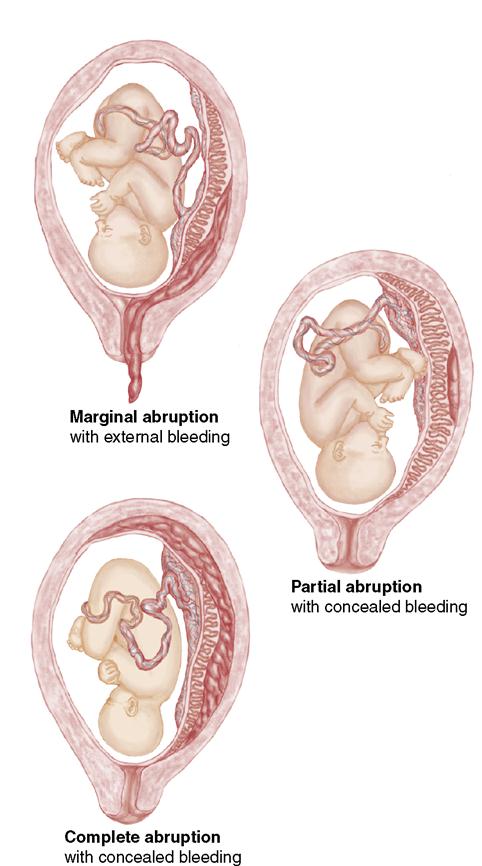
 Abruptio Placentae
Abruptio Placentae
Abruptio placentae is the premature separation of the placenta. Separation can be partial or total detachment of a normally implanted placenta and occurs after the twentieth week of pregnancy, usually during the third trimester (Table 13-2). The bleeding is accompanied by pain.
Table 13-2
Differentiation Between Placenta Previa and Abruptio Placentae*
| Signs | Placenta Previa | Abruptio Placentae |
| Placenta location | Lower third of uterus; detected by transvaginal ultrasound | Normal |
| Onset | Frequently “quiet” for first episode of bleeding | “Stormy” in moderate to severe abruptions |
| Placenta | Palpable | Nonpalpable |
| Pain | None; painless bleeding (most significant sign) | May be cramplike to severe |
| Abdomen and uterus | Soft, not tender; may be contracting normally | May be tender to rigid |
| Bleeding | External, bright red bleeding; shock with excessive bleeding | External and/or internal, either bright or dark blood; may be signs of shock that are out of proportion to bleeding |
| Abdomen and uterus | Soft, not tender, may be contracting normally | May be tender to rigid |
| Bleeding | External, bright red bleeding; shock with excessive bleeding | External and/or internal, either bright or dark blood; woman may have signs of shock that are out of proportion to bleeding |
| Blood pressure | Usually normal; with excessive bleeding, hypovolemic shock can occur | History of hypertension and toxemia; postabruption hypovolemic shock can occur |
| Fetal death | Depends on fetal maturity and amount of blood loss | Fetal distress or fetal death may occur |
| Coagulation defect | Not usually a problem | Coagulation defect (DIC) with moderate to severe abruption can be a complication |
DIC, disseminated intravascular coagulation.
*Some placental separations are mild and produce few or no symptoms; these separations are detected when the placenta is inspected after delivery.
Risk factors for abruptio placentae include maternal hypertension, prior abruption, high parity, blunt abdominal trauma, multifetal gestation, social drug use that causes vasoconstriction (particularly cocaine), cigarette smoking, hydramnios, and infection of the chorion. Researchers have suggested that placental abruption can result from degenerative changes in the small arteries that supply the intervillous space, resulting in thrombosis, decidual degeneration (causing a retroplacental hematoma), and separation of the placenta. Some placental separations are mild and produce few symptoms. In other instances, the bleeding is severe, and both fetal and maternal compromise may occur. The degree of compromise depends on the extent of the separation and the amount of blood loss.
Abruptio placentae are subdivided into the following two types (Figure 13-5):
Complications
If the blood gets into the muscles of the uterus, it may be difficult for the uterus to contract after the delivery of the newborn and placenta. Also, trapping of the blood may release thromboplastin into the maternal circulation. Thromboplastin release can result in DIC, which can be life threatening to the woman.
Assessment and Management
Classic symptoms of abruptio placentae are dark red vaginal bleeding, uterine rigidity, severe abdominal pain, maternal hypovolemia, and signs of fetal distress. Management includes assessment for excessive bleeding; continuous fetal monitoring; a coagulation profile; and laboratory workup, including blood type and cross-match. Blood for transfusion is made available. The woman is prepared for a cesarean birth or an immediate vaginal delivery (whichever is quicker) if the hemorrhage is severe or if fetal distress is evident. In Rh-negative women who have either placenta previa or abruptio placentae, the administration of RhoGAM is indicated (Box 13-2). Continuous monitoring for DIC is part of the care plan. A hysterectomy may be required in some cases. Surgery is contraindicated when uncontrollable bleeding and abnormal coagulation are present.
Disseminated Intravascular Coagulation
Disseminated intravascular coagulation (DIC) is a condition in which a coagulation defect prevents blood from clotting. This results from overstimulation of the normal coagulation process. Massive, rapid fibrin formation results. This condition causes the widespread appearance of small thrombi in the small blood vessels. Factors that prevent coagulation and factors that stimulate coagulation are activated at the same time. DIC is a life-threatening condition that occurs with some complications of pregnancy such as abruptio placenta, fetal death, amniotic fluid embolism, HELLP syndrome, and sepsis.
Assessment and Management
Because of the amount of intravascular clotting, the blood platelets and clotting factors are depleted. The process of clot lysis can have an anticoagulant effect. The following clinical problems may occur:
• Tendency toward generalized bleeding (because the clotting factors are depleted)
• Ischemia of the vital organs (caused by thrombi obstruction in the blood vessels)
DIC does not occur as the primary disorder but rather occurs secondary to another complication. It should be suspected in women with abruptio placentae, GH, retained dead fetus, hydatidiform mole, hemorrhagic shock, and septic abortion. In addition, infection can activate the coagulation pathway. This disorder can often be resolved by correcting the underlying cause, which may require terminating the pregnancy to stop the production of thromboplastin; administering blood replacement products to maintain circulatory volume; and providing intensive supportive care, including monitoring vital signs, monitoring intake and output, and administering oxygen. The platelet count and fibrinogen level are monitored closely. Replacing depleted clotting factors, including platelets, fibrinogen, and coagulation factors, by frozen plasma or cryoprecipitate may be required. Heparin is contraindicated in DIC because it will increase bleeding.
The nurse can help in the early diagnosis of DIC by being alert to signs of bleeding and vascular occlusion. Bleeding from the gums or injection sites, epistaxis, and petechiae on the skin are signs that DIC may be developing. Providing emotional support for the woman and her family is an important part of nursing care.
Blood Incompatibility (Isoimmunization)
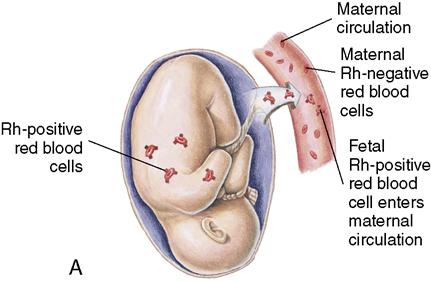
The placenta, an organ that develops during pregnancy, allows maternal and fetal blood to exchange oxygen and waste products without actually mixing together. However, small leaks may occur and allow fetal blood to enter the mother’s circulation during pregnancy. Fetal blood can also enter the mother’s blood circulation when the placenta detaches at birth.
If fetal and maternal blood types are compatible, no problem occurs. However, if the fetal and maternal blood types differ, the mother’s body produces antibodies to destroy foreign fetal red blood cells (RBCs). Rh or ABO incompatibility can occur. The phenomenon in which the Rh-negative mother develops antibodies against the Rh-positive fetus is called isoimmunization.
 Rh Incompatibility
Rh Incompatibility
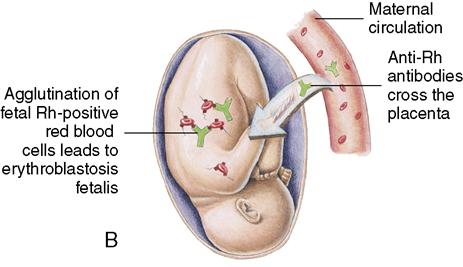
Individuals may or may not have the Rh blood factor on their erythrocytes. If they have the factor, they are Rh positive; if not, they are Rh negative. An Rh-positive person can receive Rh-negative blood if all other factors are compatible because, in Rh-negative blood, the Rh factor is absent. However, if the woman is Rh negative and the fetus is Rh positive, Rh incompatibility exists. The Rh-positive blood type is a dominant trait. This means that if the father of a fetus is Rh positive and the mother is Rh negative, there is a good chance that the newborn will be Rh positive. If fetal Rh-positive blood leaks into the mother’s circulation, her body may respond by making antibodies to destroy the Rh-positive erythrocytes, and the antibodies will be returned to fetal circulation and destroy fetal RBCs. The first Rh-positive newborn is rarely affected seriously. However, each time the woman is exposed to Rh-positive blood, such as during childbirth, amniocentesis, or abortion, her body will produce more antibodies. In future pregnancies, these antibodies in the maternal circulation will cross the placenta and cause hemolysis of the Rh-positive blood cells of the fetus. This is called erythroblastosis fetalis (Figure 13-6) (see Chapter 16).
The formation of anti-Rh antibodies can be prevented by giving Rho(D) immune globulin (RhoGAM) to the Rh-negative woman at 28 weeks’ gestation and within 72 hours after birth of an Rh-positive newborn. RhoGAM can be given to the Rh-negative woman as late as 14 to 28 days’ postpartum if not administered earlier, but to be effective it must be given before immune antibodies are developed (Gabbe, Niebyl, & Simpson, 2007). If the woman becomes sensitized (or develops antibodies) to destroy Rh-positive blood cells, the fetus can become anemic, develop heart failure, and die in utero. Several tests can be performed to determine whether too many fetal erythrocytes are being destroyed and to assess the status of the fetus. The tests include the Coombs’ test, which determines whether the woman is sensitized and has antibody formation.
At the first prenatal visit, the Rh-negative blood type of a pregnant woman is identified, and antibody titers are monitored throughout pregnancy. Amniocentesis can determine whether fetal hemolysis is present. Intrauterine blood transfusions can be performed to prevent fetal death and prolong the pregnancy until the fetus is more mature. A Doppler ultrasound focused on the middle cerebral artery of the fetus may predict fetal anemia as well as or better than an amniocentesis (Oepkes, Seaward, & Vandenbussche, 2006) (see Chapter 5).
ABO Incompatibility
ABO incompatibility can occur if the woman has group O blood and the fetus has group A, group B, or group AB blood. Anti-A and anti-B antibodies are usually already present in the woman’s body. However, fewer of these antibodies cross the placenta than those associated with Rh problems, so treatment during pregnancy is not required. However, unlike the Rh problem, the newborn is often affected during the first pregnancy. The newborn may develop jaundice within the first 24 hours. Phototherapy is usually sufficient to reduce the bilirubin level in ABO incompatibility (see Chapter 16).
Cardiovascular Disorders
Gestational Hypertension
Hypertensive disease in pregnancy complicates 12% to 20% of pregnancies (American College of Obstetricians and Gynecologists [ACOG], 2001). It is directly responsible for a significant number of maternal deaths. Maternal hypertension also is a significant cause of perinatal death. With preexisting hypertension during pregnancy, treatment is aimed at controlling hypertension; in gestational hypertension (GH), the disease process is closely monitored to prevent complications to the mother and fetus.
Classification and Risk Factors
In GH (formerly known as pregnancy-induced hypertension [PIH]), clinical subsets of the disease are recognized and traditionally have been given distinct labels related to the end-organ effects. Traditional labels include preeclampsia, when renal involvement leads to proteinuria; eclampsia, when central nervous  system involvement leads to seizures and chronic hypertension with superimposed eclampsia (Table 13-3); and the HELLP syndrome, when the disease is dominated by hematologic and hepatic clinical manifestations. The ACOG has suggested that such terminology not be used to define separate disease entities but rather manifestations of the same disorder (Perry, & Hockenberry, 2009).
system involvement leads to seizures and chronic hypertension with superimposed eclampsia (Table 13-3); and the HELLP syndrome, when the disease is dominated by hematologic and hepatic clinical manifestations. The ACOG has suggested that such terminology not be used to define separate disease entities but rather manifestations of the same disorder (Perry, & Hockenberry, 2009).
Table 13-3
Types of Hypertension in Pregnancy
| Type | Description |
| Gestational hypertension (GH) | Previously known as pregnancy-induced hypertension; blood pressure exceeding 140/90 mm Hg starts after the twentieth week of pregnancy and does not include proteinuria; blood pressure usually returns to normal by 6 weeks’ postpartum |
| Preeclampsia | GH with proteinuria present |
| Eclampsia | Preeclampsia with related seizures |
| Chronic hypertension | High blood pressure that occurs before pregnancy or before the twentieth week of pregnancy; hypertension usually lasts more than 42 days’ postpartum |
| Preeclampsia with superimposed chronic hypertension | Chronic hypertension that has new occurrence of proteinuria or occurrence of thrombocytopenia and increased liver enzymes |
Modified from American College of Obstetricians and Gynecologists. (2002). ACOG practice bulletin. Diagnosis and management of pre-eclampsia and eclampsia. Obstetrics and Gynecology, 99(1), 159-167; and National Institutes of Health. (2001). National blood pressure workshop on blood pressure education. Bethesda, MD: Author.
Pathophysiology of Preeclampsia
The pathology of GH is thought to start with placental implantation, although major signs may not be evident until 20 weeks’ gestation. There is gradual loss of resistance to angiotensin II (the resistance normally exists during pregnancy and is the reason that normal pregnancies do not cause an increase in blood pressure). The loss of resistance to angiotensin II results in changes in the ratio of the prostaglandins prostacyclin and thromboxane. Prostacyclin is a vasodilator, and thromboxane is a vasoconstrictor with platelet aggregating effects. When resistance to angiotensin II is decreased, thromboxane becomes dominant and the resulting vasospasms are the cause of the pathologic condition and symptoms seen in GH. Because the prostaglandin hormones are produced by the placenta, the condition reverses when the placenta is removed (or the pregnancy is terminated).
Vasospasm is a well-established component of preeclampsia and is likely a major cause of most of the serious end-organ effects or alterations in function. Vasospasm in the arterioles (blood vessels) leads to an increase in blood pressure and, ultimately, a decrease in blood flow to the uterus and placenta. Renal vascular changes result in the lowering of the renal blood flow, lowering of the renal glomerular filtration rate, and, ultimately, proteinuria and oliguria. Sodium is retained, resulting in edema. Edema causes a decrease in intravascular volume that results in thicker blood and a rise in hematocrit levels. Central nervous system changes may include cell damage related to vasospasms and cerebral edema, resulting in headaches and visual disturbances. Hepatic alterations include enlargement of the liver and tension on the liver capsule. These alterations cause epigastric pain, which often precedes seizures.
Severe preeclampsia or eclampsia has been associated with serious complications, such as cerebrovascular accidents (stroke), acute renal failure, abruptio placentae, DIC, and fetal and maternal death. The HELLP syndrome is found in approximately 10% of severe preeclamptic conditions. In women who exhibit the HELLP syndrome, the onset of seizures may be abrupt.
 Memory Jogger
Memory Jogger


