Alita K. Sellers
Care of the patient with a urinary disorder
Objectives
1. Describe the structures of the urinary system, including functions.
2. List the three processes involved in urine formation.
3. Name three hormones and their influence on nephron function.
4. Compare the normal components of urine with the abnormal components.
5. Identify the effects of aging on urinary system function.
8. Prioritize the special needs of the patient with urinary dysfunction.
9. Describe the alterations in kidney function associated with disorders of the urinary tract.
10. Discuss the effect of renal disease on family function.
13. Select nursing diagnoses related to alterations in urinary function.
14. Design culturally sensitive care of the patient with a urinary disorder.
Key terms
bacteriuria (b k-t
k-t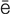 r-
r-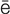 –
– -r
-r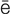 –
–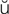 h, p. 451)
h, p. 451)
costovertebral angle (CVA) (k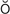 s-t
s-t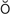 -V
-V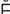 R-t
R-t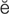 -br
-br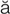 l
l 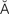 NG-g
NG-g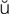 l, p. 455)
l, p. 455)
cytologic evaluation (s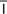 -t
-t -L
-L J-
J- k
k  -v
-v l-
l-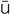 –
–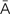 -sh
-sh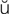 n, p. 461)
n, p. 461)
hematuria (h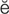 m-
m-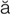 -T
-T -r
-r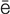 –
–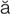 , p. 451)
, p. 451)
hydronephrosis (h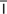 -dr
-dr -n
-n -FR
-FR -s
-s s, p. 457)
s, p. 457)
ileal conduit ( l-
l- –
– l K
l K N-d
N-d –
– t, p. 478)
t, p. 478)
micturition (m k-t
k-t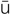 -R
-R SH-
SH-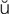 n, p. 457)
n, p. 457)
nephrotoxins (n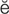 f-r
f-r -T
-T K-s
K-s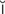 nz, p. 480)
nz, p. 480)
prostatodynia (pr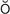 s-t
s-t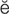 -t
-t -D
-D N-
N-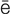 –
–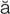 , p. 454)
, p. 454)
residual urine (r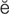 -Z
-Z -d
-d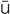 –
–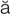 l
l  -r
-r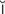 n, p. 448)
n, p. 448)
retention (r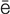 -T
-T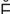 N-sh
N-sh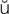 n, p. 448)
n, p. 448)
urolithiasis (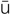 -r
-r -l
-l -TH
-TH –
– -s
-s s, p. 457)
s, p. 457)
Anatomy and physiology of the urinary system
Each day, the cells throughout the body metabolize ingested nutrients. This process provides energy for the body and produces waste products. As proteins break down, nitrogenous waste—urea, ammonia, and creatinine (a nitrogenous compound produced by metabolic processes in the body)—is produced. The primary function of the kidneys is excretion of these waste products. The kidneys also assist in regulating the body’s water, electrolytes, and acid-base balance. The urinary system is probably the most important system in maintaining homeostasis.
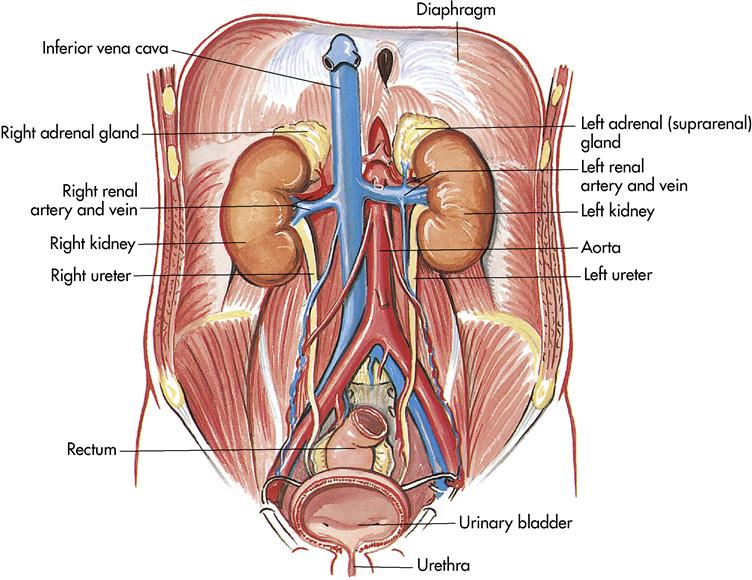
The urinary system consists of two kidneys, which produce urine by removing waste, excess water, and electrolytes from the blood; two ureters, which transport urine from the kidneys to the bladder; one bladder, which collects and stores urine; and one urethra, which transports urine from the bladder to the outside of the body for elimination (Figure 10-1). This chapter explores the filtering process, the composition of urine, and the pathway of urine removal from the body.
Kidneys
The kidneys lie behind the parietal peritoneum (retroperitoneal), just below the diaphragm on each side of the vertebral column. Kidneys are dark red, bean-shaped organs that are 4 to 5 inches (10 to 12 cm) long, 2 to 3 inches (5 to 7.5 cm) wide, and about 1 inch (2.5 cm) thick. Because of the liver, the right kidney lies slightly lower than the left. The kidneys are surrounded and anchored in place by a layer of adipose tissue. Near the center of the kidney’s medial border is a notch or indentation called the hilus where the renal artery enters and the renal vein and the ureter exit the kidney.
The adrenal glands, a part of the endocrine system, sit near the top of each kidney. The adrenal glands secrete hormones that help control blood pressure and heart rate, among other functions. The primary mineralocorticoid secreted by the adrenal cortex is aldosterone. Plasma potassium concentration is the primary regulator of aldosterone. Changes evoked through the adrenal glands create changes in kidney function (see Chapter 11).
Gross anatomical structure
The outer covering of the kidney is a strong layer of connective tissue called the renal capsule. Directly beneath the renal capsule is the renal cortex. It contains 1.25 million renal tubules, which are part of the microscopic filtration system. Immediately beneath the cortex is the medulla, which is a darker color. The medulla contains the triangular pyramids. Continuing inward, the narrow points of the pyramids (papillae) empty urine into the calyces. The calyces are cuplike extensions of the renal pelvis that guide urine into the renal pelvis. The renal pelvis is an expansion of the upper end of the ureter; the ureter in turn drains the finished product, urine, into the bladder (Figure 10-2).
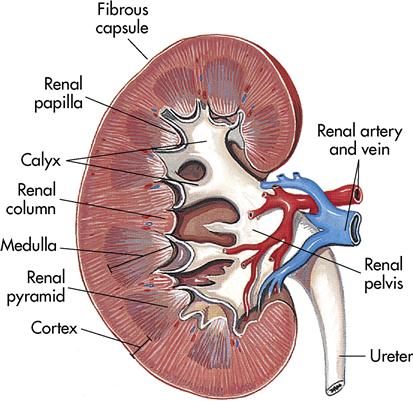
Microscopic structure
Nephron
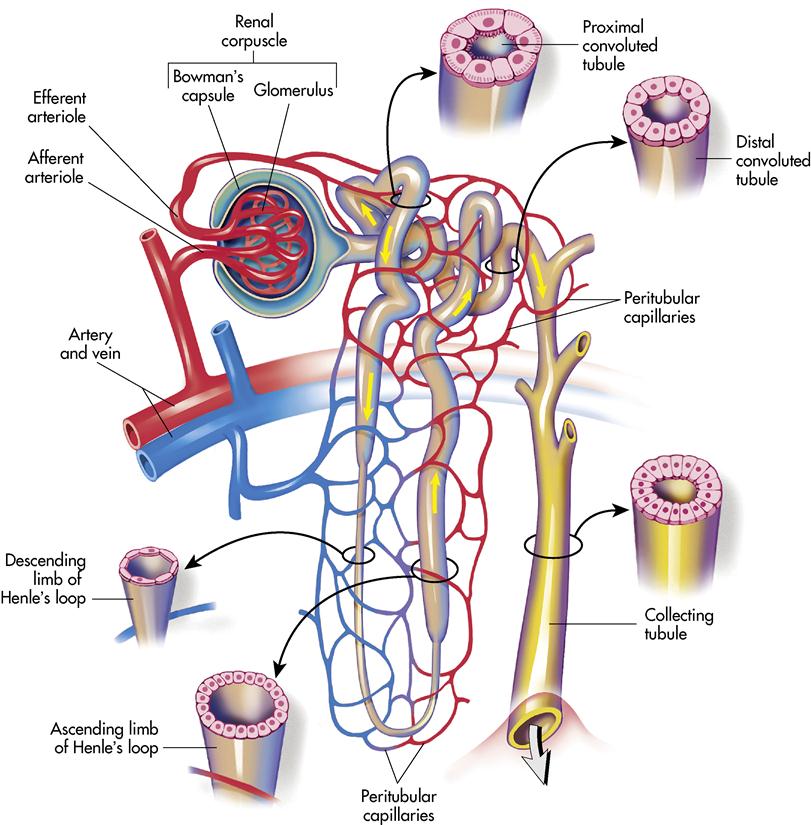
Each kidney contains more than 1 million nephrons. The nephron is the functional unit of the kidney, resembling a microscopic funnel with a long stem and two convoluted sections (Figure 10-3). It is responsible for filtering the blood and processing the urine. The nephron has three major functions: (1) controlling body fluid levels by selectively removing or retaining water, (2) assisting with the regulation of the pH of the blood, and (3) removing toxic waste from the blood. Approximately 60 times a day, the body’s entire volume of blood is filtered through the kidneys.
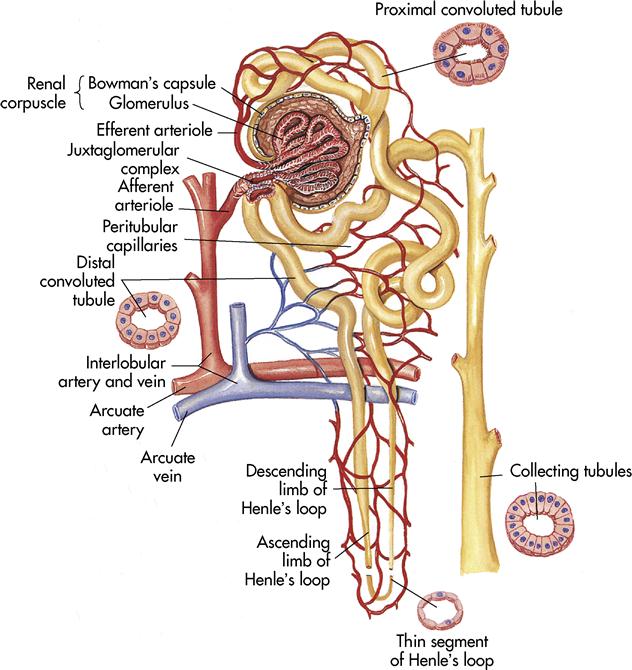
A nephron consists of two main structures: the renal corpuscle and the renal tubule. The renal corpuscle is composed of a tightly bound network of capillaries called the glomeruli that are held inside a cuplike structure, Bowman’s capsule. The renal arteries (right and left) branch off the abdominal aorta and enter the kidney at the hilus. The renal arteries continue branching until blood is delivered to the glomerulus by the afferent arteriole. The blood leaves the glomerulus through the efferent arteriole to the peritubular capillary. Blood finally reaches the renal veins and flows into the inferior vena cava.
The renal tubule becomes tightly coiled (at the proximal convoluted tubule), makes a sudden straight drop, and curves back upward like a hairpin (at Henle’s loop, or nephron loop) and becomes tightly coiled again (at the distal convoluted tubule). The convoluted tubule terminates at the collecting tubule or duct. Several collecting ducts unite in a pyramid and open at the papilla to empty urine into the associated calyx.
The juxtaglomerular apparatus is the microscopic structure in the kidney, which regulates the function of each nephron. The juxtaglomerular apparatus is named for its proximity to the glomerulus; it is found between the vascular pole of the renal corpuscle and the returning distal convoluted tubule of the same nephron. This location is critical to its function in regulating renal blood flow and glomerular filtration rate. The juxtaglomerular apparatus is where the afferent arterioles come into direct contact with the distal convoluted tubule. The juxtaglomerular apparatus works to regulate systemic blood pressure and filtrate formation.
The specialized cells of the afferent arteriole at this region are called juxtaglomerular cells. These cells contain the enzyme renin and function as mechanoreceptors to sense blood pressure.
The specialized cells of the distal convoluted tubule at the point of contact with the afferent arteriole are the macula densa cells. These cells function as chemoreceptors to sense changes in the solute concentration and flow rate of the filtrate.
When systemic blood pressure decreases, the juxtaglomerular cells have a decreased stretch, which leads to their release of renin (Figure 10-4). Renin release causes the activation of the renin-angiotensin mechanism, which ultimately leads to an increased blood pressure.
Reabsorption begins as soon as the filtrate reaches the tubule system. The filtrate contains important products needed by the body: water, glucose, and ions may be absorbed. In fact, 99% of the filtrate is returned to the body (see Figure 10-4).
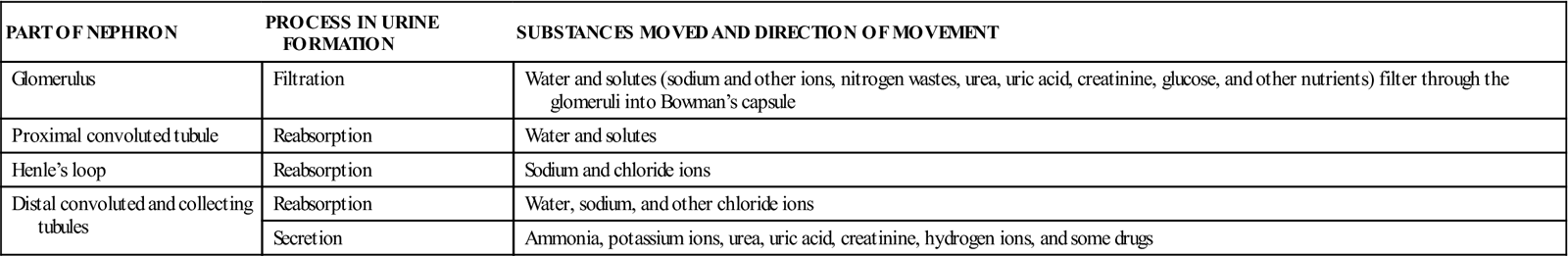
In summary, the three phases of urine formation (Table 10-1) and location of the processes are as follows:
Table 10-1
Functions of Parts of the Nephron in Urine Formation
| PART OF NEPHRON | PROCESS IN URINE FORMATION | SUBSTANCES MOVED AND DIRECTION OF MOVEMENT |
| Glomerulus | Filtration | Water and solutes (sodium and other ions, nitrogen wastes, urea, uric acid, creatinine, glucose, and other nutrients) filter through the glomeruli into Bowman’s capsule |
| Proximal convoluted tubule | Reabsorption | Water and solutes |
| Henle’s loop | Reabsorption | Sodium and chloride ions |
| Distal convoluted and collecting tubules | Reabsorption | Water, sodium, and other chloride ions |
| Secretion | Ammonia, potassium ions, urea, uric acid, creatinine, hydrogen ions, and some drugs |
Hormonal influence on nephron function.
When the body has suffered increased fluid loss through hemorrhage, diaphoresis, vomiting, diarrhea, or other means, the blood pressure drops. These events decrease the amount of filtrate produced by the kidneys. The posterior pituitary gland releases antidiuretic hormone (ADH). ADH causes the cells of the distal convoluted tubules to increase their rate of water reabsorption. This action returns the water to the bloodstream, which raises the blood pressure to a more normal level and causes the urine to become concentrated. See Box 10-1 for major functions of kidneys.
Urine composition and characteristics
The word urine comes from one of its components, uric acid. Each day, the body forms 1000 to 2000 mL of urine; this amount is influenced by several factors, including mental and physical health, oral intake, and blood pressure. Urine is 95% water; the remainder is nitrogenous wastes and salts. It is usually a transparent yellow with a characteristic odor. Normal urine is yellow because of urochrome, a pigment resulting from the body’s destruction of hemoglobin. Urine is slightly acidic, with a pH of 4.6 to 8 and a specific gravity of 1.003 to 1.030. Healthy urine is sterile, but at room temperature it rapidly decomposes and smells like ammonia as a result of the breakdown of urea.
Urine abnormalities
A urinalysis, which studies the physical, chemical, and microscopic properties of urine, can give important diagnostic information. If the body’s homeostasis has been compromised, certain substances may spill into the urine. Some of the more common substances include the following:
Ureters
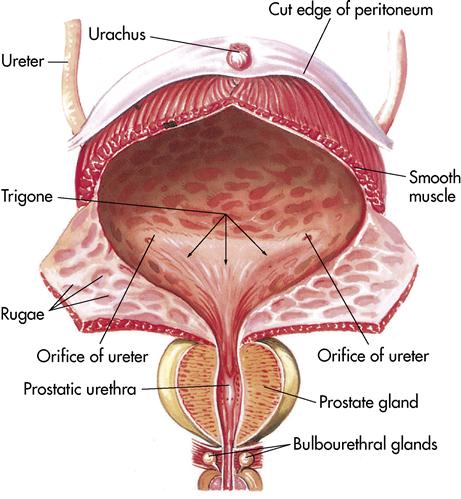
Once the urine has been formed in the nephrons, it passes to the paired ureters. Ureters are actually extensions of the renal pelvis and extend downward 10 to 12 inches (25 to 30 cm) to the lower part of the urinary bladder. As the ureters leave the kidneys, they are retroperitoneal and pass under the urinary bladder before entering it. As the ureters enter the bladder (ureterovesical junction), the mucous membrane folds, acting as a valve to prevent backflow of urine.
Urinary bladder
The urinary bladder (Figure 10-5) is a temporary storage pouch for urine. It is composed of collapsible muscle and is located anterior to the small intestine and posterior to the symphysis pubis. As the bladder fills with urine, it rises into the abdominal cavity and can be palpated. The bladder can hold 750 to 1000 mL of urine. When the bladder contains approximately 250 mL of urine, the individual has a conscious desire to urinate. This is because the stretch receptors become activated and a message is sent to the spinal cord. A moderately full bladder holds 450 mL (1 pint) of urine.
Two sphincters, the internal and external, control the release of the urine. The internal sphincter located at the bladder neck is composed of involuntary muscle. As the bladder becomes full, the stretch receptors cause contractions, pushing the urine past the internal sphincter. The urine then presses on the external sphincter, which is composed of skeletal or voluntary muscle at the terminus of the urethra.
Urethra
The urethra is the terminal portion of the urinary system. It is a small tube that carries urine by peristalsis from the bladder out of its external opening, the urinary meatus. In females it is embedded in the anterior wall of the vagina vestibule and exits between the clitoris and the vaginal opening. The female urethra is approximately 1⁄4 inch in diameter and 11⁄2 inches long. In males the urethra is approximately 8 inches long, passing through the prostate gland and extending the length of the glans penis. In the male the urethra serves two functions: as a passageway for urine and a passageway for semen.
Normal aging of the urinary system
With aging the kidneys lose part of their normal functioning capacity; in fact, by 70 years of age, the filtering mechanism is only 50% as efficient as at 40 years. This occurs because of decreased blood supply and loss of nephrons.
In the aging woman the bladder loses tone and the perineal muscles may relax, resulting in stress incontinence. In the aging man the prostate gland may become enlarged, leading to constriction of the urethra. Incomplete emptying of the bladder in both men and women increases the possibility of urinary tract infection (UTI) (see Life Span Considerations box).
Laboratory and diagnostic examinations
Diagnostic tests for urinary tract conditions include laboratory tests, diagnostic imaging, and endoscopic procedures. Nursing responsibilities vary according to the studies performed. Be aware of specific patient variables that may influence test results: state of hydration, nutritional status, or trauma (see Cultural Considerations box). Prepare patients for diagnostic testing by briefly describing the purpose of the procedure and what the patient can expect to happen.
Urinalysis
The most common urinary diagnostic study is the urinalysis. Table 10-2 describes normal and abnormal constituents in the urine and possible factors that influence test results. A urinalysis may be done during assessments of other body systems because of the role of the kidneys in maintaining homeostasis. Urine culture and sensitivity may be done to confirm suspected infections, to identify causative organisms, and to determine appropriate antimicrobial therapy. Cultures are also obtained for periodic screening of urine when the threat of a UTI persists. Various reagent strips are available to test urine for abnormal substances. The strips are a quick reference that can be used in a clinical setting or at home. Common substances measured to monitor kidney function include total urine protein, creatinine, urea, uric acid levels, and catecholamines.
Table 10-2
| CONSTITUENT | NORMAL RANGE | INFLUENCING FACTORS |
| Color | Pale yellow to amber | Diabetes insipidus, biliary obstruction, medications, diet |
| Turbidity | Clear to slightly cloudy | Phosphates, white blood cells, bacteria |
| Odor | Mildly aromatic | Medication, bacteria, diet |
| pH | 4.6-8 | Stale specimen, food intake, infection, homeostatic imbalance |
| Specific gravity | 1.003-1.030 | State of hydration, medications |
| Glucose | Negative | Diabetes mellitus, medications, diet |
| Protein | Negative | Renal disease, muscle exertion, dehydration |
| Bilirubin | Negative | Liver disease with obstruction or damage, medications |
| Hemoglobin | Negative | Trauma, renal disease |
| Ketones | Negative | Diabetes mellitus, diet, medications |
| Red blood cells | Up to 2 LPF | Renal or bladder disease, trauma, medications |
| White blood cells | 0-4 LPF | Renal disease, urinary tract infection |
| Casts | Rare | Renal disease |
| Bacteria | Negative | Urinary tract infection |
Urinalysis is completed on a clean-catch or catheterized specimen. A sterile urine specimen is obtained either by inserting a straight catheter into the urinary bladder and removing urine or by obtaining a specimen from the port of an indwelling catheter via catheter port using sterile technique. Because the kidneys excrete substances in varying amounts and rates during a 24-hour period, the nurse may be responsible for collecting a 24-hour urine sample. Discard the first voiding and note the time at the beginning of the 24-hour urine collection. For the next 24 hours collect all urine and place it in a special laboratory container.
Specific gravity
Specific gravity measures the patient’s hydration status and gives information about the kidneys’ ability to concentrate urine. Specific gravity is decreased by high fluid intake, reduced renal concentrating ability, diabetes insipidus, and diuretic use. It is increased in dehydration due to fever, diaphoresis, vomiting, diarrhea, and medical conditions such as diabetes mellitus (diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma) and inappropriate secretion of ADH. The value ranges between 1.003 and 1.030, with the lower values suggesting more dilute urine (Pagana & Pagana, 2008).
Blood (serum) urea nitrogen
Blood urea nitrogen (BUN) is a laboratory test used to determine the kidney’s ability to rid the blood of nonprotein nitrogen (NPN) waste and urea, which result from protein breakdown (catabolism). The acceptable serum range for BUN is 10 to 20 mg/dL. For a more accurate test result, the patient should receive nothing by mouth (NPO) for 8 hours before blood sampling. If the BUN is elevated, institute preventive nursing measures to protect the patient from possible disorientation or seizures.
Blood (serum) creatinine
Creatinine is a catabolic product of creatine, which is used in skeletal muscle contraction. The daily production of creatine, and subsequently creatinine, depends on muscle mass, which fluctuates little. Creatinine, as with BUN, is excreted entirely by the kidneys and is therefore directly proportional to renal excretory function. Thus, with normal renal excretory function, the serum creatinine level should remain constant and normal. Only renal disorders (such as glomerulonephritis, pyelonephritis, acute tubular necrosis, and urinary obstruction) cause an abnormal elevation in creatinine.
The serum creatinine test, as with BUN, is used to diagnose impaired kidney function. However, unlike BUN, the creatinine level is affected little by dehydration, malnutrition, or hepatic function. The creatinine level is interpreted in conjunction with the BUN. The acceptable serum creatinine range is 0.5 to 1.1 mg/dL (female) and 0.6 to 1.2 mg/dL (male) (Pagana & Pagana, 2008).
Creatinine clearance
Creatinine, an NPN substance, is present in blood and urine. Creatinine is generated during muscle contraction and then excreted by glomerular filtration. Levels are directly related to muscle mass and are usually measured for a 24-hour period. During the testing period, the patient avoids excessive physical activity. Draw a fasting blood sample at the onset of testing and another at the conclusion. Discard the initial specimen and start the 24-hour timing at that point. Collect all urine in the 24-hour period because any deviation will alter test results. An elevation in serum levels with a decline in urine levels indicates renal disease. Normal ranges are serum, 0.5 to 1.1 mg/dL (female), 0.6 to 1.2 mg/dL (male); urine, 87 to 107 mL/min (female), 107 to 139 mL/min (male) (Pagana & Pagana, 2008).
Prostate-specific antigen
Prostate-specific antigen (PSA) is an organ-specific glycoprotein produced by normal prostatic tissue. Measurement of PSA has largely replaced that of prostatic acid phosphatase because it is a more accurate test. Test results rise with tissue manipulation; therefore obtain a blood sample before physical examination. Normal range is less than 4 ng/mL. Elevated PSA levels result from prostate cancer, benign prostatic hypertrophy (BPH), and prostatitis.
Osmolality
Assessment of urine osmolality (the weight of the solute compared with its own weight) may be preferred over specific gravity. Plasma osmolality may be done in conjunction with the urine sampling when pituitary disorders are suspected. Results provide information on the concentrating ability of the kidney.
Kidney-ureter-bladder radiography
A kidney-ureter-bladder (KUB) radiograph assesses the general status of the abdomen and the size, structure, and position of the urinary tract structures. No special preparation is necessary. Explain that the procedure involves changing position on the radiography table, which may be uncomfortably firm. Abnormal findings related to the urinary system may indicate tumors, calculi, glomerulonephritis, cysts, and other conditions.
Intravenous pyelogram or intravenous urography
Intravenous pyelogram (IVP) or intravenous urography (IVU) evaluates structures of the urinary tract, filling of the renal pelvis with urine, and transport of urine via the ureters to the bladder. It is vital to determine whether the patient has an allergy to iodine (or iodine-containing foods such as iodized salt, saltwater fish, seaweed products, vegetables grown in iodine-rich soils) because it is the base of the radiopaque dye that is injected into a vein for this and other radiologic examinations. If the patient has had an allergic reaction, the physician may order administration of a corticosteroid or an antihistamine before testing or, alternatively, may order ultrasonography.
Because kidneys and ureters are positioned in the retroperitoneal space, gas and stool in the intestines interfere with radiographic visualization. Preparation usually includes eating a light supper, taking a non–gas-forming laxative, and remaining NPO 8 hours before testing. In planning the testing regimen, schedule urography before barium-based studies. When the dye is injected, the patient experiences a warm, flushing sensation and a metallic taste. During the procedure, monitor vital signs frequently. Radiographs are taken at various intervals to monitor movement of the dye. Abnormal findings may indicate structural deviations, hydronephrosis, calculi within the urinary tract, polycystic renal (kidney) disease (PKD), tumors, and other conditions.
Retrograde pyelography
Retrograde pyelography involves examination of the lower urinary tract with a cystoscope under aseptic conditions. The urologist injects radiopaque dye directly into the ureters to visualize the upper urinary tract. Urine samples can be obtained directly from the renal pelvis. Additional retrograde studies include the following:
Voiding cystourethrography
Voiding cystourethrography is used in conjunction with other diagnostic studies to detect abnormalities of the urinary bladder and the urethra. Preparation includes an enema before testing. An indwelling catheter is inserted into the urinary bladder, and dye is injected to outline the lower urinary tract. Radiographs are taken, and the catheter is then removed. The patient is asked to void while radiographs are being taken. Some patients experience embarrassment or anxiety related to the procedure and should be given the opportunity to express their feelings. Structural abnormalities, diverticula, and reflux into the ureter may be detected.
Endoscopic procedures
Endoscopic procedures are visual examinations of hollow organs using an instrument with a scope and light source. Because of the invasive nature of the procedure, informed consent is necessary, and because the procedure is most often performed in the surgical suite, preoperative preparation is indicated (see Chapter 2). The urologist performs the procedure.
Cystoscopy is a visual examination to inspect, treat, or diagnose disorders of the urinary bladder and proximal structures. Patient preparation includes a description of the procedure. Usually the procedure is carried out using a local anesthetic after the patient has been sedated. Patient safety is paramount when the patient is sedated. The patient is placed in a lithotomy position for the procedure, which may produce embarrassment and anxiety. The thought of a scope being passed while the patient is awake may intensify these feelings. Provide an opportunity for the patient to verbalize feelings.
The scope is passed under aseptic conditions after a local anesthetic is instilled into the urethra. The patient experiences a feeling of pressure as the scope is passed. Continuous fluid irrigation of the bladder is necessary to facilitate visualization. Care after the procedure includes hydration to dilute the urine. Monitor the first voiding after the procedure, assessing time, amount, color, and any dysuria (painful or difficult urination). The first voiding is occasionally blood tinged due to the trauma of the procedure.
The urologist can perform a brush biopsy via a ureteral catheter during a cystoscopy. A nylon brush is inserted through the catheter to obtain specimens from the renal pelvis or calyces. Nephroscopy (renal endoscopy) is done using the percutaneous (through the skin) route and provides direct visualization of the upper urinary structures. The urologist can obtain biopsy or urine specimens or remove calculi.
Renal angiography
Renal angiography aids in evaluating blood supply to the kidneys, evaluates masses, and detects possible complications after kidney transplantation. Withhold oral intake the night before the procedure. The procedure requires the passing of a small radiopaque catheter into an artery (usually the femoral artery) to provide a port for the injection of radiopaque dye. Therefore, when the procedure is completed, have the patient lie flat in bed for several hours to minimize the risk of bleeding. Assess the puncture site for bleeding or hematoma, and maintain the pressure dressing at the site. Assess circulatory status of the involved extremity every 15 minutes for 1 hour, then every 2 hours for 24 hours.
Renal venogram
A renal venogram provides information about the kidney’s venous drainage. Access for the radiopaque catheter is the femoral vein. Monitor the patient afterward for bleeding at the puncture site.
Computed tomography
A computed tomography (CT) scan differentiates masses of the kidney. Images are obtained by a computer-controlled scanner. A radiopaque dye may be injected to enhance the image. A serum urea and creatinine level are obtained before use of radiopaque dye. The dye is not used if inadequate kidney function is noted. Inform the patient that the table on which he or she is placed and the machine “taking pictures” will move at intervals and that it is important to lie still. The CT body-scanning unit takes multiple cross-sectional pictures at several different sites, creating a three-dimensional map of the renal structure. The adrenals, the bladder, and the prostate may also be visualized.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) uses nuclear magnetic resonance as its source of energy to obtain a visual assessment of body tissues. The patient requires no special preparation other than removal of all metal objects that might be attracted by the magnet. Patients with metal prostheses (such as heart valves, orthopedic screws, or cardiac pacemakers) cannot undergo MRI.
Emphasize that the examination area will be confining and that a repetitive “pounding” sound will be heard (somewhat like the sound of a muffled jackhammer). MRI can be used for diagnoses of pathologic conditions of the renal system.
Renal scan
A radionuclide tracer substance that will be taken up by renal tubular cells or excreted by the glomerular filtrate is injected intravenously. A series of computer-generated images is then made. The scan provides data related to functional parenchyma (the essential parts of an organ that are concerned with its function). No special preparation is needed. Check facility policy concerning the disposal of the patient’s urine for the first 24 hours. Pregnant nurses should refrain from caring for this patient during this time.
Ultrasonography
Ultrasonography is a diagnostic tool that uses the reflection of sound waves to produce images of deep body structures. Inform the patient that a conducting jelly will be applied on the skin over the area to be studied; this improves the transmission of sound waves. The sound waves are high frequency and inaudible to the human ear; the waves are converted into electrical impulses that are photographed for study.
Ultrasonography can visualize size, shape, and position of the kidney and delineate any irregularities in structure. Deviations from normal findings may indicate tumor, congenital anomalies, cysts, or obstructions. No special preparations are necessary.
Transrectal ultrasound
Transrectal ultrasound instrumentation of the prostate gland provides clear images of prostatic tumors that otherwise might go undiagnosed. Transrectal ultrasound–guided biopsy is performed to obtain samples of prostatic tissue from various areas with minimal discomfort to the patient.
Renal biopsy
The kidney can be biopsied by an open procedure similar to other surgical procedures on the kidney or by the less invasive method of needle biopsy, also called a percutaneous biopsy. Tell the patient that he or she may experience pain during the procedure and should follow instructions, such as holding the breath. Bed rest is instituted for 24 hours after the procedure. Mobility is restricted to bathroom privileges for the next 24 hours, and gradual resumption of activities is allowed after 48 to 72 hours.
Urodynamic studies
Urodynamic studies are indicated when neurologic disease is suspected of being an underlying cause of incontinence. The studies evaluate detrusor reflex. The patient may experience embarrassment and slight discomfort. During cystometrogram a catheter is inserted into the bladder, then connected to a cystometer, which measures bladder capacity and pressure. The examiner asks the patient about sensations of heat, cold, and urge to void and instructs the patient at times to void and change position.
Cholinergic and anticholinergic medications may be administered during urodynamic studies to determine their effects on bladder function. (A cholinergic drug, such as bethanechol [Urecholine], stimulates the atonic bladder; an anticholinergic drug, such as atropine, brings an overactive bladder to a more normal level or function.)
Associated testing includes rectal electromyography, which involves placement of an electrode; and urethral pressure profile, in which a special catheter connected to a transducer evaluates urethral pressures.
Medication considerations
The kidneys filter a wide range of water-soluble products from the blood, including medications. The kidneys’ effectiveness in removing certain medications from the blood may be affected by various conditions, such as renal disease, changes in the pH of urine, and age. Patients with renal disease are given reduced dosages of medications to minimize further damage or drug toxicity. Alteration in urinary pH affects the absorption rate of certain medications. Older patients may have decreased physiologic functioning, diminishing the kidneys’ capacity to excrete drugs. Diminished kidney function interferes with the filtration of water-soluble medications.
The medications included in this discussion are representative of those that directly affect the function of the kidney or are used to treat urinary disorders (Table 10-3).
![]() Table 10-3
Table 10-3
Medications that Affect the Urinary System
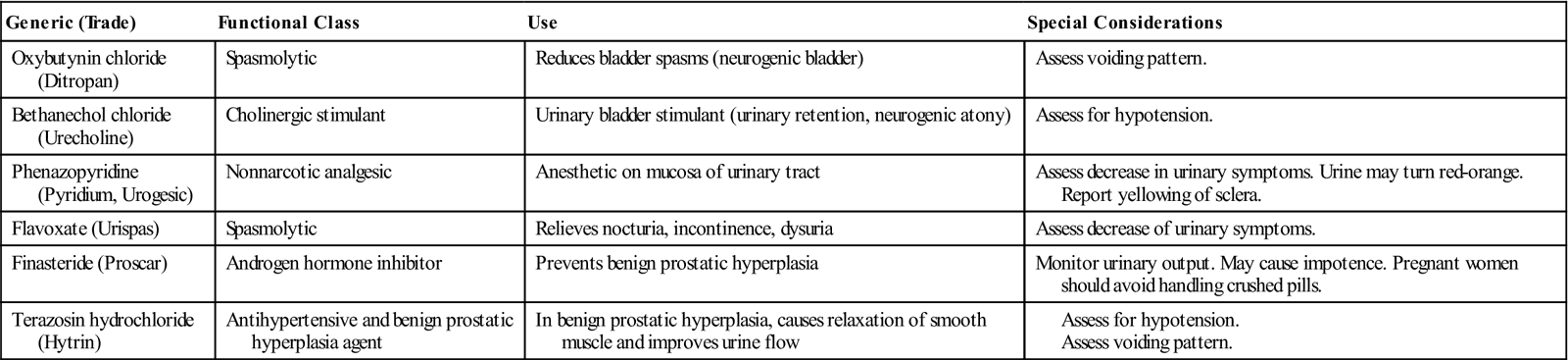
Diuretics to enhance urinary output
Diuretics are administered to enhance urinary output. They achieve this by increasing the kidney’s filtration of sodium, chloride, and water at different sites in the kidney. Diuretics are used in the management of a variety of disorders, such as heart failure and hypertension. Diuretics are classified by chemical structure and by the site and type of action on the kidney.
Thiazide diuretics
Thiazide diuretics act at the distal convoluted tubule to impair sodium and chloride reabsorption, leading to excretion of electrolytes and water. The thiazide diuretic chlorothiazide (Diuril) affects electrolytes to cause hypokalemia (extreme potassium depletion in blood), hyponatremia (decreased sodium concentration in blood), and/or hypercalcemia (excessive amounts of calcium in blood). Hypochloremic alkalosis occurs from a deficiency of chloride. The main uses are management of systemic edema and control of mild to moderate hypertension, although it may take a month to achieve the full antihypertensive effect. Chlorothiazide is contraindicated in anuria.
Loop (or high-ceiling) diuretics
Loop, or high-ceiling, diuretics act primarily in the ascending Henle’s loop to inhibit tubular reabsorption of sodium and chloride. This group is the most potent of all diuretics and may lead to significant electrolyte depletion. These diuretics are effective for use in patients with impaired kidney function.
The loop diuretic furosemide (Lasix) affects electrolytes to cause hypokalemia, hypochloremia, hyponatremia, hypocalcemia (abnormally low blood calcium), and/or hypomagnesemia (decreased magnesium in the blood). The effect on acid-base balance is the development of hypochloremic alkalosis. Furosemide is used in nephrotic syndrome, heart failure, and pulmonary edema. Side effects are those associated with rapid fluid loss: vertigo, hypotension, and possible circulatory collapse.
Potassium-sparing diuretics
Potassium-sparing diuretics act on the distal convoluted tubule to inhibit sodium reabsorption and potassium secretion. Potassium-sparing diuretics decrease the sodium-potassium exchange. Although the actions of these medications vary, they all conserve potassium that is usually lost with sodium in diuresis. But, because they are weak, they are usually used in combination with other diuretics. Potassium-sparing diuretics are contraindicated in patients who experience hyperkalemia, since further retention of potassium could cause a fatal cardiac dysrhythmia. There are two types of potassium-sparing diuretics: aldosterone antagonists and nonaldosterone antagonists.
The aldosterone antagonist spironolactone (Aldactone) blocks aldosterone in the distal tubule to promote potassium uptake in exchange for sodium secretion. Although it can be used in combination with other diuretics, primarily in the treatment of hypertension and edema, spironolactone is most frequently used for its potassium-sparing quality.
The nonaldosterone antagonist triamterene (Dyrenium) directly reduces ion transportation in the tubule, though it has little diuretic effect. Triamterene is instead used to help limit the potassium-wasting effect of other diuretics.
Osmotic diuretics
Osmotic diuretics act at the proximal convoluted tubule to increase plasma osmotic pressure, causing redistribution of fluid toward the circulatory vessels. Osmotic diuretics are used to manage edema, promote systemic diuresis in cerebral edema, decrease intraocular pressure, and improve kidney function in acute renal failure (ARF). In ARF, osmotics are used to prevent irreversible failure, but they are contraindicated in advanced states of renal failure.
The osmotic diuretic mannitol (Osmitrol) increases osmolarity of glomerular filtrate; decreases reabsorption of water electrolytes; and increases urinary output, sodium, and chloride, which actually has minimal effect on acid-base balance. Mannitol is used to prevent or treat the oliguric phase of ARF, promote systemic diuresis in cerebral edema, and decrease intraocular pressure. Careful assessment of the cardiovascular system before administering mannitol is essential because of the high risk of inducing heart failure. Avoid extravasation (escape of the medication from the blood vessel into the tissues), which may lead to tissue irritation or necrosis.
Carbonic anhydrase inhibitor diuretics
The carbonic anhydrase inhibitor diuretic acetazolamide (Diamox) interferes with the bonding of water and carbon dioxide by the enzyme carbonic anhydrase (present in red blood cells) at the proximal convoluted tubule. Although it has limited usefulness as a diuretic, acetazolamide is used to lower intraocular pressure.
Nursing interventions
Because patients receiving diuretics often have complicated disease conditions such as heart failure and pulmonary edema, monitor for signs and symptoms of fluid overload: changes in pulse rate, respirations, cardiac sounds, and lung fields. Record daily morning weights for the patient receiving diuretics. Keep accurate intake and output (I&O) records, and document blood pressure, pulse, and respirations four times a day until the medication is regulated and the vital signs stabilize. Assess BUN, serum electrolytes, and urine as ordered. Diet instruction to the patient and the family should include a warning to avoid overuse of salt in cooking or as a table additive. A number of salt substitutes are currently on the market; however, the long-term effects of those potassium preparations are not known and could further complicate the renal patient’s condition. The use of most diuretics, with the exception of the potassium-sparing diuretics, requires adding daily potassium sources (e.g., baked potatoes, raw bananas, apricots, or navel oranges). In some cases the physician orders potassium supplements to be taken with the diuretic.
When a diuretic is effective, the serum concentration of other medications may increase as a result. Carefully monitor this potentiating effect to prevent toxicity from other medications. For example, as diuretics effectively decrease the volume of extracellular fluid, the serum level of digoxin may increase proportionately, resulting in digitoxicity. Special care is required in the selection and management of diuretics in the treatment of children, adolescents, and older adults.
Medications for urinary tract infections
Certain antimicrobial agents are administered primarily to treat infections within the urinary tract. The appropriate medication is selected according to Gram-stain sensitivity of the organism. Urinary antiseptics inhibit bacteria growth and are used to prevent and treat urethritis and cystitis. Caution should be used to determine if the patient is pregnant, since all of these agents have not been sufficiently tested for use during pregnancy.
Urinary antiseptics are divided into four groups: quinolones, nitrofurantoins, methenamines, and fluoroquinolones. Examples of each group follow.
Quinolone
Nalidixic acid (NegGram) is used to treat UTIs caused by gram-negative microbes (e.g., Escherichia coli and Proteus mirabilis). The common side effects are drowsiness, vertigo, weakness, nausea, and vomiting. The use of nalidixic acid is contraindicated in renal impairment.
Nitrofurantoin
Nitrofurantoin compound (Macrodantin) is effective against both gram-positive and gram-negative microbes (e.g., Streptococcus faecalis, E. coli, and P. mirabilis) in the urinary tract. Common side effects are loss of appetite, nausea, and vomiting.
Methenamine
Methenamine mandelate (Mandelamine) suppresses fungi and gram-negative and gram-positive organisms (e.g., E. coli, staphylococci, and enterococci). Acidification of the urine with an acid-ash diet or other acidifiers to a pH of less than 5.5 is necessary for effective action. Methenamine mandelate is used for patients with chronic, recurrent UTIs as a preventive measure after antibiotics have cleared the infection. Although side effects are rare, they include nausea, vomiting, skin rash, and urticaria (hives).
Fluoroquinolone
Norfloxacin (Noroxin) is a broad-spectrum antibiotic effective against gram-positive and gram-negative organisms (e.g., E. coli, P. mirabilis, Pseudomonas organisms, Staphylococcus aureus, and Staphylococcus epidermidis). It is used in the treatment of UTIs, gonorrhea, and gonococcal urethritis. It is administered with a full glass of water 1 hour before or 2 hours after meals or with antacids.
Nursing interventions
Before administering antibiotics for UTIs, be certain to check all medications the patient is using for potential negative drug interactions. Instruct the patient to take all the medication, even though the symptoms may subside quickly. Hydrate the patient to produce daily urinary output of 2000 mL, unless contraindicated. When indicated, teach the patient to use the acid-ash diet to help maintain a urine pH of 5.5. Soothe skin irritations with cornstarch or a bath of bicarbonate of soda or dilute vinegar. Report continuing signs of infection.
Observe the patient receiving nalidixic acid for visual disturbances and offer appropriate assistance for ambulation or transfer. Monitor the patient receiving nitrofurantoin for signs of allergic response (such as erythema, chills, fever, and dyspnea). If these signs or symptoms develop, discontinue the medication and notify the physician (trial doses of this medication may be used to detect possible allergic reaction before administering full dosage).
Nutritional considerations
The nutritional needs of the patient with a urinary tract disorder vary with each disease process. Some general guidelines include provision of food choices and number of servings as recommended by the U.S. Department of Agriculture’s MyPyramid nutrition planning tool (www.mypyramid.gov) and daily intake of 2000 mL of water, unless contraindicated. Unique nutritional requirements are discussed with each disorder. Box 10-2 gives an example of dietary modifications for urinary lithiasis. Patients with other systemic diseases, such as diabetes mellitus, require strict adherence to those restrictions as well.

 n-
n- -S
-S R-k
R-k ,
,  -N
-N -r
-r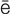 –
–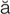 ,
, 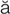 s-TH
s-TH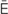 -n
-n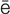 –
–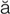 ,
, 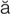 -z
-z -T
-T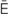 -m
-m –
– ,
,  –
– L-
L- -s
-s s,
s,  s-
s- -r
-r –
–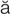 ,
, 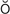 k-T
k-T -r
-r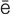 –
–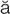 ,
, 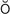 l-
l-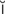 -G
-G -r
-r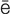 –
–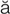 ,
, 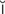 –
– -r
-r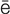 –
–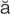 ,
,