Drug Definitions, Standards, and Information Sources
Objectives
Key Terms
pharmacology (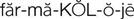 ) (p. 1)
) (p. 1)
therapeutic methods (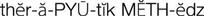 ) (p. 1)
) (p. 1)
drugs (p. 1)
chemical name ( ) (p. 1)
) (p. 1)
generic name (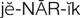 ) (p. 1)
) (p. 1)
brand name (p. 1)
over-the-counter (OTC) drugs (p. 2)
illegal drugs ( ) (p. 2)
) (p. 2)
![]() http://evolve.elsevier.com/Clayton
http://evolve.elsevier.com/Clayton
Pharmacology (from the Greek pharmakon, meaning “drugs,” and logos, meaning “science”) deals with the study of drugs and their actions on living organisms. Diseases that cause illness may be treated in several different ways, which are referred to as therapies. The various approaches to therapy are called therapeutic methods. Examples of therapeutic methods include the following:
• Drug therapy: treatment with drugs
• Diet therapy: treatment with diet (e.g., a low-salt diet for patients with cardiovascular disease)
• Physiotherapy: treatment with natural physical forces (e.g., water, light, heat)
Most illnesses caused by diseases require a combination of therapeutic methods for successful treatment.
Drugs (from the Dutch droog, meaning “dry”) are chemical substances that have an effect on living organisms. Therapeutic drugs, which are often called medicines, are those drugs that are used for the prevention or treatment of diseases. Up until the early to mid twentieth century, dried plants were the most abundant source of medicines; thus, the word drug was applied to them.
Drug Names, CLASSIFICATIONS, Standards, Legislation, and Development in the United States
Drug Names
All drugs have several names, which may cause confusion. When administering the prescribed drug, the spelling on the drug package must correspond exactly with the spelling of the drug ordered to ensure that the proper medicine is administered.
Each drug has three names: (1) a chemical name; (2) a generic name; and (3) a brand name. The chemical name is most meaningful to the chemist. By means of the chemical name, the chemist understands the exact chemical constitution of the drug as well as the exact placement of its atoms or molecular groupings.
Before a drug becomes official, it is given a generic name or common name. The generic name is simpler than the chemical name. It may be used in any country and by any manufacturer. The first letter of the generic name is not capitalized. Students are strongly encouraged to learn and refer to drugs by their generic names, because formularies (i.e., lists of medicines available through a pharmacy) are maintained by generic names. When a therapeutically equivalent drug becomes available in generic form, the generic medicine is routinely substituted for the brand-name medicine.
Generic names are provided by the U.S. Adopted Names Council, which is an organization sponsored by the U.S. Pharmacopeial Convention, the American Medical Association, and the American Pharmacists Association. The official name, which is virtually always the generic name in the United States, is the name under which the drug is listed by the U.S. Food and Drug Administration (FDA). The FDA is empowered by federal law to name the drugs for human use in the United States.
A trademark or brand name is followed by the symbol ®. This symbol indicates that the name is registered and that the use of the name is restricted to the owner of the drug, which is usually the manufacturer. Most drug companies place their products on the market under brand names rather than generic names. The brand names are deliberately made easier to pronounce, spell, and remember. The first letter of the brand name is capitalized.
EXAMPLE: See Figure 1-1.
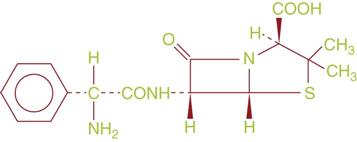
Chemical name: 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxy-lic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, [2S-[2α,-5α,6β(S*)]]-
Generic name: ampicillin
Brand names: Principen, Polycillin
Drug Classifications
Drugs may be classified by a variety of methods according to:
Drugs may be further classified as prescription or nonprescription. Prescription drugs require an order by a health professional who is licensed to prescribe drugs, such as a physician, a nurse practitioner, a physician assistant, a pharmacist, or a dentist. Nonprescription or over-the-counter (OTC) drugs are sold without a prescription in a pharmacy or in the health section of department or grocery stores. Illegal drugs, which are sometimes referred to as recreational drugs, are drugs or chemical substances used for nontherapeutic purposes. These substances are obtained illegally or have not received approval for use by the FDA. See Chapter 49 for further information about substance abuse.
Sources of Drug Information
Objectives
3 List official sources of drug standards in the United States.
4 List literature resources for researching prescription and nonprescription drugs.
5 Cite sources of credible drug information on the Internet.
Drug products made by different manufacturers or in different batches by the same manufacturer must be uniformly pure and potent. The United States Pharmacopeial Convention is a nongovernment organization that promotes public health by establishing state-of-the-art standards to ensure the quality of medicines and other health care technologies. These standards are developed by a unique process of public involvement, and they are accepted worldwide. The Convention publishes a single volume text, the United States Pharmacopeia (USP)/National Formulary (NF), which is revised annually. The primary purpose of this volume is to provide standards for the identity, quality, strength, and purity of substances used in the practice of health care. The standards described in the USP/NF are enforced by the FDA as the official standards for the manufacture and quality control of medicines and nutritional supplements produced in the United States. The USP/NF is also recognized by the ![]() Canadian Food and Drugs Act as an authoritative source of drug standards in Canada.
Canadian Food and Drugs Act as an authoritative source of drug standards in Canada.
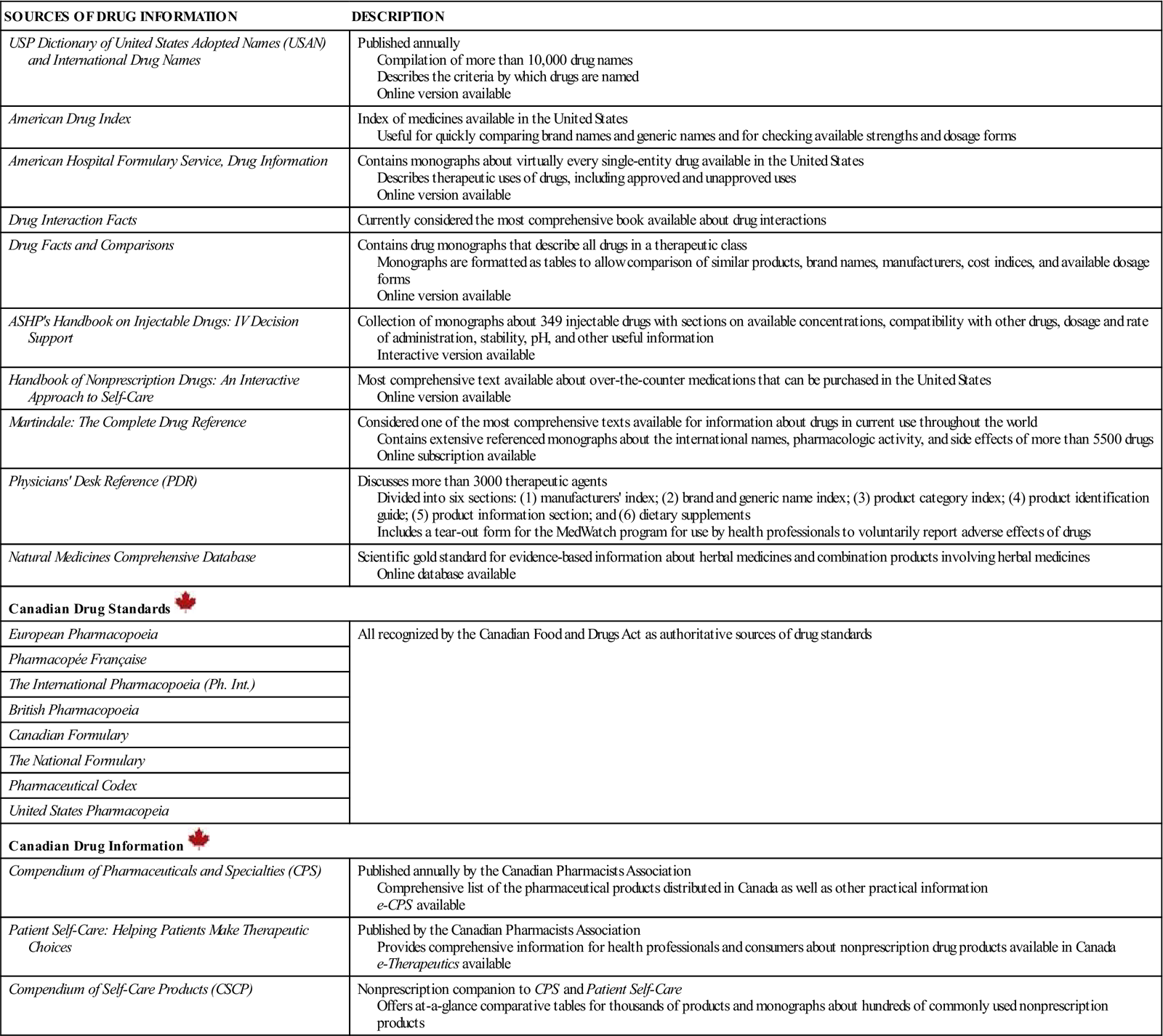
Table 1-1 lists and describes the common sources of drug information available for the professional health care provider; additional resources are described in the following sections.
Table 1-1
Sources of Drug Information for Health Care Providers
| SOURCES OF DRUG INFORMATION | DESCRIPTION |
| USP Dictionary of United States Adopted Names (USAN) and International Drug Names | Published annually Compilation of more than 10,000 drug names Describes the criteria by which drugs are named Online version available |
| American Drug Index | Index of medicines available in the United States Useful for quickly comparing brand names and generic names and for checking available strengths and dosage forms |
| American Hospital Formulary Service, Drug Information | Contains monographs about virtually every single-entity drug available in the United States Describes therapeutic uses of drugs, including approved and unapproved uses Online version available |
| Drug Interaction Facts | Currently considered the most comprehensive book available about drug interactions |
| Drug Facts and Comparisons | Contains drug monographs that describe all drugs in a therapeutic class Monographs are formatted as tables to allow comparison of similar products, brand names, manufacturers, cost indices, and available dosage forms Online version available |
| ASHP’s Handbook on Injectable Drugs: IV Decision Support | Collection of monographs about 349 injectable drugs with sections on available concentrations, compatibility with other drugs, dosage and rate of administration, stability, pH, and other useful information Interactive version available |
| Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care | Most comprehensive text available about over-the-counter medications that can be purchased in the United States Online version available |
| Martindale: The Complete Drug Reference | Considered one of the most comprehensive texts available for information about drugs in current use throughout the world Contains extensive referenced monographs about the international names, pharmacologic activity, and side effects of more than 5500 drugs Online subscription available |
| Physicians’ Desk Reference (PDR) | Discusses more than 3000 therapeutic agents Divided into six sections: (1) manufacturers’ index; (2) brand and generic name index; (3) product category index; (4) product identification guide; (5) product information section; and (6) dietary supplements Includes a tear-out form for the MedWatch program for use by health professionals to voluntarily report adverse effects of drugs |
| Natural Medicines Comprehensive Database | Scientific gold standard for evidence-based information about herbal medicines and combination products involving herbal medicines Online database available |
| Canadian Drug Standards | |
| European Pharmacopoeia | All recognized by the Canadian Food and Drugs Act as authoritative sources of drug standards |
| Pharmacopée Française | |
| The International Pharmacopoeia (Ph. Int.) | |
| British Pharmacopoeia | |
| Canadian Formulary | |
| The National Formulary | |
| Pharmaceutical Codex | |
| United States Pharmacopeia | |
| Canadian Drug Information | |
| Compendium of Pharmaceuticals and Specialties (CPS) | Published annually by the Canadian Pharmacists Association Comprehensive list of the pharmaceutical products distributed in Canada as well as other practical information e-CPS available |
| Patient Self-Care: Helping Patients Make Therapeutic Choices | Published by the Canadian Pharmacists Association Provides comprehensive information for health professionals and consumers about nonprescription drug products available in Canada e-Therapeutics available |
| Compendium of Self-Care Products (CSCP) | Nonprescription companion to CPS and Patient Self-Care Offers at-a-glance comparative tables for thousands of products and monographs about hundreds of commonly used nonprescription products |
Package Inserts
Manufacturers of drugs have to develop a comprehensive but concise description of the drug, indications and precautions for clinical use, recommendations for dosage, known adverse reactions, contraindications, and other pharmacologic information relating to the drug. Federal law requires that this material be approved by the FDA before the product is released for marketing and that it be presented on an insert that accompanies each package of the product.
In 2006, the FDA adopted a new format for package inserts to help reduce medication errors and to improve patient education. The new labeling reduces practitioners’ time looking for information, decreases the number of preventable medical errors, and improves treatment effectiveness and patient education. Because these labeling revisions represent considerable effort and are most critical for newer and less familiar drugs, the program applies only to relatively new prescription drug products.
Nursing Journals
Many specialty journals have articles about drug therapy as it relates to a specific field of interest (e.g., Geriatric Nursing, American Journal of Critical Care). Nursing journals such as RN and American Journal of Nursing provide drug updates and articles that discuss nursing considerations related to drug therapy and drugs. Nurses must keep in mind that the purpose of using resources like journals is for professional knowledge of current evidence-based practice changes and not as a primary source for drug information; they must be mindful of the accuracy of the information contained and should check the dates on articles to validate the currency of the information.
Electronic Databases
With the exponential growth of information about medicines and health, it is almost impossible to make the information available without the use of electronic databases. The U.S. National Library of Medicine provides Medline and other searchable databases at no cost. Most of the drug information sources listed in Table 1-1 are also available via electronic retrieval from libraries. Many college libraries subscribe to CINAHL, which is a cumulative index of nursing and allied health literature. These sources give nurses access to a wealth of information from sources published in the United States as well as other countries.
Databases for practitioners are also available by subscription. UpToDate, Lexi-Comp, and ePocrates are three vendors with several different packages of regularly updated information (see Online Resources on p. 10). Lexi-Comp has a particularly strong database, because the American Hospital Formulary Service is available through its portal.
The DailyMed system (see Online Resources on p. 10) was developed in collaboration with federal agencies—including the FDA, the National Library of Medicine, the Agency for Healthcare Research and Quality, the National Cancer Institute in the Department of Health and Human Services, and the Department of Veterans Affairs—to provide high-quality information about marketed drugs. DailyMed makes available to health care providers and the public a standard, comprehensive, up-to-date resource about medicines.
Drug Legislation
Objectives
Key Term
Drug legislation protects the consumer from false claims made by the drug manufacturer. The need for such protection is great, because manufacturers and advertisers may make unfounded claims about the benefits of their products.
Federal Food, Drug, and Cosmetic Act
The Federal Food, Drug, and Cosmetic Act of 1938 (passed on June 25, 1938, and amended in 1952 and 1962) requires the FDA to determine the safety of drugs before marketing and to ensure that certain labeling specifications and standards in advertising are met in the marketing of products. Manufacturers are required to submit new drug applications to the FDA for the review of safety studies before the products can be released for sale.
The Kefauver-Harris Drug Amendment was brought about in 1962 as a result of the thalidomide tragedy. Thalidomide was an incompletely tested drug that had been approved for use as a sedative-hypnotic during pregnancy. Fetuses exposed to thalidomide were born with serious birth defects. This amendment provides greater control and surveillance of the distribution and clinical testing of investigational drugs and requires that a product be proven both safe and effective before release for sale.
Controlled Substances Act
The Comprehensive Drug Abuse Prevention and Control Act, which is commonly referred to as the Controlled Substances Act, was passed by Congress in 1970. This statute repealed almost 50 other laws written between 1914 and 1970 that related to the control of drugs. The new composite law was designed to improve the administration and regulation of the manufacturing, distribution, and dispensing of drugs that require control by the government because of their high incidence of abuse. The basic structure of the Controlled Substances Act consists of five classifications or schedules of controlled substances. The degree of control, the conditions of record keeping, the particular order forms required, and other regulations depend on these classifications.
Schedule I  Drugs
Drugs
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 ) (
) (