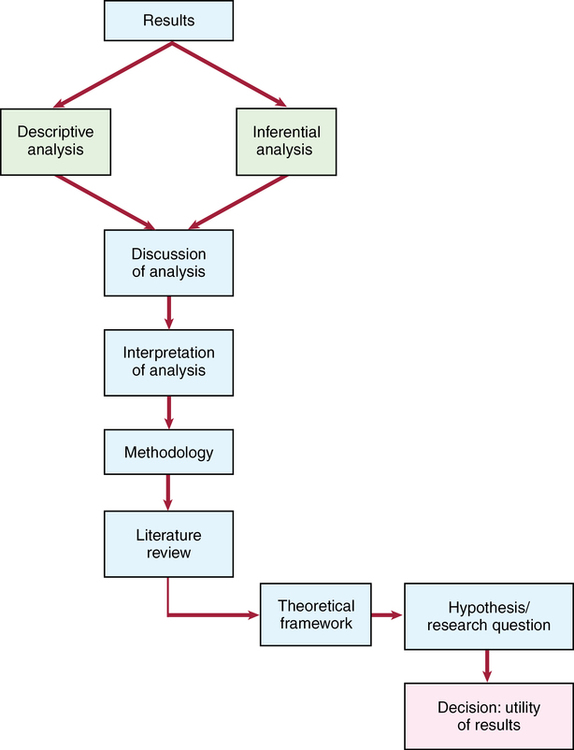
CHAPTER 17 After reading this chapter, you should be able to do the following: • Discuss the difference between the “Results” and the “Discussion” sections of a research article. • Identify the format and components of the “Results” section. • Determine if both statistically supported and statistically unsupported findings are appropriately discussed. • Determine whether the results are objectively reported. • Describe how tables and figures are used in a research report. • List the criteria of a meaningful table. • Identify the format and components of the “Discussion” section. • Determine the purpose of the “Discussion” section. • Discuss the importance of including generalizability and limitations of a study in the report. • Determine the purpose of including recommendations in the study report. • Discuss how the strength, quality, and consistency of evidence provided by the findings are related to a study’s results, limitations, generalizability, and applicability to practice. Go to Evolve at http://evolve.elsevier.com/LoBiondo/ for review questions, critiquing exercises, and additional research articles for practice in reviewing and critiquing. The final sections of published research studies are generally titled “Results” and “Discussion,” but other topics, such as conclusions, limitations of findings, recommendations, and implications for future research and nursing practice, may be separately addressed or subsumed within these sections. The presentation format of these areas is a function of the author’s and the journal’s stylistic considerations. The function of these final sections is to integrate all aspects of the research process, as well as to discuss, interpret, and identify the limitations, the threats related to bias, and generalizability relevant to the investigation, thereby furthering evidence-based practice. The process that both an investigator and you use to assess the results of a study is depicted in the Critical Thinking Decision Path. The “Results” section of a research study is considered to be the data-bound section of the report and is where the quantitative data or numbers generated by the descriptive and inferential statistical tests are presented. Other headings that may be used for the results section are “Statistical Analyses,” “Data Analysis,” or “Analysis.” The results of the data analysis set the stage for the interpretations or discussion and the limitations sections that follows the results. The “Results” section should reflect analysis of each research question and/or hypothesis tested. The information from each hypothesis or research question should be sequentially presented. The tests used to analyze the data should be identified. If the exact test that was used is not explicitly stated, the values obtained should be noted. The researcher does this by providing the numerical values of the statistics and stating the specific test value and probability level achieved (see Chapter 16). Examples of these statistical results can be found in Table 17-1. These numbers and their signs should not frighten you. The numbers are important, but there is much more to the research process than the numbers. They are one piece of the whole. Chapter 16 conceptually presents the meanings of the numbers found in studies. Whether you only superficially understand statistics or have an in-depth knowledge of statistics, it should be obvious that the results are clearly stated, and the presence or lack of statistically significant results should be noted. TABLE 17-1 EXAMPLES OF REPORTED STATISTICAL RESULTS At times the researchers will begin the “Results” or “Data Analysis” section by identifying the name of the statistical software program they used to analyze the data. This is not a statistical test but a computer program specifically designed to analyze a variety of statistical tests. For example, Alhusen and colleagues (2012; Appendix B) state that “data were analyzed using PASW Statistics 18, Release Version 18.00.” PASW Statistics 18 was the statistical program, and the statistical tests used were Pearson correlations and point biserial coefficient and logistic regression (see Chapter 16). The investigator should also demonstrate objectivity in the presentation of the results. For example, the following quote by Alhusen and colleagues (2012; Appendix B) is an appropriate way to express results: “As hypothesized, there was a significant negative relationship between MFA and adverse neonatal outcomes supporting our first hypothesis.” The investigators would be accused of lacking objectivity if they had stated the results in the following manner: “The results were not surprising as we found that the mean scores were significantly different in the comparison group, as we expected.” Opinions or reactionary statements about the data in the “Results” section are therefore avoided. Box 17-1 provides examples of objectively stated results. As you appraise a study, you should consider the following points when reading the “Results” section: • Investigators responded objectively to the results in the discussion of the findings. • The investigator interpreted the evidence provided by the results, with a careful reflection on all aspects of the study that preceded the results. • The data presented are summarized. Much data are generated, but only the critical summary numbers for each test are presented. Examples of summarized demographic data are the means and standard deviations of age, education, and income. Including all data is too cumbersome. The results can be viewed as a summary. • The reduction of data is in both the written text and through the use of tables and figures. Tables and figures facilitate the presentation of large amounts of data. • Results for the descriptive and inferential statistics for each hypothesis or research question are presented. No data should be omitted even if they are not significant. • Any untoward events during the course of the study should be reported. In their study, Alhusen and colleagues (2012) developed tables to present the results visually. Table 17-2 provides a portion of the descriptive results about the subjects’ demographics. Table 17-3 provides the correlations among the study’s variables. Tables allow researchers to provide a more visually thorough explanation and discussion of the results. If tables and figures are used, they must be concise. Although the article’s text is the major mode of communicating the results, the tables and figures serve a supplementary but independent role. The role of tables and figures is to report results with some detail that the investigator does not explore in the text. This does not mean that tables and figures should not be mentioned in the text. The amount of detail that an author uses in the text to describe the specific tabled data varies according to the needs of the author. A good table is one that meets the following criteria: TABLE 17-2 DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS
Understanding research findings
Findings
Results
STATISTICAL TEST
EXAMPLES OF REPORTED RESULTS
Mean
m = 118.28
Standard deviation
SD = 62.5
Pearson correlation
r = .49, P < .01
Analysis of variance
F = 3.59, df = 2, 48, P < .05
t test
t = 2.65, P < .01
Chi-square
χ2 = 2.52, df = 1, P < .05
VARIABLE
N
%
Race
African American
155
93
White non-Hispanic
9
5
Other
2
2
Education
Less than high school
110
67
High school graduate/GED
45
27
Some college/trade school
5
3
College/trade school graduate
6
3
Marital Status
Single
90
54
Partnered/not married
56
34
Married
17
10
Other
3
2
Employment Status
Unemployed
127
77
Employed full-time
25
15
Employed part-time
14
8 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree