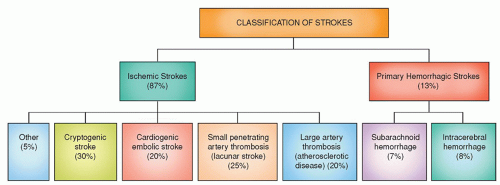
Each year about 7,95,000 people experience a new or recurrent stroke (6,10,000 first attack and 1,85,000 recurrent); by gender about 55,000 more women than men have a stroke.
On the average, every 40 seconds, someone in the United States has a stroke.
From 1998 to 2008, the stroke death rate fell 34.8%, and the actual number of stroke deaths declined 19.4%. However, there continues to be increased numbers of strokes annually related to population growth and increased numbers of older Americans.
First-time stroke for African Americans is almost double that for Caucasians.
Stroke age-adjusted rates by state report a death rate per 1,00,000 of 51.5 to 58.1 for the so called “stroke belt” states which includes the Carolinas, Georgia, Tennessee, Alabama, Mississippi, Louisiana, Arkansas, and Oklahoma. The stroke belt has the highest incidence of stroke compared to other parts of the country.
Projections for 2030 suggest an additional 4 million people will have a stroke, a 24.9% increase in prevalence from 2010.2
comprehensive overview of nursing and interdisciplinary rehabilitation care, and other guidelines that address the management of stroke patients along a continuum of care through rehabilitation. They are available at the AHA website and are updated periodically to reflect the latest scientific information to assist health care providers in providing best practices in managing patients.3, 4, 5 Other respected groups such as the Veterans Administration have also published evidence-based guidelines that are available at a variety of websites.6
use a standardized method for delivering care based on the Brain Attack Coalition recommendations for the establishment of PSCs.
support a patient’s self-management activities.
tailor treatment and interventions to individual needs.
promote the flow of patient information across settings and providers, while protecting patient rights, security, and privacy.
analyze and use standardized performance measure data to continually improve treatment plans.
demonstrate application and compliance with the clinical practice guidelines published by the AHA/ASA or equivalent evidence-based guidelines.
Data supporting minimum volume of cases including evidence of subarachnoid patients, craniotomies for aneurysm clippings, endovascular coiling procedures, and administration of intravenous (IV) thrombolytics.
Capacity of advanced imaging capabilities such as carotid ultrasound, catheter angiography, CT angiography, MRA, MRI, transcranial Doppler (TCD), transesophageal echocardiography (TEE), and others.
Posthospital care coordination of patients.
Dedicated neurological intensive care unit beds for complex stroke patients.
Peer review process.
Participation in stroke research.
Collection of additional performance measures as identified for CSC.
Stroke is a preventable health problem; it is a treatable condition, in most cases, if treatment is prompt and evidence based. A well-developed public education program is critical to have an informed public who can recognize the signs and symptoms of a stroke and know how to respond. In addition, the health care system must be organized to provide evidence-based care provided by stroke-competent health care providers. The following recommendations are made by the AHA.5, 10 Activation of the 911 system by patients and others is strongly supported because it speeds treatment of stroke.
Approved resources are available. The National Stroke Association’s Stroke Rapid Response is a new innovative program that teaches Emergency Medical System and prehospital providers basic and advanced stroke information, including field assessment and hospital treatment, and provides reference tools that can be used when caring for stroke patients; it is available online and includes other tools to assist rapid delivery of appropriate care.
The American Association of Neuroscience Nurses (AANN) now offers a stroke certification program for nurses to demonstrate expertise and competency in the delivery of stroke care.9
Public education programs to increase public awareness of stroke are supported to increase the number of patients who can be seen and treated in the first few hours after stroke.
Education of all health care providers and EMS personnel will increase the number of patients promptly and properly treated.
Since EMS personnel are often the first responders, education in brief assessment according to an established protocol will facilitate communication of information for decisions about transport to
the appropriate health care facility and needed care that alerts health providers.
EMS personnel should begin the initial management of stroke in the field according to approved protocols.
The use of a stroke identification algorithm such as the Los Angeles Prehospital Stroke Scale (LAPSS) or the Cincinnati Prehospital Stroke Scale (CPSS) screens is encouraged.
Patients should be transported for evaluation and treatment to the closest facility that provides emergency stroke care, even if it means bypassing other health care facilities not prepared to provide emergency stroke care.
Many states have enacted legislation guiding the transportation and treatment of acute stroke patients based on the ASA Stroke Systems of Care work in the 2000s.
The latest ASA guidelines support the triage and transport of stroke patients to the nearest PSC or CSC.9
TABLE 22-1 COMPARISON OF SIGNS AND SYMPTOMS OF CAROTID AND VERTEBROBASILAR TRANSIENT ISCHEMIC ATTACKS | ||||
|---|---|---|---|---|
|
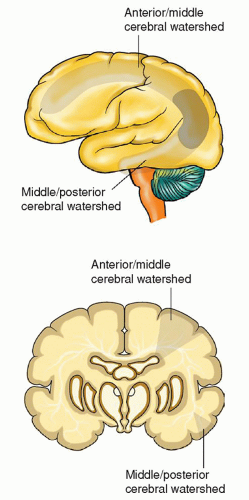
fit a known vascular territory and that last for 24 hours or more. Stroke occurs when oxygen supply to a localized area in the brain is interrupted, resulting in a series of intricate processes that lead to the destruction of neural tissue and consequent brain damage.
TABLE 22-2 ABCD SCORE | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
TABLE 22-3 ABCD SCORE AND RISK OF NEW ISCHEMIC STROKE WITHIN 48 HOURS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
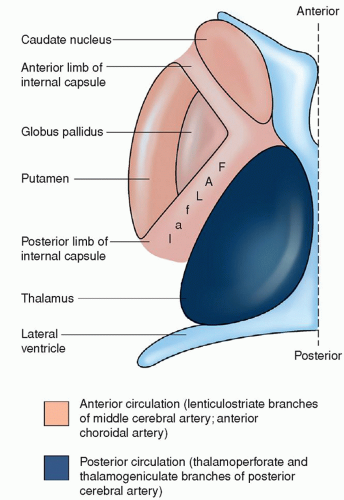
a condition characterized by pathologic thickening of these small vessels, and leads to a specific clinical stroke syndrome. Hypertension is the principal risk factor for lacunar strokes. Diabetes mellitus is also associated with the development of lacunar strokes. Lacunar strokes are seen predominately in the basal ganglia, especially the putamen, the thalamus, and the white matter of the internal capsule and pons; they occur occasionally in the white matter of the cerebral gyri. They are rare in the gray matter of the cerebral surface, the corpus callosum, visual radiations, or medulla (Fig. 22-3). Most lacunae occur in the lenticulostriate branches of the anterior cerebral artery (ACA) and middle cerebral artery (MCA), the thalamoperforate branches of the posterior cerebral arteries, and the paramedian branches of the basilar artery (BA).15
can lead to infarction follows two distinct pathways. The first is a necrotic pathway in which cellular cytoskeletal breakdown is rapid, due primarily to energy failure of the cell. The second is an apoptotic pathway in which cells become programmed to die.21
TABLE 22-4 FACTORS CONTRIBUTING TO POOR OUTCOME FOLLOWING ACUTE ISCHEMIA | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
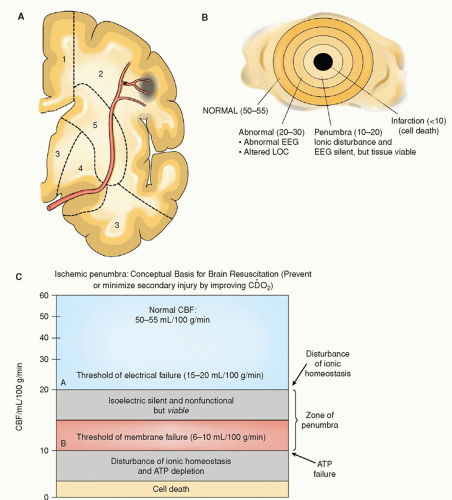
period of time. The critical time period during which the penumbra is at risk is referred to as the “window of opportunity” because the neurological deficits created by the ischemia can be partly or completely reversed if reperfusion of the ischemic area occurs within a critical time frame of 2 to 4 hours.24 Lesser degrees of ischemia, seen within the ischemic penumbra, result in apoptotic cellular death causing cells to die days to weeks later if a timely reversal of ischemia did not occur.21
the plasma and mitochondrial membrane is intact until later in the process when mitochondrial damage occurs. Ischemia activates proteins within the nuclei, triggers an autolytic process which results in cell death.19
TABLE 22-5 THRESHOLDS OF CEREBRAL ISCHEMIA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Otherwise, they should not be considered a routine part of the work-up of acute ischemic stroke.30
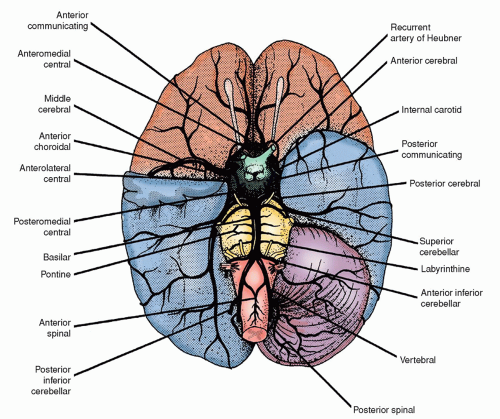
TABLE 22-6 NAME, DESCRIPTION, AND SIGNS/SYMPTOMS RELATED TO STROKE BY INVOLVED VESSEL | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 22-7 COMPARISON OF SIGNS AND SYMPTOMS ASSOCIATED WITH RIGHT-SIDED AND LEFT-SIDED HEMIPLEGIA | ||||
|---|---|---|---|---|
|
TABLE 22-8 DIAGNOSTIC PROCEDURES FOR TRANSIENT ISCHEMIC ATTACKS (TIAs) AND STROKE | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree