CHAPTER 23 Tissue viability and managing chronic wounds
Introduction
The impact of a wound on an individual can be considerable. Pain, fear and scarring are the most obvious, but individuals vary in their response to having a wound. For some, restriction in social activity, or the loss of earnings, should also be considered alongside the psychological effects of altered body image (Wilson 2000).
The presence of a wound will inevitably have some effect on well-being. There may be no difficulty when a wound is small and heals rapidly, but many patients experience anxiety relating to the wound (Atkinson 2002). This can disrupt sleep, increase pain perception and impair immune function. In more serious wounds, a disturbance of body image may also cause lasting distress, particularly if the patient is unprepared for this (Wilson 2000).
Clinical effectiveness and the delivery of evidence-based care are key to wound management.
Wound definition, types and classification
There is no clear-cut method of classifying wounds. Some practitioners refer to wounds by anatomical site, e.g. abdominal wall wounds. Others classify wounds by their depth, e.g. epidermal loss, subcutaneous wounds. Another possible classification is by degree of tissue loss (Dealey 1999). In the first group, there are wounds with little or no tissue loss where the skin edges can be brought together and sutured. In the second, there is substantial tissue loss and the skin edges cannot be brought together. A further description may be to group wounds by their potential to heal, with wounds being described as acute or chronic (Fletcher 2008a).
Epidemiology
The epidemiology of wounds is not clearly documented. Because people with wounds can be found in almost every specialty, information relating specifically to wounds is not consistently collected. Posnett & Franks (2007) identify that there are over 200 000 individuals at any one time with a chronic wound in the UK, with costs estimated to be as high as £2.3–3.1 bn per year (at 2005/2006 prices), around 3% of total estimated expenditure on health (£89.4 bn). These figures do not address acute wounds which would add a considerable additional burden.
Physiology of wound healing
A number of cell types are involved in the healing process (Table 23.1).
Table 23.1 Important cells in wound healing
| Cell | Function |
|---|---|
| Endothelial cells | Help to achieve haemostasis |
| Polymorphonuclear leucocytes (‘polymorphs’) | Take part in the initial inflammatory response |
| Macrophages | Digest debris and stimulate other cells to function; orchestrate wound healing processes |
| Fibroblasts | Produce collagen |
| Myofibroblasts | Aid wound contraction by producing mature collagen |
Tissue repair
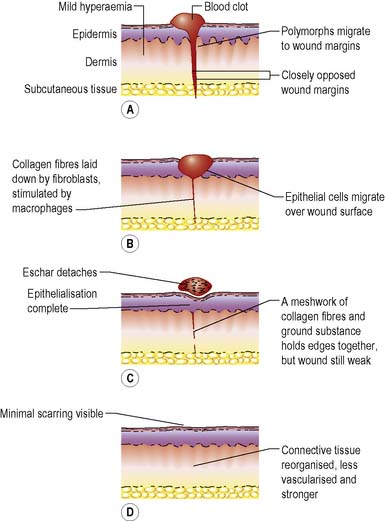
The wound healing process comprises a complex series of events whereby the continuity and strength of damaged tissues are restored by the formation of connective tissue and regrowth of epithelium. The process can be divided into four phases (Figure 23.1) but, since wound healing is a continuous biological process, there is some overlap between them.
Phase II – inflammation
The fibrin clot begins to degrade and the surrounding capillaries dilate and become permeable, allowing fluid into the wound site. This activates the complement system, several interacting soluble proteins found in serum and extracellular fluid that induce lysis and destruction of target cells, such as bacteria. Cytokines and proteolytic fragments are also found in the wound space (Steed 1997), which initiate a massive influx of other cells. The two main inflammatory cells are neutrophils and macrophages:
Phase III – proliferation/reconstruction
Tissue repair takes place during this phase. It usually begins at around day three and lasts for some weeks. Proliferation is characterised by the formation of granulation tissue in the wound space. This new tissue consists of a matrix of fibrin, fibronectin, collagens, proteoglycans and glycosaminoglycans, and other glycoproteins (Hart 2002). Fibroblasts move into the wound space and proliferate. Their function is to synthesise and deposit extracellular proteins, producing growth factors and angiogenic factors that regulate cell proliferation and angiogenesis (Stephens & Thomas 2002). Fibroblasts will multiply rapidly in the well-nourished individual and, to be most effective, need adequate amounts of vitamin C, iron, oxygen and nutrients. Granulation tissue also contains elastin, providing the wound with elasticity and resilience (Wysocki 2007).
Angiogenesis
is the formation of new blood vessels in the wound space which are essential for the delivery of oxygen and other nutrients. The key cells involved in angiogenesis are the vascular endothelial cells, which arise from the damaged end of vessels and capillaries (Neal 2001). New vessels sprout from existing small vessels at the wound edge, endothelial cells detaching from these small vessels and penetrating the wound space. These sprouts are then extended in length until they meet other capillaries, connecting together to form new vascular loops and networks.
Phase IV – maturation
Maturation usually begins around 7 days post injury and continues for many months or years, long after the wound looks to be closed or healed. The immature collagen laid down earlier is gradually replaced by a mature collagen. The formation of new collagen and the lysis of immature collagen are balanced so that the amount of collagen present remains constant. The immature collagen is laid down in a random, haphazard fashion, its function being to fill the wound defect as quickly as possible. The remodelling process involves the balance between synthesis and degradation of collagen, where the cells producing the different types of collagen are subjected to apoptosis (programmed cell death) (Tjero-Trujeque 2001). Mature collagen is laid down following lines of tension within the wound, and is cross-linked to give strength. Tensile strength at 14 days in sutured wounds is approximately 10% of the original strength of the skin. Within 3 weeks this increases to 20%, gradually reaching a maximum of 70–80% about 1 year later.
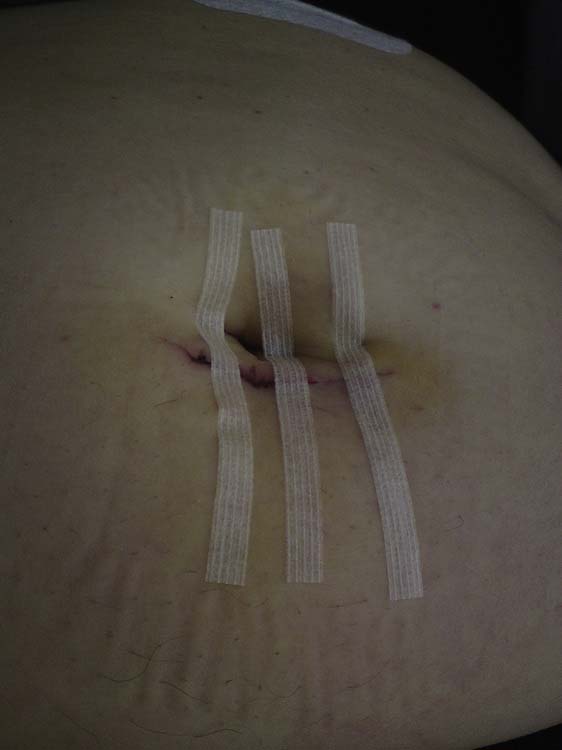
Healing by primary/first intention
Following wounding, treatment aims to effect complete healing as quickly as possible with minimal scarring. To achieve this, the method of choice is healing by primary intention, which occurs when wound edges are in apposition. There is minimal formation of granulation tissue and, once the wound has healed, only a thin scar remains. Healing by primary intention is only possible where there is adequate, mobile tissue and no complicating bacterial contamination. In situations where contamination is suspected, wound closure is accompanied by the use of prophylactic systemic antibiotics. For healing to take place by primary intention, the wound edges need to be closely approximated and held together until the wound has healed sufficiently. The skin may be closed by using tapes, clips, continuous or interrupted sutures, or glue (Figure 23.2).
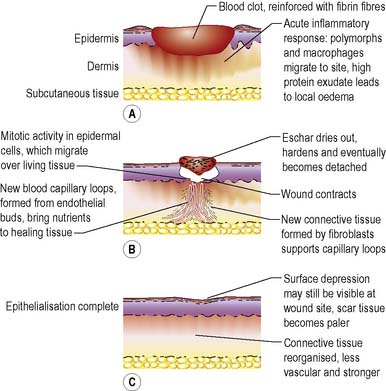
Healing by secondary intention
Where there is significant tissue loss and/or bacterial contamination, wounds are usually left open to heal by secondary intention through the formation of granulation tissue and, later, wound contraction (Figure 23.3). Due to the amount of tissue excised or lost during injury, wound healing by secondary intention is a longer process, taking weeks or even months to complete. The healing process proceeds in much the same way as for healing by primary intention. The proliferative phase is much extended, as this is when granulation tissue forms and fills the wound defect. It was established many years ago (Marks et al 1983) that, for some wounds, the length of time taken to heal depends on the original size, i.e. small wounds heal more quickly than larger ones, and it is therefore possible in some wound types (pilonidal sinus, abdominal and axillary wounds) to predict when wounds of a given size that are free from infection will heal.
Scar tissue
Box 23.1 outlines factors influencing scarring.
Factors that adversely affect healing
Many factors can adversely affect the normal rate of healing, slowing it down and, in severe cases, impairing it altogether. These factors can be described as pathophysiological (see below), psychological or practical (Harding 2007).
Pathophysiological factors
Intrinsic factors
Advanced age
With advanced age the dermis gradually thins and the underlying structural support, collagen, reduces (see Ch. 34).Reduced collagen production results in loss of skin elasticityand its ability for elastic recoil, leading to creases and wrinkles. The amount of subcutaneous fat reduces, and there is less of a cushion for underlying bone. The natural moisture from sebum secretions lessens, leading to increasing dryness of the skin. The consequence of ageing is dry, thin, inelastic skin that is susceptible to damage, with a reduced metabolic rate and prolonged healing.
Malnutrition
Malnutrition may result in delayed wound healing and the production of weak, poor quality scars (Pinchcofsky-Devin 1994).
Protein–energy malnutrition (PEM)
is caused by an absolute or relative deficiency of energy and protein that affects between 19 and 50% of hospitalised patients (McLaren 1997). Several factors contribute to PEM including a reduced intake of nutrients, reduced digestion and absorption of nutrients, and increased metabolic use. McWhirter & Pennington (1994) reported that 200 out of 500 patients admitted to hospital were undernourished, and just over 100 lost weight during their admission. Other research supports these findings. McLaren (1997) estimates that although 70% of patients admitted to hospital are malnourished prior to admission, the remaining 30% develop PEM during their hospital stay.
Nutrient deficiency
The European Pressure Ulcer Advisory Panel (EPUAP) (2003) recommends a minimum daily intake of 30–35 g/kg body weight, with 1–1.5 g/kg/day of protein and 1 mL/kcal/day of fluid intake. These guidelines also recommend that nurses consider the quality of the food that patients are offered, along with removing the physical or social barriers to its consumption. The latest guideline states ‘Offer high-protein mixed oral nutritional supplements and/or tube feeding, in addition to the usual diet, to individuals with nutritional risk and pressure ulcer risk because of acute or chronic diseases, or following a surgical intervention’ (European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel [NPUAP] 2009, p. 14).
Some people may have difficulty in maintaining adequate nutrition, e.g. an older person living alone may lose interest in cooking or a patient having chemotherapy may be unable to eat due to nausea. The importance of diet cannot be overemphasised, and wherever possible the nurse should ensure that the patient receives all the nutrients required for healing. The advice of a dietitian may be sought in an attempt to improve or supplement the nutritional status of vulnerable individuals (see Ch. 21).
Smoking
Smoking adversely affects the healing process in a number of ways. Smoking and the absorption of nicotine has a vasoconstricting effect. The main influences of nicotine and carbon dioxide relate to the effects on peripheral tissues, with a reduction in tissue oxygen tension and the formation of thrombi. Nicotine has been demonstrated to inhibit epithelialisation (Waldrop & Doughty 2000) and the healing of abdominal wounds, the overall cosmetic effect of a scar being poorer in patients who were smokers (Siana et al 1992).
Psychosocial factors
There is a close association between psychological and physical well-being. Stress and anxiety can impair immune function through elevation of stress hormones glucocorticoids and catecholamines (e.g. adrenaline, noradrenaline) (Webster Marketon & Glaser 2008). The same authors describe the effects of increased levels of stress hormones including reduced lymphocyte proliferation, reduced antibody production and decreased activity of natural killer (NK) cells. One of the many adverse effects of the changes to immune function is delay in wound healing (Webster Marketon & Glaser 2008). Sleep disturbances are linked to stress, and sleep is thought to be essential for healing and tissue repair (Dealey 1999).
Wound infection
It is inevitable that most wounds contain microorganisms, but it is only pathogenic organisms, usually bacteria, which delay healing and cause systemic illness (Carville et al 2008). Established infection in a healing wound often delays healing and may even cause wound breakdown, herniation of the wound or complete wound dehiscence. The clinical signs and symptoms of a wound infection are summarised in Box 23.2. Despite all the technological advances that have been made in surgery and wound management, the problem of wound infection persists with surgical site infections accounting for 13.8% of health care-associated infections (HCAIs) in acute settings (Hospital Infection Society 2007). It is suggested that at least 5% of patients undergoing surgery develop a surgical site infection. This relates to advances in surgical and anaesthetic techniques which have allowed patients with many co-morbidities and therefore greater risk levels to be considered for surgery (National Institute for Health and Clinical Excellence [NICE] 2008).
Box 23.2 Information
Signs of wound infection (reproduced with permission from Principles of Best Practice: Wound Infection in clinical practice. An international consensus. London: MEP Ltd, 2008)
Acute Wounds, e.g. surgical or traumatic wounds, or burns
| Localised Infection | Spreading Infection |
|---|---|
| Classic signs and symptoms: Pyrexia – in surgical wounds, typically 5–7 days post surgery Delayed (or stalled) healing Abscess Malodour | As for localised infection plus Further extension of erythema Lymphangitis Crepitus in soft tissue Wound breakdown/dehiscence |
Notes:
Burns – also skin graft rejection, pain is not always a feature of infection in full-thickness burns.
Deep wounds – induration, extension of the wound, unexplained increased white cell count or signs of sepsis may be signs of a deep wound infection.
Immunocompromised patients – signs and symptoms may be modified and less obvious.
Chronic Wounds, e.g. diabetic foot ulcers, venous leg ulcers, arterial leg/foot ulcers, pressure ulcers
| Localised Infection | Spreading Infection |
|---|---|
| New, increased or altered pain* Delayed or stalled healing* Periwound oedema Bleeding or friable (easily damaged) granulation tissue Distinctive malodour or change in odour Wound bed discolouration Increased or altered/purulent exudate Induration Pocketing Bridging | As for localised infection plus Wound breakdown* Erythema extending from the wound edge Crepitus, warmth, induration or discolouration spreading into periwound area Lymphangitis Malaise or other non-specific deterioration in patient’s general condition |
Notes:
In patients who are immunocompromised and/or who have motor or sensory neuropathies, symptoms may be modified and less obvious. For example, in a diabetic patient with an infected foot ulcer and peripheral sensory neuropathy, pain may not be a prominent feature.
Arterial ulcers – previously dry ulcers may become wet when infected.
Clinicians should also be aware that in the diabetic foot inflammation is not always indicative of infection. For example inflammation may be associated with Charcot’s arthropathy.
* Individually highly indicative of infection. Infection is also highly likely if the patient has two or more of the other signs listed.
Factors that predispose to wound infection
Factors may be associated with the patient or the hospital environment.
Factors associated with the patient
Factors specifically related to the hospital environment
Sources of wound infection
Endogenous
Organisms found on the patient’s own skin are endogenous sources of wound infection. These organisms, usually Staphylococcus aureus or gut commensals, are either present under normal circumstances or are hospital pathogens that colonise the body after admission to hospital. Shortening the time between admission and surgery reduces the possibility of skin contamination by hospital-acquired bacteria (Dealey 1999).
Exogenous
![]() See website Critical thinking question 23.1
See website Critical thinking question 23.1
Nursing management and health promotion: holistic wound care
All patients should have access to a minimum standard of care, regardless of where that care is provided or by whom, in order to optimise the chance of achieving a straightforward, uncomplicated and timely healing process (Fletcher 2008b). This should be a systematic process which is adapted to best suit the local circumstances, for example the care pathway proposed by Chadwick et al (2008, p. 7).
Research has been undertaken using different study designs to answer a diverse range of clinical questions, and to explore patients’ views. Best available evidence is used not only to inform practice but also to develop clinical guidelines (NICE 2001, EPUAP 2003, EPUAP & NPUAP 2009).
Patient assessment
Whether the patient is being cared for at home, other community setting or hospital, or is young or old, assessment of the patient’s general condition should be undertaken. This is done to identify any of the factors that might impair the wound-healing process (pp. 636–638): for those factors seen as reversible, treatment should be sought; for those factors where no treatment is possible, some degree of delay in healing should be anticipated and allowed for in the care plan.
Wound assessment
Where wound specific assessment tools are used, for example a leg ulcer assessment chart, other data such as the ankle brachial pressure index (see p. 650) would also be included. Once a thorough assessment has been completed objectives of care can be set in conjunction with the patient’s needs and wishes.
Wound measurement
There are a variety of ways of measuring wounds; most commonly a simple length × width is recorded using a ruler. It is possible to convert this into a surface area; however, unless the wound is square or rectangular this is a very inaccurate measurement (Langemo et al 2008). A simple and more accurate practice is to trace the wound outline onto an acetate sheet with grid squares; these can then be counted and the area calculated.