Chapter 1. The use of pharmaceuticals
At the end of this chapter, the reader should be able to:
• describe the factors that dictate the choice of dose
• list the various routes of drug administration
• list the factors that affect absorption and distribution of drugs in the body
• discuss the consequences of metabolism and excretion on drug efficacy and duration
• define the terms agonist, antagonist, partial agonist and ligand
• explain the basic properties of the log 10 dose–response curve
Drug development
The science of pharmacology is the discovery and characterization of chemicals to the point where they can be used to treat or prevent illness (e.g. aspirin to treat pain or inhibit platelet aggregation). Drugs are also designed to intervene in the normal functions of the body (e.g. contraceptives). The pharmacologist is involved during virtually every stage of this process.
Medicinally useful drugs can be discovered quite by accident, as was the case with penicillin and the oral sulphonylureas for the treatment of adult-onset (type II) diabetes (see pp. 191 and 306, respectively). More usually, however, a deliberate attempt is made to introduce a new drug by modifying an existing natural or synthetic drug to increase its potency, improve its absorption and reduce unwanted effects. Once the chemical structure of morphine from the opium poppy was worked out, this led to the use of pethidine for the treatment of pain (see p. 138). Advances in the understanding of physiological processes can also result in new drugs. The discovery that Parkinson’s disease results from the selective destruction of certain dopaminergic brain pathways led to the invention of the drug levodopa to try to counteract some of the distressing symptoms of the disease, such as tremor, slowness of movement and rigidity (see p. 257). The discovery of the physiological role of the peptide tumour necrosis factor (TNF-α) in the human immune system has led to the use of the very potent and effective anti-TNF-α drugs for the treatment of rheumatoid arthritis (see p. 156).
The testing of new drugs before use
Nurses play an important role in clinical trials and must be aware of the related regulations, recommendations and procedures. The Independent Expert Scientific Group which was convened by the Secretary of State for Health has made several new recommendations for improving the safety of phase I clinical trials following the very serious adverse immune system-related effects suffered by volunteers who took a monoclonal antibody called TGN 1412 in London in March 2006.
Once a potential drug has been identified, it should be rigorously tested to ensure that it is not teratogenic (causes birth defects), carcinogenic (causes cancer) or otherwise harmful to any of the organs and systems of the body (toxicity studies). It should be specific in action and its mechanism of action understood as completely as possible ( pharmacodynamic studies; see below). It is imperative to find the optimal route of administration, dose and frequency of dosing in order to maintain effective concentrations in the body.
The pharmacokinetics of the drug must be determined. This entails finding out how the drug is distributed in the body compartments and how long its effective concentrations will be maintained after a dose.
Once all this has been done, it is time to test the drug in human volunteers and patients by means of strictly controlled clinical trials. These are set up through collaboration between pharmaceutical companies and the medical professions, and the nurse figures very prominently in these trials. If the drug passes this final test, then government-controlled regulatory bodies will grant a licence for the drug to be routinely prescribed. Even with all these precautions, some drugs slip through the net. The tragic consequences of the introduction of the drug thalidomide for use by pregnant women remains as an indelible reminder of the risks taken when drugs are allowed into the body.
Basic pharmacokinetics
Pharmacokinetics is about how the body deals with the drug. It helps to answer a most important question: What are the factors that determine the maintenance of a therapeutically useful level of the drug in the bloodstream? To answer this question, the following questions must be investigated:
• The dose: How much of the drug should be used to get the desired effect without getting unwanted effects?
• Route of administration: By what route should the drug be administered?
• Absorption and distribution: How is the drug absorbed and compartmentalized in the body, e.g. does most of it dissolve in the aqueous (water) or in the lipid (fatty) compartments? How is it carried in the blood? Is it concentrated in any particular organ? For example, when iodine is administered, most of it is concentrated in the thyroid gland.
• Metabolism and excretion: How long does the drug stay in the body? The body’s way of dealing with drugs is to try and get them out of it as soon as possible through metabolism and excretion. How often does the patient need to repeat the dose in order to sustain therapeutically effective concentrations of the drug?
The dose
The aim is to give the patient a dose of the drug that achieves the desired effect without causing harmful side-effects. This dose is found experimentally using isolated tissues or cells, experimental animals, and human volunteers and patients. The dose–response curve is described below.
The therapeutic index
The therapeutic index is a measure of the danger of poisoning, and the higher it is, the safer the drug is. The therapeutic index is a ratio of a therapeutic dose to one that is actually toxic. The therapeutic index for aspirin is around 3.5. For digoxin (from the foxglove), which is used for cardiac arrhythmias and congestive heart failure, the index is dangerously low (less than 2), and patients taking digoxin need regular and frequent monitoring of blood levels of the drug.
Choosing and adjusting the dose
In a perfect world, all adults would respond equally to a given dose. In practice, however, there may be considerable interperson variation in plasma concentrations of a given dose of a drug. With some drugs, the therapeutic index is low, and this too demands regular plasma monitoring of the drug and dose adjustment as needed. Some commonly used drugs that usually require close monitoring include:
• ciclosporin, used for suppressing transplant rejection and for rheumatoid arthritis
• digoxin, for treatment of cardiac disease
• gentamicin and other aminoglycoside antibiotics
• lithium, for treatment of manic-depression psychosis
• methotrexate for treatment of rheumatoid art-hritis and cancer
• phenytoin, for treatment of epilepsy.
Route of administration – terminology
Before describing the routes of administration it is important to understand the related terminology:
• the internal and external environments
• bioavailability
• topical and parenteral application of drugs.
Internal and external environments
Where the body is concerned, physiologists talk about the internal and external environments. When anything is swallowed, it remains in the external environment until it or one of its breakdown products passes a cell membrane and gets into a cell of the body. A cherry pip, if swallowed, may be inside the body for a while, but normally it will never get into the internal environment of the body from the gastrointestinal tract.
Bioavailability
Another important concept is that of bioavailability. The term bioavailability generally means that the drug has reached the circulation and is therefore available to all the tissues. The patient may take 600 mg of aspirin, but after the drug passes into the circulation from the gastrointestinal tract and then emerges from the liver, less than 600 mg of aspirin is available to the body.
Topical application of drugs
The term topical application of drugs means the application of drugs directly to the surface where its action is wanted. For example:
• Ointments, patches or creams that are applied to the skin.
• Inhalation of drugs to treat asthma directly by dilating airway passages.
• Drops or ointments into the eyes.
• Suppositories and pessaries, which are inserted into the rectum or vagina, respectively (see also below). Some of the drug will get absorbed, but most of the drug will remain at the surface where it is applied unless it has been formulated to penetrate to the bloodstream.
Parenteral administration of drugs
The term parenteral administration is used to describe the injection of drugs into the tissues, including directly into the blood. Oral administration mainly means the taking of drugs to be absorbed through the gastrointestinal tract, although, as will be mentioned below, some drugs are absorbed into the body directly from the oral mucosa.
When considering the route of administration, several questions need to be addressed:
• Which is the most convenient route for the patient?
• How quickly does the prescriber want the drug to reach its site of action?
• For how long does the prescriber want the dose of drug to work, i.e. to stay in the body?
• Which organs is the drug to be kept away from?
The route of administration can have profound effects on:
• the onset of the action of the drug
• the plasma concentrations achieved
• the length of time that the drug will spend in the body (see Fig. 1.1).
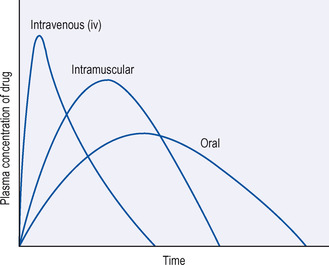 |
| Figure 1.1 Effects of the route of administration of a single dose of a drug on its plasma levels with time. |
Routes of administration – details
The routes of administration are as follows:
• topical
• oral
• transdermal
• rectal and vaginal
• inhalation
• injection (parenteral)
• intravenous
• intramuscular
• intradermal
• subcutaneous
• intrathecal.
Topical
The skin is traditionally the most accessible part of the body for the administration of drugs. Most of the preparations for topical administration to the skin are used for treating skin disorders. There is a huge range of non-pharmaceutical topical products for cosmetic or recreational use and these will not be described in any detail except when their use results in the need for treatment. Skin preparations are formulated mainly into lotions, creams, ointments and powders and are used for a wide variety of purposes, e.g. for the symptomatic relief of an itch or to treat fungal infections such as athlete’s foot. They are convenient for the patient, who can use them at home, and, because the drug is generally poorly absorbed, this makes them relatively safe. The one tissue most at risk from the use of these preparations is the skin itself, and a salutary tale is that of the prolonged use of the anti-inflammatory steroid hydrocortisone, which when it was introduced in the 1950s was found to be dramatically effective in reducing inflammation in, for example, eczema. It took some time before it was realized that the prolonged topical use of hydrocortisone causes thinning of skin.
Strictly speaking, the inhalation of drugs for use on lung tissue (see below) is a form of topical application. However, since inhalation is also used as a route for drugs that are to be absorbed (e.g. gaseous anaesthetics), inhalation is treated separately below.
Oral
The medication is taken by mouth and preparations comprise:
• tablet
• capsule
• powder
• mixture
• emulsion
• linctus.
Tablets and capsules
Tablets are prepared by mixing a drug with a base that binds the two together so that the tablets will not disintegrate in the body before they are meant to. They are usually coated and may be coloured. Capsules are made of gelatine or some similar substance and contain a drug that is liberated when the wall of the capsule is digested in the stomach or intestine. The actual formulation of tablets and capsules is very important and determines how satisfactorily the drug is released, which governs their absorption and bioavailability. A great deal of care is taken in the manufacture of tablets to ensure the maximum bioavailability. It is also possible by coating the tablets, modifying the capsule or by binding the drug to some inert substance, to slow down the release of the active ingredient and thus prolong its absorption and effect. These may be called sustained-release or retard preparations.
Mixtures
Mixtures are liquids that contain several ingredients dissolved or diffused in water or some other solvent. An example is kaolin mixture for diarrhoea, when insoluble kaolin powder is suspended in the aqueous liquid. An emulsion is a mixture of two immiscible liquids (e.g. oil and water) in which one is dispersed through the other in a finely divided state, e.g. Milk of Magnesia. A linctus is a liquid that contains some sweet syrupy substance used for its soothing effect on coughs. It may also contain a cough suppressant such as dextromethorphan.
When liquid drugs are prescribed, they are accompanied by a standard oral syringe, which measures up to 5 ml and is marked with 0.5 ml divisions. This should be used to draw up the fluid to achieve the exact dose. For doses of 5 ml or more, the standard 5 ml plastic spoon can be used. The syringe is issued with a manufacturer’s information leaflet advising on use and storage, but verbal explanations are still valuable, especially as liquid medicines are usually given to children and frequently administered by anxious parents. Families appreciate practical demonstrations of the use of the syringe provided by the nurse. Medicines for oral administration to babies are most usually in liquid form.
Advantages of oral administration
• Oral administration of drugs is extremely convenient for the patient. The drug can be taken at home and does not need the attendance of a carer or health professional unless the patient is physically or mentally incapable of self-medication.
• Oral administration avoids the fear of needles.
• The gastrointestinal tract provides a huge surface area for absorption and drugs are absorbed by diffusion and in some cases by active transport processes, when drugs are pumped from the gastrointestinal tract lumen into the gastric mucosa against concentration gradients (see below).
Disadvantages of oral administration
• Absorption can be variable and depends on the chemical nature of the drug, e.g. its ionization, solubility and stability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
