The Normal Newborn
Adaptation and Assessment
Learning Objectives
After studying this chapter, you should be able to:
• Describe thermoregulation in the newborn.
• Compare gastrointestinal functioning in the newborn and adult.
• Explain the causes and effects of hypoglycemia.
• Describe kidney functioning in the newborn.
• Explain the functioning of the newborn’s immune system.
• Describe the periods of reactivity and behavioral states of the newborn.
• Describe nursing assessments of the newborn.
• Explain the importance and the components of gestational-age assessment.
![]()
http://evolve.elsevier.com/McKinney/mat-ch/
At birth, neonates must make profound physiologic changes to adapt to extrauterine life and meet their own respiratory, digestive, and regulatory needs. During nursing assessments, nurses must be aware of those changes so they can identify behaviors signifying problems or abnormalities.
Initiation of Respirations
The first vital task in newborn adaptation is the initiation of respirations. Forces occurring throughout pregnancy and during birth bring about this change.
Development of the Lungs
During fetal life, the alveoli produce fetal lung fluid that expands the alveoli and aids in lung development. As the fetus nears term, production of lung fluid decreases. During labor, the fluid begins to move into the interstitial spaces, where it is absorbed. Absorption is accelerated by the process of labor and may be delayed after cesarean birth that occurs without labor. This continues throughout labor and during the early hours after birth. At birth only about 35% of the original amount of fetal lung fluid remains (Blackburn, 2013).
Surfactant, a slippery detergent-like combination of lipoproteins, is detectable by 24 to 25 weeks of gestation (Blackburn, 2013). It reduces surface tension within the alveoli. Without surfactant, the alveoli collapse as the infant exhales. They must be reexpanded with each breath, greatly increasing the work of breathing. Sufficient surfactant is usually produced beginning at 34 to 36 weeks of gestation to prevent respiratory distress syndrome (Gardner, Enzman-Hines, & Dickey, 2011). Surfactant secretion increases during labor and immediately after birth to enhance the transition from fetal to neonatal life.
Steroids may be given to women in preterm labor to help increase surfactant production and lung maturation. Some complications, such as hypertension, placental insufficiency, maternal infection, and rupture of membranes greater than 48 hours may cause accelerated lung maturity. Diabetes may delay surfactant production (Gardner et al., 2011)
Causes of Respirations
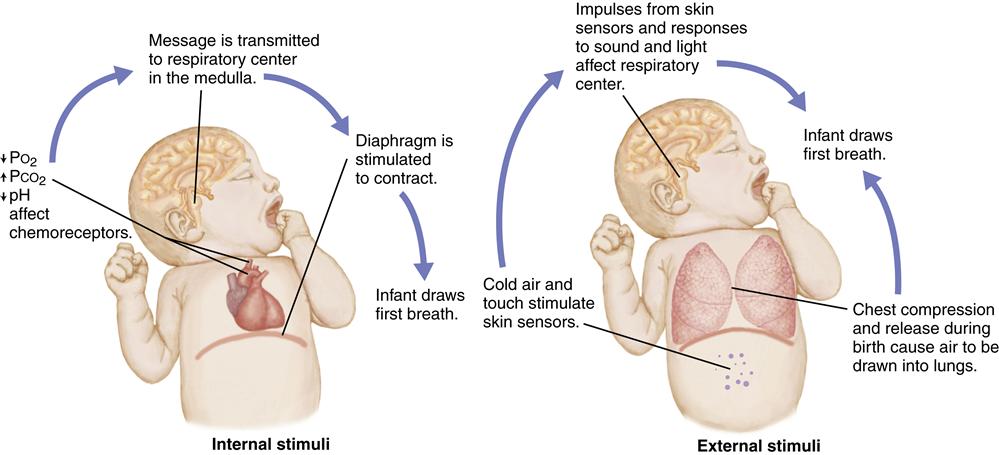
The infant’s first breath at birth must force the remaining fetal lung fluid out of the alveoli and into the interstitial spaces to allow air to enter the lungs. This requires a much larger negative pressure (suction) than subsequent breathing. Breathing is initiated by chemical, mechanical, thermal, and sensory factors that stimulate the respiratory center in the medulla of the brain and trigger respirations (Figure 21-1).
Chemical Factors
Chemoreceptors in the carotid arteries and the aorta respond to changes in blood chemistry brought about by the hypoxia that occurs with normal birth. The decrease in the level of the partial pressure of oxygen (PO2) and pH and an increase in the partial pressure of carbon dioxide (PCO2) in the blood cause stimulation of the respiratory center in the medulla. A forceful contraction of the diaphragm results, causing air to enter the lungs. However, stimulation of the respiratory center and breathing do not occur if prolonged hypoxia causes central nervous system depression.
Mechanical Factors
During a vaginal birth, the fetal chest is compressed by the narrow birth canal. Approximately one third of the fetal lung fluid is forced out of the lungs into the upper air passages and expelled during birth. When the pressure against the chest is released at birth, recoil of the chest draws a small amount of air into the lungs. This reduces the amount of negative pressure needed for the first breath after birth.
Thermal Factors
The temperature change that occurs with birth also stimulates the initiation of respirations. Sensors in the skin respond to this sudden change in temperature by sending impulses that stimulate the respiratory center of the brain and breathing.
Sensory Factors
Tactile stimuli that occur during birth stimulate skin sensors. Nurses hold, dry, and place infants skin to skin with the mother or wrap them in blankets, providing further stimulation to skin sensors. The stimulation of the sound, light, smell, and pain at delivery also may aid in initiating respirations.
Continuation of Respirations
As the alveoli expand, surfactant allows them to remain partially open between respirations. Much of the air from the first breath remains in the lungs to become the functional residual capacity. Subsequent breaths require less effort than the first one because the alveoli remain partly open.
As the infant cries, the pressure within the lungs increases, causing remaining fetal lung fluid to move into the interstitial spaces, where it is absorbed by the pulmonary circulatory and lymphatic systems. Although most fluid is absorbed within a few hours, complete absorption may take as long as 24 hours. Therefore the lungs may sound moist when first auscultated but become clear a short time later.
Cardiovascular Adaptation: Transition from Fetal to Neonatal Circulation
During fetal life, three shunts, the ductus venosus, foramen ovale, and ductus arteriosus carry much of the blood away from the lungs and some blood away from the liver. High pressures within the collapsed, fluid-filled lungs permit only a small amount of blood flow into the narrow pulmonary vessels.
Ductus Venosus
Oxygenated blood from the placenta enters fetal circulation through the umbilical vein. About a third of the blood is directed away from the liver into the ductus venosus (DV) which connects to the inferior vena cava (IVC). The rest of the umbilical vein flow goes through the liver before entering the IVC. Near the end of pregnancy, the liver needs more perfusion, and 70% to 80% of the oxygenated blood from the umbilical vein flows through to the liver (Blackburn, 2013).
As blood from the DV or the portal system enters the IVC, it joins blood from the lower part of the body to travel to the heart in separate streams so that there is little mixing of the blood. When the blood enters the right atrium, the more highly oxygenated blood is directed across the atrium to the foramen ovale (Blackburn, 2013).
Foramen Ovale
The foramen ovale is a flap in the septum between the right and left atria of the fetal heart. About 50% to 60% of the blood from the right atrium moves through the foramen ovale to the left atrium (Blackburn, 2013). The blood flows from the left atrium to the left ventricle and leaves through the ascending aorta. The majority of this better oxygenated blood flows to the heart, brain, head, and upper body.
Blood that does not cross the foramen ovale moves to the right ventricle but flow is restricted to the lungs by the narrow pulmonary artery and pulmonary blood vessels. This elevates the pressure in the right side of the heart. Pressure is low on the left side of the heart because there is little resistance as blood leaves the left ventricle to travel to the rest of the body and into the widely dilated placental vessels. This difference in pressure between the right and left sides of the heart allows blood flow through the foramen ovale.
Pulmonary Blood Vessels
Blood from the superior vena cava and the less oxygenated blood from the inferior vena cava flow into the right atrium, to the right ventricle, and into the pulmonary artery. Approximately 10% to 12% of the blood goes to the lungs and the rest passes through the ductus arteriosus to the aorta (Blackburn, 2013). Little blood is allowed into the lungs because the pulmonary artery and other blood vessels are constricted, causing high pulmonary vascular resistance. Blood perfusing the lungs returns to the left atrium by the pulmonary veins.
Ductus Arteriosus
The ductus arteriosus connects the pulmonary artery and the aorta. Most of the blood that enters the pulmonary artery passes into the aorta through the widely dilated ductus arteriosus. Dilation of the ductus arteriosus is maintained by prostaglandins from the placenta and the low oxygen content of the blood.
Changes at Birth
At birth, the shunts close and the pulmonary vessels dilate. These changes occur in response to increases in blood oxygen and shifts in pressure within the heart, pulmonary, and systemic circulations, as well as clamping of the umbilical cord. The changes necessary for transition from fetal to neonatal circulation occur simultaneously within the first few minutes after birth. They are discussed separately here (see Figure 12-9).
As the newborn takes the first breaths at birth, the rise in oxygen level causes the ductus arteriosus to constrict, preventing entry of blood from the pulmonary artery. The pulmonary blood vessels respond to the increased oxygenation by dilating. At the same time, fetal lung fluid begins to shift into the interstitial spaces and is removed by blood and lymph vessels. These changes decrease pulmonary vascular resistance and allow more room for dilation of the pulmonary blood vessels. As a result, the pulmonary vessels can expand to hold the suddenly increased blood flow from the pulmonary artery.
At birth, pressures between the right and left sides of the heart are reversed. The sudden dilation of the vessels of the lungs allows blood to enter freely from the right ventricle and decreases pressure in the right side of the heart. Clamping of the umbilical cord further decreases pressure in the right side of the heart. Increased blood flow from the pulmonary veins into the left atrium causes pressure in the left side of the heart to build. Systemic resistance increases as blood flow to the placenta ends with clamping of the cord, further elevating pressure in the left heart.
Because the foramen ovale opens only from right to left, it closes when the pressure in the left atrium is higher than that in the right atrium. This change forces the blood from the right atrium into the right ventricle and pulmonary artery. Thus blood flow through the heart and lungs changes from fetal to neonatal circulation and is similar to that in the normal adult.
The foramen ovale is functionally closed soon after birth because the unequal pressures between the atria prevent it from opening. Conditions such as asphyxia (insufficient oxygen and excess carbon dioxide in the blood and tissues) and persistent pulmonary hypertension, however, may reverse the pressures in the heart and cause the foramen ovale to reopen. It is permanently closed within several months (Kenney, Hoover, Williams, et al., 2011). The ductus arteriosus closes gradually as oxygenation improves and prostaglandins, which helped keep it open, are metabolized. Functional closure occurs for most term infants at about 72 hours and permanent closure occurs within 1 to 2 weeks (Kenney et al., 2011).
Until closure is complete, the blood that does flow through the ductus arteriosus usually reverses, moving from the aorta to the pulmonary artery and increasing blood flow to the lungs. This sequence occurs because pressure in the aorta is now higher than that in the pulmonary artery. A murmur may be heard as a result of blood flow through the partially open vessel.
Low levels of oxygen in the blood may cause the ductus arteriosus to dilate and the pulmonary vessels to constrict, increasing resistance to blood flow to the lungs. The result may be opening of the foramen ovale to allow a right-to-left shunt of blood and flow from the pulmonary artery through the ductus arteriosus and into the aorta. The ductus venosus closes shortly after birth. Permanent closure occurs by 1 to 2 weeks after birth (Kenney et al., 2011).
Neurologic Adaptation: Thermoregulation
Although the fetus produces heat in utero, the consistently warm temperature of the amniotic fluid and the mother’s body makes thermoregulation, the maintenance of body temperature, unnecessary. When the neonate moves from the warm uterus to the cooler outside environment it must produce and maintain heat to prevent the serious effects of cold stress.
Newborn Characteristics Leading to Heat Loss
Certain newborn characteristics predispose them to heat loss. The skin is thin, blood vessels are close to the surface, and there is little subcutaneous (white) fat to provide a barrier to loss of heat. Heat is readily transferred from the warmer internal areas of the body to the cooler skin surfaces and then to the surrounding air. Newborns have three times more surface area to body mass than the adult and the rate of heat loss is four times greater than in adults (Carlo, 2011a).
The flexed position of the healthy full-term infant reduces the amount of skin surface exposed to the surrounding temperatures and decreases heat loss. Because of decreased muscle tone, the sick or preterm infant does not maintain a flexed position and is more susceptible to loss of heat.
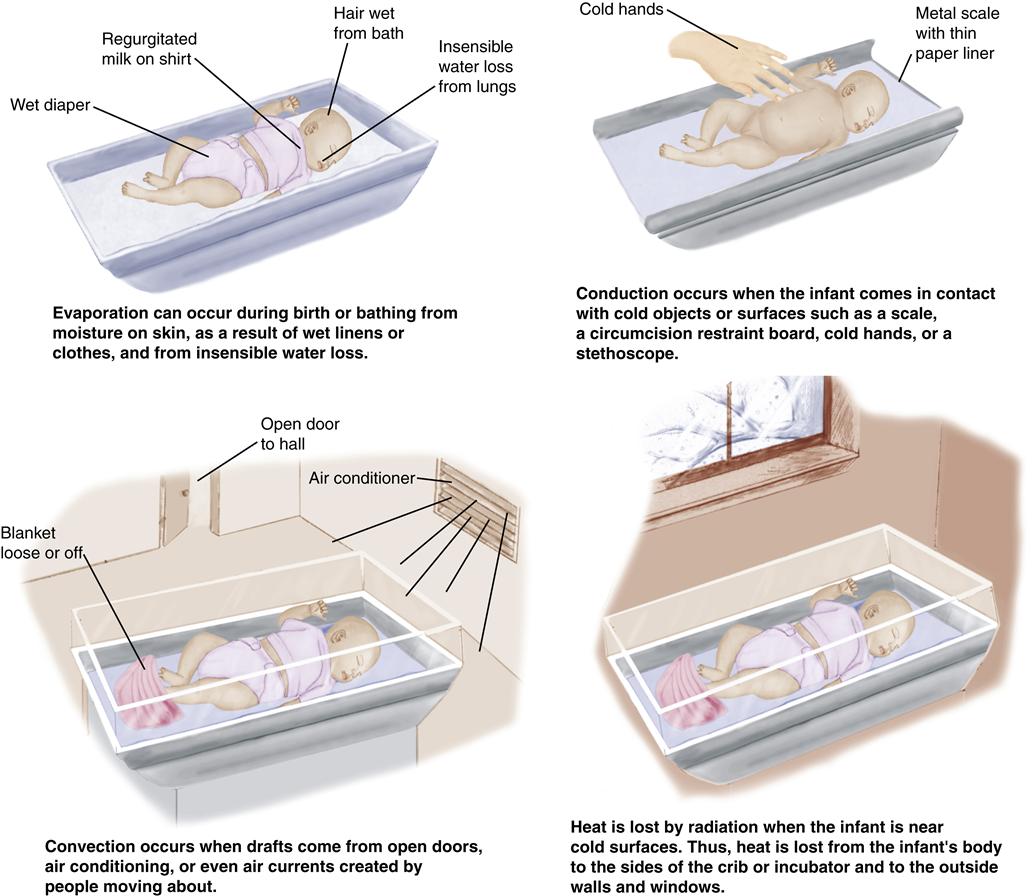
Methods of Heat Loss
The four methods of heat loss in the neonate are (Figure 21-2):
Nonshivering Thermogenesis
When newborns become cold they become restless and cry, increasing flexion and activity to help maintain heat. Vasoconstriction occurs to decrease heat loss and acrocyanosis (bluish discoloration of the hands and feet) may result. Body metabolism rises increasing the need for oxygen and glucose (Blackburn, 2013). Adults shiver when they are cold, but shivering is rare in newborns. It is seen only after prolonged exposure to cold and is not an important method of heat production. The primary method of heat production is nonshivering thermogenesis (NST), the metabolism of brown fat to produce heat. Newborns can increase heat production by 100% using NST (Blackburn, 2013).
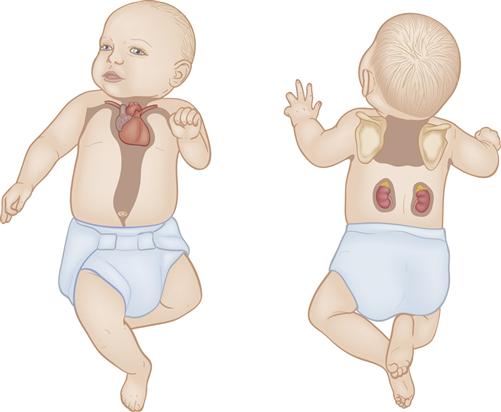
Brown fat, also called brown adipose tissue, or BAT, is the vascular specialized fat that provides heat when metabolized. It is located primarily around the back of the neck, in the axillae, between the scapulae, along the abdominal aorta, and around the kidneys, adrenals, and sternum (Figure 21-3). As brown fat is metabolized, it generates more heat than white subcutaneous fat. Blood passing through brown fat is warmed and carries heat to the rest of the body.
NST begins when thermal receptors in the skin detect a drop in skin temperature. Thermal receptor stimulation causes release of norepinephrine in brown fat, which initiates its metabolism. The process goes into effect even before a change occurs in core or interior body temperature, as measured with a rectal thermometer. Therefore NST may begin in an infant when skin temperature has been cooled, even though a temperature taken rectally shows a normal reading. A decreased core temperature will not occur until NST is no longer effective.
Preterm infants and those with intrauterine growth restriction may have inadequate brown fat stores. Hypoxia, hypoglycemia, and acidosis may interfere with an infant’s ability to generate heat.
These infants are not able to raise their body temperature if they are subjected to cold stress and may have serious complications.
Effects of Cold Stress
Cold stress causes many body changes (Figure 21-4, Box 21-1). An increase in metabolic rate and metabolism of brown fat can lead to a significant rise in the need for oxygen. If an infant is having even mild respiratory distress, the problem may be increased as oxygen is used for heat production. Cold stress also causes diminished production of surfactant, impeding lung expansion and leading to more respiratory distress.
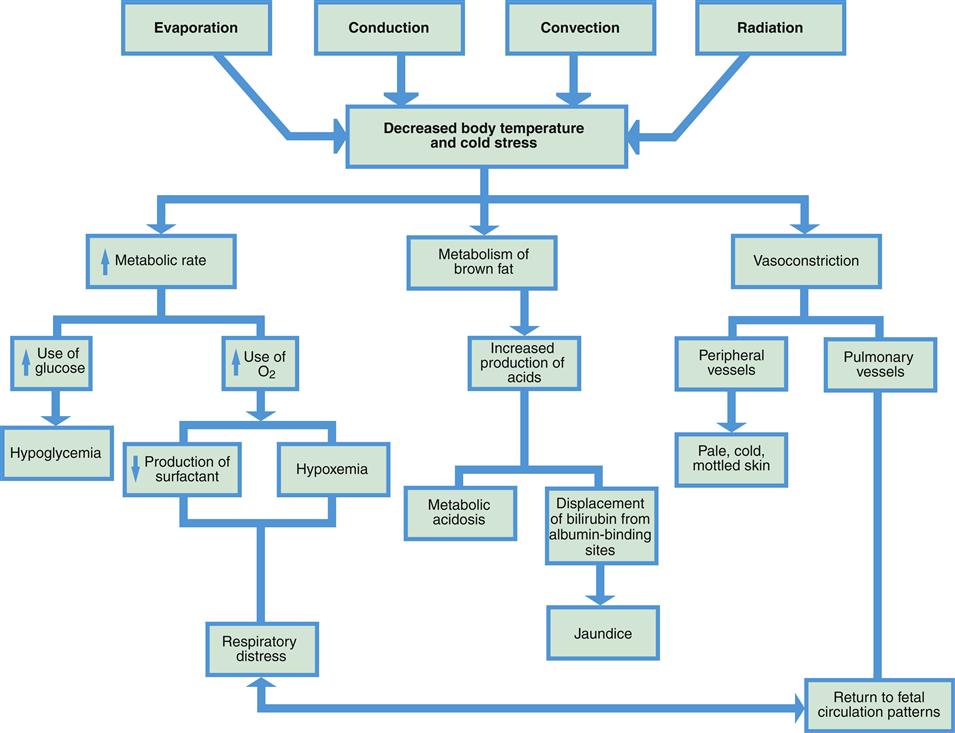
Glucose is also necessary in larger amounts when the metabolic rate rises to produce heat. When glycogen stores are converted to glucose, they may be quickly depleted, causing hypoglycemia. Continued use of glucose for temperature maintenance leaves less glucose available for growth. Metabolism of glucose in the presence of insufficient oxygen causes increased production of acids.
Metabolism of brown fat also releases fatty acids. This release can cause metabolic acidosis, which can be a life-threatening condition. Elevated fatty acids in the blood also can interfere with transport of bilirubin (unusable component of hemolyzed erythrocytes) to the liver, increasing the risk of jaundice, a yellow discoloration of the skin and sclera from excessive bilirubin in the blood.
As the infant’s body attempts to conserve heat, vasoconstriction of the peripheral blood vessels occurs to reduce heat loss from the skin’s surface. Decreased oxygen in the blood, however, may also cause vasoconstriction of the pulmonary vessels, leading to further respiratory distress.
Neutral Thermal Environment
A neutral thermal environment is one in which the infant can maintain a stable body temperature with minimal oxygen need and without an increase in metabolic rate. The range of environmental temperature that allows this maintenance is called the thermoneutral zone. In healthy unclothed full-term newborns, an environmental temperature of 32° C to 33.5° C (89.6° F to 92.3° F) provides a thermoneutral zone. When the infant is dressed, the thermoneutral range is 24° C to 27° C (75.2° F to 80.6° F) (Blackburn, 2013). The thermoneutral zone varies according to an infant’s gestational age, size, and postnatal age.
Hyperthermia
Infants also respond poorly to hyperthermia. With an elevated temperature, the metabolic rate rises, causing an increased need for oxygen and glucose. In addition, peripheral vasodilation leads to increased insensible fluid losses. Sweating may occur but is often delayed because sweat glands are immature.
Newborns may be overheated by poorly regulated equipment designed to keep them warm. When radiant warmers, warming lights, or warmed incubators are used, the temperature mechanism must be set to vary the heat according to the infant’s skin temperature and thus prevent heat that is too high or too low. Alarms to signal that the infant’s temperature is too high or too low should be functioning properly.
Hematologic Adaptation
Factors Affecting the Blood
The blood volume of the term newborn is 80 to 100 mL/kg, but this varies according to the time of cord clamping, the position of the infant when the cord is clamped, and the gestational age of the infant (Diehl-Jones & Askin, 2010). Preterm infants have a greater blood volume per kilogram than term infants.
Blood samples drawn from the heel, where the circulation is sluggish, show higher hemoglobin and hematocrit levels than samples taken from central areas. Venous blood samples are more accurate and are taken when precise measurement is essential. (Newborn values for common laboratory tests are listed in Table 21-1.)
TABLE 21-1
LABORATORY VALUES IN THE NEWBORN
| TEST, SPECIMEN, AND UNIT OF MEASUREMENT | AGE | NORMAL RANGES |
| Erythrocyte (red blood cell [RBC]) count, whole blood | Newborn | 4.8-7.1 (million/microliter) |
| Hemoglobin, whole blood | Newborn | 15-24 g/dL |
| Hematocrit, whole blood | Newborn | 44%-70% |
| Leukocytes, whole blood | Birth | 9.1-34 (thousand/mm3) |
| Leukocyte differential count, whole blood | ||
| Myelocytes | 0% | |
| Neutrophils (“bands”) | 3%-5% | |
| Neutrophils (“segs”) | 54%-62% | |
| Lymphocytes | 25%-33% | |
| Monocytes | 3%-7% | |
| Eosinophils | 1%-3% | |
| Basophils | 0%-0.75% | |
| Platelet count, whole blood | Newborn | 84-478 (thousand/mm3) |
| Glucose, serum | Cord Newborn at 1 day Newborn, >1 day | 45-96 mg/dL 40-60 mg/dL 50-90 mg/dL |
| Calcium, total serum | Cord 3-24 hr 24-48 hr 4-7 days | 9-11.5 mg/dL 9-10.6 mg/dL 7-12 mg/dL 9-10.9 mg/dL |
| Magnesium, plasma | 0-6 days | 1.2-2.6 mg/dL |
| Bilirubin | Cord | <2 mg/dL |
Adapted from Lo, S. F. (2011). Reference intervals for laboratory tests and procedures. In R. M. Kliegman, B. E. Stanton, et al. (Eds.), Nelson textbook of pediatrics (19th ed., p. 2466). Philadelphia: Saunders; Pagana, K. D., & Pagana, T. J. (2011). Mosby’s diagnostic and laboratory test reference (10th ed.). St. Louis: Mosby; Blackburn, S. T. (2013). Maternal, fetal, and neonatal physiology: A clinical perspective (4th ed.). St. Louis: Saunders.
Blood Values
Erythrocytes and Hemoglobin
At birth, an infant has comparatively more erythrocytes (red blood cells [RBCs]) and higher hemoglobin and hematocrit levels than an adult. This difference is necessary because the partial pressure of oxygen of fetal blood is much lower than the normal adult level. The large number of erythrocytes (4.8 to 7.1 million/mm3) and higher hemoglobin level (15 to 24 g/dL) enable the fetal cells to receive enough oxygen (Pagana & Pagana, 2011; Lo, 2011). In addition, fetal hemoglobin (hemoglobin F) has a greater affinity for oxygen than adult hemoglobin (Verklan, 2011).
The newborn’s erythrocytes have a shorter life span than those of the adult. Excess bilirubin caused by the hemolysis of large numbers of RBCs may lead to jaundice.
Hematocrit
The hematocrit level in the normal newborn is 44% to 70% (Lo, 2011). A level above 65% from a central site indicates polycythemia, an abnormally high erythrocyte count (Luchtman-Jones & Wilson, 2011). Polycythemia increases the risk of jaundice and injury to the brain and other organs as a result of blood stasis. Respiratory distress and hypoglycemia are more common in these infants. Laboratory testing is performed if the infant has risk factors or signs of polycythemia or anemia.
Leukocytes
The leukocyte (white blood cell [WBC]) count at birth is 9,100 to 34,000/mm3 (Lo, 2011). The WBC count falls to an average of 12,000/mm3 by 4 to 5 days after birth (Blackburn, 2013). In newborns, an elevated WBC (leukocyte) count does not necessarily indicate infection. In fact, the WBC count may decrease in sepsis (Lott, 2010). Increased numbers of immature leukocytes are a sign of infection or sepsis. Platelets (thrombocytes) may decrease as a result of infections.
Risk of Clotting Deficiency
Newborns have low levels of vitamin K, which is necessary to activate several of the clotting factors (factors II [prothrombin], VII, IX, and X). Vitamin K is synthesized in the intestines, but food and normal intestinal flora are necessary for this process (Luchtman-Jones & Wilson, 2011). To decrease the risk of hemorrhagic disease of the newborn, vitamin K is administered intramuscularly to most newborns. Drugs such as phenytoin (Dilantin), phenobarbital, and antituberculosis drugs taken by the mother during pregnancy interfere with clotting ability in the infant after birth.
Although the platelet counts in term newborns are near adult levels, platelet response to stimuli is decreased during the first few days of life.
Gastrointestinal System
Stomach
The newborn’s stomach capacity is approximately 6 mL/kg at birth. Gastric emptying may be delayed at first. It is more rapid after ingestion of human milk than after formula and slower if the infant has swallowed mucus (Blackburn, 2013). The gastrocolic reflex is stimulated when the stomach fills, causing increased intestinal peristalsis. Infants frequently pass a stool during or after a feeding. The cardiac sphincter between the esophagus and the stomach is relaxed, which explains the tendency to regurgitate feedings easily.
Intestines
The newborn’s intestines are long in proportion to the infant’s size and compared with those of the adult. The added length allows more surface area for absorption, but it also makes infants more prone to water loss should diarrhea develop. Air enters the gastrointestinal tract soon after birth, and bowel sounds are present within the first hour.
The digestive tract is sterile at birth. Once the infant is exposed to the external environment and begins to take in fluids, bacteria enter the gastrointestinal tract. Normal intestinal flora are established within the first few days of life.
Digestive Enzymes
Maturation of the ability to digest and absorb occurs at different rates for various nutrients. Pancreatic amylase, needed to digest complex carbohydrates is deficient for the first 4 to 6 months after birth (Blackburn, 2013). As a result, newborn digestion of complex carbohydrates such as those in cereals is limited. Amylase is also produced by the salivary glands, but in low amounts until about the third month of life. Amylase is present in breast milk.
The newborn is also deficient in pancreatic lipase, limiting fat absorption significantly. Lipase present in the mouth and stomach helps with some digestion of fat. Lipase is present in breast milk, which may make it more digestible for the newborn than formula. Protein and lactose, the major carbohydrate in the infant’s milk diet, are both well digested.
Stools
Meconium is the first stool excreted by the newborn. It consists of particles from amniotic fluid such as vernix, skin cells, and hair, along with cells shed from the intestinal tract, bile, and other intestinal secretions. Meconium is greenish black with a thick, sticky, tarlike consistency. The first meconium stool is usually passed within 12 hours of birth, and 99% of newborns have the first stool within 48 hours (Carlo, 2011b). If meconium is not passed within that time, obstruction is suspected.
Meconium stools are followed by transitional stools, a combination of meconium and milk stools. Transitional stools are greenish brown and of a looser consistency than meconium. They are followed by milk stools characteristic of the type of feeding the infant receives.
The stools of infants fed with breast milk are seedy, and the color and consistency of mustard with a sweet-sour smell. The breastfed infant generally has more frequent stools than the infant who is formula fed. A stool may be passed with each feeding. Some older infants pass only one stool every 2 to 3 days. The normal breastfed newborn should have at least four or more stools daily (Lawrence & Lawrence, 2011).
The formula-fed infant excretes pale yellow to light brown stools. They are firmer in consistency than those of the breastfed infant. The infant may excrete several stools daily, or only one or two. The stools have the characteristic odor of feces.
Hepatic System
Important liver functions include maintenance of blood glucose levels, conjugation of bilirubin, production of factors necessary for blood coagulation, storage of iron, and metabolism of drugs.
Blood Glucose Maintenance
During the last trimester, glucose is stored in the fetal liver as glycogen for use after birth. Glucose is used rapidly by the newborn for energy during the stress of delivery and for breathing, heat production, movement against gravity, and activation of all the functions that the neonate must take on at birth.
Until newborn feedings are adequate to meet energy requirements, glucose present in the body is used, and stored glycogen is converted by the liver to glucose for use. In the term infant, glucose levels should be 40 to 60 mg/dL at day 1 and 50 to 90 mg/dL thereafter (Lo, 2011). There is no general consensus about glucose level that defines hypoglycemia, but a level less than 40 to 45 mg/dL in the term infant is often used as the lower limit of normal plasma glucose (Kubicka & Little, 2009; McGowan, Rozance, Price-Douglas, et al, 2011).
Many newborns are at increased risk for hypoglycemia. In the preterm, late preterm (born between 34 weeks and 366⁄7 weeks of gestation), and small-for-gestational-age infant, adequate stores of glycogen may not have accumulated. Stores may be used up before birth in the postterm infant because of poor intrauterine nourishment from a deteriorating placenta. Large-for-gestational-age infants and those with diabetic mothers may produce excessive insulin that consumes available glucose quickly (see Chapters 29 and 30). Infants exposed to such stressors as asphyxia or infection may exhaust their stores of glycogen. Cold-stressed infants may deplete glycogen to increase metabolism and raise body temperature.
Conjugation of Bilirubin
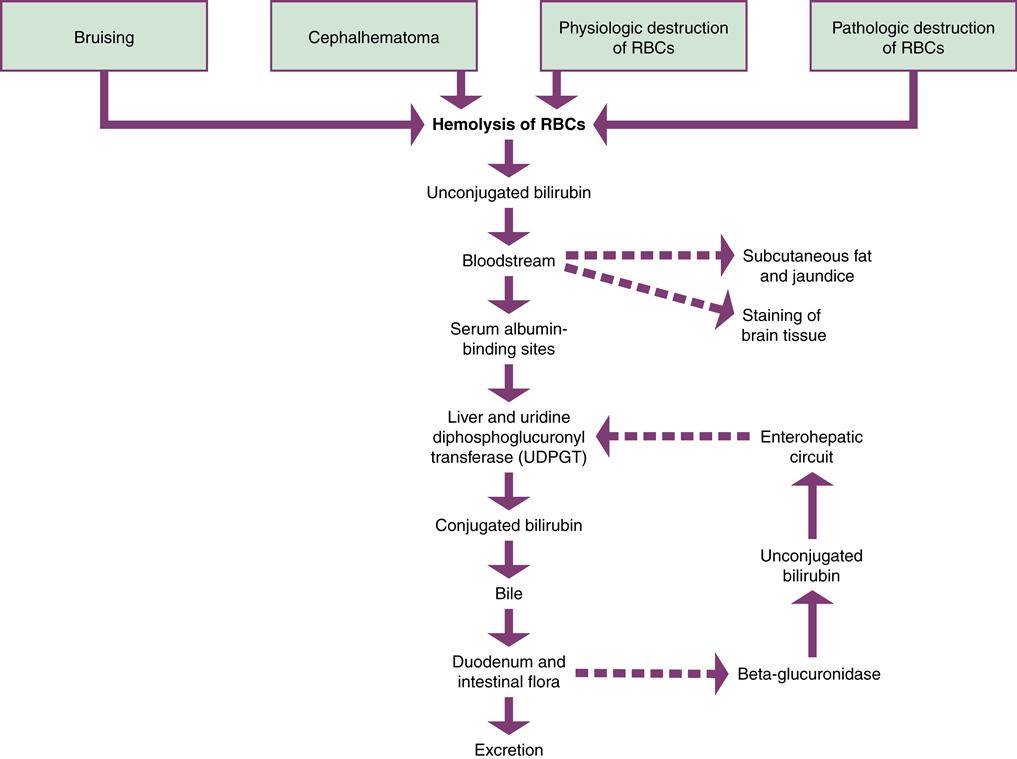
A major function of the liver is the conjugation of bilirubin (Figure 21-5). The newborn’s liver may not be mature enough to prevent jaundice during the first week of life. Jaundice results from hyperbilirubinemia, excessive bilirubin in the blood. Jaundice (or icterus) occurs in 60% of term newborns and 80% of preterm infants (Ambalavanan & Carlo, 2011).
Source and Effect of Bilirubin
The principal source of bilirubin is the hemolysis of erythrocytes. This is a normal occurrence after birth, when fewer erythrocytes are needed than during fetal life. Bilirubin is toxic to the body and must be excreted.
Bilirubin is released in an unconjugated form. Unconjugated bilirubin, also called indirect bilirubin, is not soluble in water. Before excretion can occur, the liver must change it to a water-soluble form by a process called conjugation. The bilirubin is then known as conjugated or direct bilirubin. Conjugated bilirubin is not toxic to the body and can be excreted.
Unconjugated bilirubin is fat soluble and may be absorbed by the subcutaneous fat, causing the yellowish discoloration of the skin called jaundice. If enough unconjugated bilirubin accumulates in the blood, staining of the tissues in the brain may occur. This may cause acute bilirubin encephalopathy, a neurologic condition resulting from bilirubin toxicity. If this condition becomes chronic, it is permanent neurologic injury known as kernicterus. The level of bilirubin necessary to cause injury to the central nervous system is unknown and may be different for various infants.
Normal Conjugation
When unconjugated bilirubin is released into the bloodstream, it attaches to binding sites on albumin in the plasma and is carried to the liver. If there are not enough albumin-binding sites, bilirubin circulates as unbound or free unconjugated bilirubin. Bilirubin can be displaced from albumin by some medications. Free fatty acids, acidosis, and infection also decrease albumin binding of bilirubin (Kamuth, Thilo, & Hernandez, 2011).
When the albumin-bound bilirubin reaches the liver it is changed to the conjugated form of bilirubin by the enzyme uridine diphosphate glucuronosyltransferase (UDPGT). Conjugated bilirubin is excreted into the bile and then into the duodenum. In the intestines, the normal flora acts on bilirubin to reduce it to urobilinogen and stercobilin, which are excreted in the stool. A small amount of urobilinogen is excreted by the kidneys.
A small percentage of conjugated bilirubin may be deconjugated or converted back to the unconjugated state by the intestinal enzyme beta-glucuronidase. This enzyme is important in fetal life because only unconjugated bilirubin can be cleared by the placenta for conjugation by the mother’s liver. In the newborn, deconjugated bilirubin in the intestines is absorbed into the portal circulation and carried back to the liver, where it again undergoes the conjugation process. This recirculation of bilirubin is called the enterohepatic circuit, and it creates additional work for the liver.
Blood tests for bilirubin measure total serum bilirubin (TSB) and direct (conjugated) bilirubin in the serum. TSB is a combination of indirect (unconjugated) and direct bilirubin.
Risk Factors for Elevated Bilirubin
Factors that increase the risk for jaundice in the newborn include:
• Excess production: Twice as much bilirubin is produced in newborns as in adults. The rate of production remains higher in relation to their size for 3 to 6 weeks (Blackburn, 2013). Polycythemia increases RBC breakdown even more.
• Red blood cell life: Fetal RBCs break down more quickly than do adult erythrocytes.
• Blood incompatibility: Rh, ABO, or others
• Gestation: Preterm and late preterm infants have immature conjugation abilities.
Hyperbilirubinemia
Physiologic Jaundice
Physiologic jaundice is also called nonpathologic or developmental jaundice. It is caused by transient hyperbilirubinemia and is considered normal. It is not present during the first 24 hours of life in term infants but appears on the second or third day after birth. Jaundice becomes visible when the serum bilirubin is 5 mg/dL to 6 mg/dL (Blackburn, 2013).
Cord blood has an indirect bilirubin level of 1 to 3 mg/dL. In physiologic jaundice, the bilirubin peaks at 5 to 6 mg/dL between the second and fourth days of life. The bilirubin then begins to fall, declining to less than 2 mg/dL by 5 to 7 days (Ambalavanan & Carlo, 2011). Bilirubin normally rises higher and falls more slowly in Asian infants.
Nonphysiologic (Pathologic) Jaundice
Jaundice that is physiologic or normal must be differentiated from nonphysiologic or pathologic jaundice that requires further investigation. One of the most important differences is the time at which jaundice appears as pathologic jaundice may occur during the first 24 hours. Bilirubin that rises more rapidly and to higher levels than is expected or stays elevated for longer than normal is more likely to lead to severe hyperbilirubinemia and may need earlier treatment.
Nonphysiologic jaundice is the result of abnormalities causing excessive destruction of RBCs or problems in bilirubin conjugation. These include incompatibilities between the mother’s and infant’s blood types (see Chapter 25, p. 601), infection, and metabolic disorders. Nonphysiologic jaundice is often treated with phototherapy (discussed in Chapter 30, p. 721).
Charts are available that show the rise and fall of bilirubin and the degree of risk for various levels of TSB according to the age of the infant in hours. For example, a full-term infant with no complications who is 24 hours old is considered at low risk if the TSB is 5 mg/dL or less and at high risk if the TSB is greater than 8 mg/dL. At 48 hours of age, that infant would be low risk if the TSB were 8.5 mg/dL but high risk if the TSB were that high before 48 hours. Infants who are preterm, late preterm, or who have other risk factors may receive treatment for hyperbilirubinemia at lower TSB levels than full-term infants.
Jaundice Associated with Breastfeeding
The breastfed infant has a higher risk of developing jaundice, which may begin early or late after birth.
Breastfeeding or Early Onset Jaundice
Bilirubin levels greater than 12 mg/dL develop in 13% of breastfed infants by 1 week of age (Ambalavanan & Carlo, 2011). The most common cause of jaundice in breastfed infants is insufficient intake. Jaundice begins within the first week of life, and serum bilirubin may rise above 12 mg/dL and reach dangerous levels if intake is not increased.
Infants who are sleepy, have a poor suck, or nurse infrequently may not receive enough colostrum, the substance that precedes true breast milk, to benefit from its normal laxative effect in eliminating bilirubin-rich meconium. Lack of adequate suckling depresses production of breast milk and increases the problem further. Helping the mother with breastfeeding to stimulate milk production and increase the infant’s intake is essential. Supplementing with formula interferes with milk production. If breastfeeding is not adequate, weight loss is excessive, or the infant is dehydrated, supplementing with expressed breast milk or formula may be necessary. Glucose water will not reduce bilirubin levels and should be avoided.
True Breast Milk Jaundice
True breast milk jaundice, also called late-onset breast milk jaundice, occurs after the first 3 to 5 days of life. It lasts 3 weeks to as long as 3 months for some infants. The TSB usually peaks at 5 to 10 mg/dL and falls gradually over several months. Some infants reach levels of 20 to 30 mg/dL (Kaplan et al., 2011). The exact cause of true breast milk jaundice is unknown. Substances in the breast milk may increase absorption of bilirubin from the intestine or interfere with conjugation. This may be a form of physiologic jaundice in breastfed infants. Infants have no signs of illness.
Treatment of breast milk jaundice includes close monitoring of TSB and at least 8 to 12 feedings each 24 hours. If bilirubin levels rise too high, phototherapy is begun while the mother continues frequent breastfeeding. Interruption of breastfeeding is not generally recommended. However, if the TSB levels are dangerously high, the health care provider may order formula feeding be given for 1 to 3 days while the mother uses a breast pump to maintain milk supply. Formula supplementation may be used instead. These measures should cause a rapid drop in bilirubin. If the level rises while breastfeeding is interrupted, jaundice from another cause should be investigated. The TSB may rise again when breastfeeding resumes, but usually not high enough to interfere with further breastfeeding.
Blood Coagulation
Prothrombin and coagulation factors II, VII, IX, and X are produced by the liver and activated by vitamin K, which is deficient in the newborn (see p. 473).
Iron Storage
Iron is stored in the fetal liver and spleen during the last months of pregnancy. Full-term infants who are breastfeeding usually do not need added iron until 4 to 6 months of age. At that time, they should begin iron-containing foods or iron supplements. All infants who are not breastfeeding should be given iron-fortified formula (American Academy of Pediatrics [AAP] & American College of Obstetricians and Gynecologists [ACOG], 2007; Holt, Wooldridge, Story, et al., 2011).
Drug Metabolism
The liver metabolizes drugs inefficiently in the newborn. Breastfeeding mothers should alert their physician or lactation consultant before taking medications because harmful amounts of some drugs may be transferred to the infant through the breast milk.
Urinary System
Kidney Development
The kidney’s nephrons are formed by 34 to 36 weeks of gestation (Frost, Fashaw, Hernandez, et al., 2011). Full kidney function, however, does not occur until after birth. Blood flow to the kidneys increases after birth because of decreased resistance in the renal vessels. The improved perfusion results in a steady improvement in kidney function during the first few days of life.
Kidney Function
The newborn’s kidney function is immature compared with that of the adult. The ability of the glomeruli to filter and the renal tubules to reabsorb is considerably less than in adults. The glomerular filtration rate doubles or triples during the first weeks after full term birth, but does not reach adult levels until 1 to 2 years of age (Frost et al., 2011). Therefore infants have a decreased ability to remove waste products from the blood.
Small amounts of substances such as glucose and amino acids may escape into the urine of the neonate (Blackburn, 2013; Frost et al., 2011). Uric acid crystals may give a reddish color to the urine that is sometimes mistaken for blood.
Voiding occurs within 12 hours for 50% of newborns, 92% void within 24 hours, and 99% void within 48 hours of life (Frost et al., 2011). Absence of kidneys or abnormalities that interfere with excretion of urine are usually discovered before birth because they cause low amniotic fluid volume. Only one or two voidings may occur during the first 2 days of life. The infant voids at least six times a day by the fourth day.
Fluid Balance
Newborns have a lower tolerance for changes in total volume of body fluid than do older infants. In addition, the fluid turnover rate is greater than that in adults (Box 21-2). To maintain fluid balance, full-term infants need 60 to 100 mL/kg (27 to 45 mL/lb) daily during the first 3 to 5 days of life and 150 to 175 mL/kg (68 to 80 mL/lb) a day by 7 days of age (Halbardier, 2010).
Water Distribution
Seventy-five percent of the newborn’s body is composed of water (Jones, Hayes, Starbuck, et al., 2011). Because infants have more fluid for their size than adults and because a larger proportion of it is located outside the cells, total body water is easily depleted. Conditions such as vomiting and diarrhea can quickly result in life-threatening dehydration. At birth, normal diuresis causes a 5% to 10% weight loss as excess extracellular water is lost (Halbardier, 2010; Jones et al., 2011).
Insensible Water Loss
Water lost from the skin and respiratory tract contributes to insensible water loss. The newborn’s large body surface area and rapid respiratory rate cause increased insensible water loss. Fluid losses increase greatly when infants are placed under radiant warmers or phototherapy lights, which accelerate evaporation from the skin. An elevated respiratory rate or low humidity in the air surrounding the infant raises insensible water losses even further.
Urine Dilution and Concentration
The ability of a newborn’s kidneys to dilute urine is similar to that of adults, but they have only half the adult’s ability to concentrate urine (Blackburn, 2013). Therefore, a newborn’s kidneys cannot handle large increases in fluids, which result in fluid overload. This is most likely to happen if infants receive too much intravenous fluid. When abnormal conditions such as diarrhea cause excessive loss of fluid, the newborn’s limited ability to conserve water may result in dehydration more quickly than in the older infant or child. Normal newborn urine specific gravity is 1.002 to 1.01, and normal urine output is 2 to 5 mL/kg/hr (Jones et al., 2011).
Acid-Base and Electrolyte Balance
The maintenance of acid-base and electrolyte balance is a primary function of the kidneys and may be precarious in neonates. Newborns’ tendency to lose bicarbonate at lower levels than adults and decreased ability to reabsorb it increases their risk for metabolic acidosis. The excretion of solutes is less efficient in newborns as well. Although newborns conserve needed sodium well, they are limited in excretion of sodium, especially if they receive excessive amounts.
Immune System
The neonate is less effective in fighting off infection than the older infant or child. Leukocytes are delayed in moving to the site of invasion and are inefficient in destroying the invader. The infant’s decreased ability to localize infection leads to a tendency toward sepsis.
Fever and leukocytosis, which occur during infection in the older child, are often not present in the newborn with infection. This lack of response is the result of immaturity of the hypothalamus and the inflammatory response. Signs of infection in the neonate are nonspecific including subtle changes in activity, tone, color, or feeding.
Because of their immature immune system, infants are susceptible to pathogens that do not usually affect older children. Full-term newborns received antibodies from the mother during the last trimester of pregnancy. If the mother breastfeeds, the infant continues to receive antibodies in breast milk that provide passive immunity. Immunoglobulins (serum globulins with antibody activity) help protect the newborn from infection. The major immunoglobulins are IgG, IgM, and IgA.
IgG
IgG, the only immunoglobulin that crosses the placenta, provides the fetus with passive temporary immunity to bacteria, bacterial toxins, and viruses to which the mother has immunity. Preterm infants have less IgG because transfer is greatest during the third trimester. Although the fetus makes some IgG, production at significant levels is delayed until after 6 months of age (Blackburn, 2013). The passive immunity from the mother gradually disappears over the first 6 to 8 months of life (Buckley, 2011).
IgM
IgM helps protect against gram-negative bacteria. Production increases rapidly a few days after birth as the infant is exposed to environmental antigens. IgM reaches adult levels at about 1 year of age (Buckley, 2011). If IgM is found in larger-than-normal amounts in the neonate, exposure to infection in utero is probable because IgM does not cross the placenta.
IgA
IgA also does not cross the placenta and must be produced by the infant. Because IgA is important in protection of the gastrointestinal and respiratory systems, newborns are particularly susceptible to infections of those systems. The immunoglobulin is produced beginning about 2 weeks of age. Secretory IgA is included in colostrum and breast milk (Kapur, Yoder, & Polin, 2011). Therefore breastfed infants may receive protection that formula-fed infants do not.
Psychosocial Adaptation
Periods of Reactivity
In the early hours after birth, the infant goes through changes called periods of reactivity. The two periods of reactivity are separated by a period of sleep or decreased activity (Gardner & Hernandez, 2011).
First Period of Reactivity
The first period of reactivity begins at birth. Infants are wide awake, alert, and seem interested in their surroundings. Parents enjoy this phase, as the infant gazes directly at them when held in the en face (face-to-face) position. Infants move their arms and legs energetically, root, and appear hungry. If allowed to nurse, many infants latch on to the nipple and suck well.
The temperature may be decreased and heart rate may be elevated to 180 beats per minute (bpm). Respirations may be as high as 80 breaths per minute. Rales, retractions, nasal flaring, and increased mucous secretions may be present. The pulse and respirations gradually slow, and the infant becomes sleepy.
Period of Sleep or Decreased Activity
After the first period of reactivity, infants fall into a deep sleep or have much decreased activity. During this time, the pulse and respirations drop to the normal range.
Second Period of Reactivity
During the second period of reactivity, infants become interested in feeding and may pass meconium. The pulse and respiratory rates may increase, mucous secretions increase, and infants may gag or regurgitate.
Behavioral States
Six gradations in the infant’s behavioral state, ranging from quiet sleep to crying, have been identified.
Deep or Quiet Sleep State
In the deep or quiet sleep state, the infant has no eye movements. Respirations are quiet, regular, and slower than in the other states. Although startles occur at intervals, the infant’s body is quiet. Little or no response to noise or stimuli occurs, and the infant is difficult to arouse.
Light or Active Sleep State
In the light or active sleep state, infants move their extremities, stretch, change facial expressions, make sucking movements, and may fuss briefly. During this period, respirations tend to be more rapid and irregular, and rapid eye movements (REMs) occur. Infants are more likely to startle from noise or disturbances and may return to sleep or move to an awake state.
Drowsy State
The drowsy state is a transitional period between sleep and waking. The eyes may remain closed or, if open, appear glazed and unfocused. Infants startle and move their extremities slowly. They may go back to sleep or, with gentle stimulation, gradually awaken.
Quiet Alert State
The quiet alert state should be pointed out to parents because it is an excellent time to increase bonding. Infants focus on objects or people and seem bright and interested in their surroundings. They respond to stimuli and interaction with others. Body movements are minimal as infants seem to concentrate on the environment. Full-term infants often are in this state shortly after birth.
Active Alert State
In the active alert state, infants are often fussy. They seem restless, have faster and more irregular respirations, may hiccup or regurgitate, and seem more aware of feelings of discomfort from hunger or cold. Although their eyes may be open, infants seem less focused on visual stimuli than during the quiet alert state.
Crying State
The crying state may quickly follow the active alert state if no intervention occurs to comfort the infant. The cries are continuous and lusty, and the infant does not respond positively to stimulation. It may take a period of comforting to move the infant to a state in which feeding or other activities can be accomplished.
Early Assessments
Immediately after birth the infant is examined quickly for cardiorespiratory problems and obvious anomalies. The nurse determines whether resuscitation (see Chapter 30) or other immediate intervention is necessary. When the infant is stable and oxygenating well, a more thorough assessment can be performed. Table 21-2 on pp. 479-483 summarizes newborn assessments.
TABLE 21-2
| NORMAL | ABNORMAL (POSSIBLE CAUSES) | NURSING CONSIDERATIONS |
| Initial Assessment | ||
| Assess for obvious problems first. If infant is stable and has no problems that require immediate attention, continue with complete assessment. | ||
| Vital Signs | ||
| Temperature | ||
| Axillary: 36.5° C-37.5° C (97.7° F-99.599.1° F). Axilla is preferred site. | Decreased (cold environment, hypoglycemia, infection, CNS problem). Increased (infection, environment too warm). | Decreased: Institute warming measures and check in 30 min. Check blood glucose. Increased: Remove excessive clothing. Check for dehydration. Decreased or increased: Look for signs of infection. Check radiant warmer or incubator temperature setting. Check thermometer for accuracy if skin is warm or cool to touch. Report abnormal temperatures to physician. |
| Pulses | ||
| Heart rate 120-160 bpm (100 sleeping, 180 crying). Rhythm regular. PMI at third to fourth intercostal space lateral to the midclavicular line. Brachial, femoral, and pedal pulses present and equal bilaterally. | Tachycardia (respiratory problems, anemia, infection, cardiac conditions). Bradycardia (asphyxia, increased intracranial pressure). PMI to right (dextrocardia, pneumothorax). Murmurs (normal or congenital heart defects). Dysrhythmias. Absent or unequal pulses (coarctation of the aorta). | Note location of murmurs. Refer abnormal rates, rhythms and sounds, pulses. |
| Respirations | ||
| Rate 30-60 (average 40-49) breaths per min. Respirations irregular, shallow, unlabored. Chest movements symmetric. Breath sounds present and clear bilaterally. | Tachypnea, especially after the first hour (respiratory distress) Slow respirations (maternal medications). Nasal flaring (respiratory distress) Grunting (respiratory distress syndrome). Gasping (respiratory depression). Periods of apnea more than 20 sec or with change in heart rate or color (respiratory depression, sepsis, cold stress). Asymmetry or decreased chest expansion (pneumothorax). Intercostal, xiphoid, or supraclavicular retractions or seesaw (paradoxical) respirations (respiratory distress). Moist, coarse breath sounds (crackles, rhonchi) (fluid in lungs). Bowel sounds in chest (diaphragmatic hernia). | Mild variations require continued monitoring and usually clear in early hours after birth. If persistent or more than mild, suction, give oxygen, call physician, and initiate more intensive care. |
| Blood Pressure | ||
| Varies with age, weight, activity, and gestational age. Average systolic 65-95 mm Hg, average diastolic 30-60 mm Hg. | Hypotension (hypovolemia, shock, sepsis). BP 20 mm Hg or more higher in arms than legs (coarctation of the aorta). | Refer abnormal blood pressures. Prepare for intensive care if very low. |
| Measurements | ||
| Weight | ||
| Weight 2500-4000 g (5 lb, 8 oz to 8 lb, 13 oz). Weight loss up to 10% in early days. | High (LGA, maternal diabetes). Low (SGA, preterm, multifetal pregnancy, medical conditions in mother that affected fetal growth). Weight loss above 10% (dehydration, feeding problems). | Determine cause. Monitor for complications common to cause. |
| Length | ||
| 48-53 cm (19-21 in). | Below normal (SGA, congenital dwarfism). Above normal (LGA, maternal diabetes). | Determine cause. Monitor for complications common to cause. |
| Head Circumference | ||
| 32-38 cm (12.5-15 in). Head and neck are approximately one fourth of infant’s body surface. | Small (SGA, microcephaly, anencephaly). Large (LGA, hydrocephalus, increased intracranial pressure). | Determine cause. Monitor for complications common to cause. |
| Chest Circumference | ||
| 30-36 cm (12-14 in). Is 2 cm less than head circumference. | Large (LGA). Small (SGA). | Determine cause. Monitor for complications common to cause. |
| Posture | ||
| Flexed extremities move freely, resist extension, return quickly to flexed state. Hands usually clenched. Movements symmetric. Slight tremors on crying. Breech: extended, stiff legs. “Molds” body to caretaker’s body when held, responds by quieting when needs met. | Limp, flaccid, “floppy,” or rigid extremities (preterm, hypoxia, medications, CNS trauma). Hypertonic (neonatal abstinence syndrome, CNS injury). Jitteriness or tremors (low glucose or calcium level). Opisthotonos, seizures, stiff when held (CNS injury). | Seek cause, refer abnormalities. |
| Cry | ||
| Lusty, strong. | High pitched (increased intracranial pressure). Weak, absent, irritable, catlike “mewing” (neurologic problems). Hoarse or crowing (laryngeal irritation). | Observe for changes, report abnormalities. |
| Skin | ||
| Color pink or tan with acrocyanosis. Vernix caseosa in creases. Small amounts of lanugo over shoulders, sides of face, forehead, upper back. Skin turgor good with quick recoil. Some cracking and peeling of skin. Normal variations: Milia. Skin tags. Erythema toxicum (“flea bite” rash). Puncture on scalp (from electrode). Mongolian spots. | Color: Cyanosis of mouth and central areas (hypoxia). Facial bruising (nuchal cord). Pallor (anemia, hypoxia). Gray (hypoxia, hypotension). Red, sticky, transparent skin (very preterm). Ruddy (polycythemia). Greenish brown discoloration of skin, nails, cord (possible fetal compromise, postterm). Harlequin color (normal transient autonomic imbalance). Mottling (normal or cold stress, hypovolemia, sepsis). Jaundice (pathologic if first 24 hr). Yellow vernix (blood incompatibilities). Thick vernix (preterm). Delivery marks: Bruises on body (pressure), scalp (vacuum extractor), or face (cord around neck). Petechiae (pressure, low platelet count, infection). Forceps marks. Birthmarks: Mongolian spots. Nevus simplex (salmon patch, “stork bite” ). Nevus flammeus (port-wine stain). Nevus vasculosus (strawberry hemangioma). Café au lait spots (6 or more) larger than 0.5 cm in size (neurofibromatosis). Other: Excessive lanugo (preterm). Excessive peeling, cracking (postterm). Pustules or other rashes (infection). “Tenting” of skin (dehydration). | Differentiate facial bruising from cyanosis. Central cyanosis requires suction, oxygen, and further treatment. Refer jaundice in first 24 hr or more extensive than expected for age. Watch for respiratory problems in infants with meconium staining. Look for signs and complications of preterm or postterm birth. Record location, size, shape, color, type of rashes and marks. Differentiate mongolian spots from bruises. Check for facial movement with forceps marks. Watch for jaundice with bruising. Point out and explain normal skin variations to parents. |
| Head | ||
| Sutures palpable with small separation between each. Anterior fontanel diamond-shaped, 4-5 cm, soft, and flat. May bulge slightly with crying. Posterior fontanel triangular, 0.5-1 cm. Hair silky and soft with individual hair strands. Normal variations: Overriding sutures (molding). Caput succedaneum or cephalhematoma (pressure during birth). | Head large (hydrocephalus, increased intracranial pressure) or small (microcephaly). Widely separated sutures (hydrocephalus) or hard, ridged area at sutures (craniosynostosis). Anterior fontanel depressed (dehydration, molding), full or bulging at rest (increased intracranial pressure). Woolly, bunchy hair (preterm). Unusual hair growth (genetic abnormalities). | Seek cause of variations. Observe for signs of dehydration with depressed fontanel; increased intracranial pressure with bulging of fontanel and wide separation of sutures. Refer for treatment. Differentiate caput succedaneum from cephalhematoma, and reassure parents of normal outcome. Observe for jaundice with cephalhematoma. |
| Ears | ||
| Ears well formed and complete. Area where upper ear meets head even with imaginary line drawn from outer canthus of eye. Startle response to loud noises. Alerts to high-pitched voices. | Low-set ears (chromosomal disorders). Skin tags, preauricular sinuses, dimples (may be associated with kidney or other abnormalities). No response to sound (deafness). | Check voiding if ears abnormal. Look for signs of chromosomal abnormality if position abnormal. Refer for evaluation if no response to sound. |
| Face | ||
| Symmetric in appearance and movement. Parts proportional and appropriately placed. | Asymmetry (pressure and position in utero). Drooping of mouth or one side of face, “one-sided cry” (facial nerve injury). Abnormal appearance (chromosomal abnormalities). | Seek cause of variations. Check delivery history for possible cause of injury to facial nerve. |
| Eyes | ||
| Symmetric. Eyes clear. Transient strabismus. Scant or absent tears. Pupils equal, react to light. Alerts to interesting sights. Doll’s-eye sign, red reflex present. May have subconjunctival hemorrhage or edema of eyelids from pressure during birth. | Inflammation or drainage (chemical or infectious conjunctivitis). Constant tearing (plugged lacrimal duct). Unequal pupils. Failure to follow objects (blindness). White areas over pupils (cataracts). Setting-sun sign (hydrocephalus). Yellow sclera (jaundice). Blue sclera (osteogenesis imperfecta). | Clean and monitor any drainage; seek cause. Reassure parents that subconjunctival hemorrhage and edema will clear. Refer other abnormalities. |
| Nose | ||
| Both nostrils open to air flow. May have slight flattening from pressure during birth. | Blockage of one or both nasal passages (choanal atresia). Malformations (congenital conditions). Flaring, mucus (respiratory distress). | Observe for respiratory distress. Report malformations. |
| Mouth | ||
| Mouth, gums, tongue pink. Tongue normal in size and movement. Lips and palate intact. Sucking pads. Sucking, rooting, swallowing, gag reflexes present. Normal variations: Precocious teeth, Epstein’s pearls. | Cyanosis (hypoxia). White patches on cheeks or tongue (candidiasis). Protruding tongue (Down syndrome). Diminished movement of tongue, drooping mouth (facial nerve paralysis). Cleft lip or palate, or both. Absent or weak reflexes (preterm, neurologic problem). Excessive drooling (tracheoesophageal fistula, esophageal atresia). | Oxygen for cyanosis. Expect loose teeth to be removed. Obtain order for antifungal medication for candidiasis. Check mother for vaginal or breast infection. Refer anomalies. |
| Feeding | ||
| Good suck/swallow coordination. Retains feedings. | Poorly coordinated suck and swallow (prematurity). Duskiness or cyanosis during feeding (cardiac defects). Choking, gagging, excessive drooling (tracheoesophageal fistula, esophageal atresia). | Feed slowly. Stop frequently if difficulty occurs. Suction and stimulate if necessary. Refer infants with continued difficulty. |
| Neck/Clavicles | ||
| Short neck turns head easily side to side. Infant raises head when prone. Clavicles intact. | Weakness, contractures, or rigidity (muscle abnormalities). Webbing of neck, large fat pad at back of neck (chromosomal disorders). Crepitus, lump, or crying when clavicle or other bones palpated, diminished or absent arm movement (fractures). | Fracture of clavicle more frequent in large infants with shoulder dystocia at birth. Immobilize arm. Look for other injuries. Refer abnormalities. |
| Chest | ||
| Cylinder shape. Xiphoid process may be prominent. Symmetric. Nipples present and located properly. May have engorgement, white nipple discharge (maternal hormone withdrawal). | Asymmetry (diaphragmatic hernia, pneumothorax). Supernumerary nipples. Redness (infection). | Report abnormalities. |
| Abdomen | ||
| Rounded, soft. Bowel sounds present within first hour after birth. Liver palpable 1-2 cm below right costal margin. Skin intact. Three vessels in cord. Clamp tight and cord drying. Meconium passed within 12-48 hr. Urine generally passed within 12-24 hr. Normal variation: “Brick dust” staining of diaper (uric acid crystals). | Sunken abdomen (diaphragmatic hernia). Distended abdomen or loops of bowel visible (obstruction, infection, enlarged organs). Absent bowel sounds after first hour (paralytic ileus). Masses palpated (kidney tumors, distended bladder). Enlarged liver (infection, heart failure, hemolytic disease). Abdominal wall defects (umbilical or inguinal hernia, omphalocele, gastroschisis, exstrophy of bladder). Two vessels in cord (other anomalies). Bleeding (loose clamp). Redness, drainage from cord (infection). No passage of meconium (imperforate anus, obstruction). Lack of urinary output (kidney anomalies) or inadequate amounts (dehydration). | Refer abnormalities. Assess for other anomalies if only two vessels in cord. Tighten or replace loose cord clamp. If stool and urine output abnormal, look for missed recording, increase feedings, report. |
| Genitals | ||
| Female | ||
| Labia majora dark, cover clitoris and labia minora. Small amount of white mucous vaginal discharge. Urinary meatus and vagina present. Normal variations: Vaginal bleeding (pseudomenstruation). Hymenal tags. | Clitoris and labia minora larger than labia majora (preterm). Large clitoris (ambiguous genitalia). Edematous labia (breech birth). | Check gestational age for immature genitalia. Refer anomalies. |
| Male | ||
| Testes within scrotal sac, rugae on scrotum, prepuce nonretractable. Meatus at tip of penis. | Testes in inguinal canal or abdomen (preterm, cryptorchidism). Lack of rugae on scrotum (preterm). Edema of scrotum (pressure in breech birth). Enlarged scrotal sac (hydrocele). Small penis, scrotum (preterm, ambiguous genitalia). Empty scrotal sac (cryptorchidism) Urinary meatus located on upper side of penis (epispadias), underside of penis (hypospadias), or perineum. Ventral curvature of the penis (chordee). | Check gestational age for immature genitalia. Refer anomalies. Explain to parents why no circumcision can be performed with abnormal placement of meatus. |
| Extremities | ||
| Upper and Lower Extremities | ||
| Equal and bilateral movement of extremities. Correct number and formation of fingers and toes. Nails to ends of digits or slightly beyond. Flexion, good muscle tone. | Crepitus, redness, lumps, swelling (fracture). Diminished or absent movement, especially during Moro reflex (fracture, nerve injury, paralysis). Polydactyly (extra digits). Syndactyly (webbing). Fused or absent digits. Poor muscle tone (preterm, neurologic injury, hypoglycemia, hypoxia). | Refer all anomalies, look for others. |
| Upper Extremities | ||
| Two transverse palm creases. | Simian crease (normal or Down syndrome). Diminished movement (injury). Diminished movement of arm with extension and forearm prone (Erb-Duchenne paralysis). | Refer all anomalies, look for others. |
| Lower Extremities | ||
| Legs equal in length, abduct equally, gluteal and thigh creases and knee height equal, no hip “clunk.” Normal position of feet. | Ortolani and Barlow tests abnormal, unequal leg length, unequal thigh or gluteal creases (developmental dysplasia of the hip). Malposition of feet (position in utero, talipes equinovarus). | Refer all anomalies, look for others. Check malpositioned feet to see if they can be gently manipulated back to normal position. |
| Back | ||
| No openings observed or felt in vertebral column. Anus patent. Sphincter tightly closed. | Failure of one or more vertebrae to close (spina bifida), with or without sac with spinal fluid and meninges (meningocele) or spinal fluid, meninges, and cord (myelomeningocele) enclosed. Tuft of hair over spina bifida occulta. Pilonidal dimple or sinus. Imperforate anus. | Refer abnormalities. Observe for movement below level of defect. If sac, cover with sterile dressing wet with sterile saline. Protect from injury. |
| Reflexes | ||
| See Table 21-3. | Absent, asymmetric, or weak reflexes. | Observe for signs of fractures, nerve injury, or injury to CNS. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree