Chapter 7
The Eye1
Who would believe that so small a space could contain the images of all the universe? O mighty process!
Leonardo da Vinci (1452–1519)
Historical Considerations
The eyes are the windows to the world. Most of the sensory input to the brain comes from the eyes. For centuries, the eye has been considered the essence of the person, representing the “I.” In mythology and the writings of ancient times, the eye is an organ associated with mystical powers.
The eye has long been associated with mythical gods. In ancient Egypt, the eye was the symbol of the Great Mother Goddess Maat, goddess of law, morality, and justice. The Egyptians believed that Maat held the universe together. The Eye of Maat later became known as the Eye of Horus. The Eye of Horus was believed to protect against all evil and to ensure success. The “evil eye” from the myth of Medusa was an expression of envy and greed (see Fig. 27-3). To this day, people wear talismans to ward off the “evil eye.”
Another interesting association is the subconscious linking of “eyeball” with genitalia. Blindness can symbolize castration because testicles and eyeballs have the same shape and are important in the development of the sense of identity. This linking goes back to the legend of Oedipus, who pierced his eyeballs when he discovered that he had been married to his mother and had killed his father. This can be thought of as an act of self-castration, as well as a means of cutting oneself off from all worldly relationships. Throughout literature, the blinding of an individual was frequently a form of punishment for lust. The age-old notion that masturbation causes blindness further reinforces this close association of these organs.
Structure and Physiology
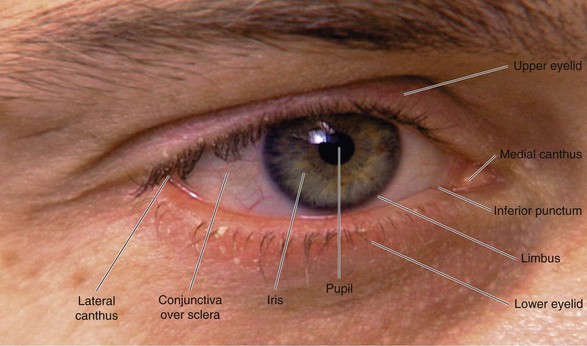
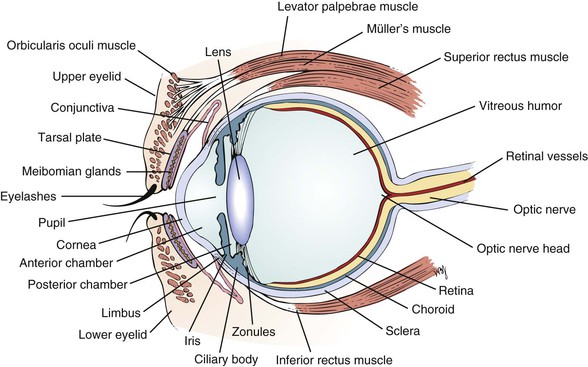
The external landmarks of the eye are shown in Figure 7-1, and the cross-sectional anatomy of the eye is shown in Figure 7-2.
The eyelids and eyelashes protect the eyes. The eyelids cover the globe and lubricate its surface. The meibomian glands, which are modified sebaceous glands in the eyelids, secrete meibum, an oily lubricating substance that retards evaporation. The openings of these glands are at the eyelid margins. This is illustrated in Figure 7-28B.
The orbicularis oculi muscle encircles the lids and is responsible for their closure. This muscle group is the reason we have such expressive eyes. This muscle is supplied by the facial, or seventh cranial, nerve. The levator palpebrae muscle elevates the lids and is innervated by the oculomotor, or third cranial, nerve. Müller’s muscle is a small part of the levator muscle that has sympathetic innervation.
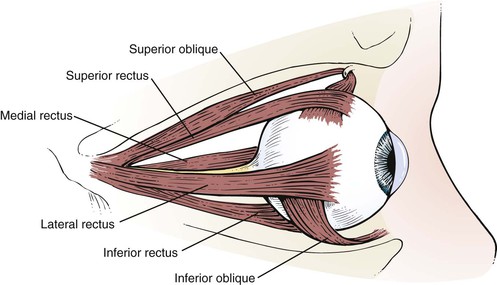
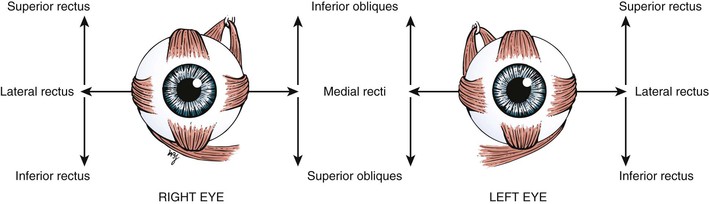
The globe has six extraocular muscles that control its motion. There are four rectus and two oblique muscles: the medial rectus, the lateral rectus, the superior rectus, the inferior rectus, the superior oblique, and the inferior oblique muscles. These six extraocular muscles are shown in Figure 7-3.
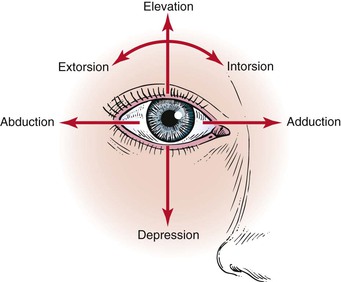
The extraocular muscles work in a parallel, conjugate manner to maintain single, binocular vision. When the head is turned to look left, for example, the left lateral rectus and the right medial rectus contract to turn the eyes to the left. The actions and innervations of the extraocular muscles are listed in Table 7-1, and the extraocular movements are illustrated in Figure 7-4.
Table 7–1
Actions and Innervations of the Extraocular Muscles*
| Muscle | Action | Cranial Nerve Innervation |
| Medial rectus | Adduction (eye moves nasally) | Oculomotor (III) |
| Lateral rectus | Abduction (eye moves temporally [away from the nose]) | Abducens (VI) |
| Inferior rectus | Depression (eye moves down) Extorsion (the 12 o’clock position on the cornea rotates temporally) Adduction | Oculomotor (III) |
| Superior rectus | Elevation (eye moves up) Intorsion (the 12 o’clock position on the cornea rotates nasally) Adduction | Oculomotor (III) |
| Superior oblique | Intorsion Depression Abduction | Trochlear (IV) |
| Inferior oblique | Extorsion Elevation Abduction | Oculomotor (III) |
* Remember “LR6SO4.” This mnemonic means that the lateral rectus (LR) muscle is innervated by the sixth cranial nerve, and the superior oblique (SO) muscle is innervated by the fourth cranial nerve. All the other muscles are innervated by the third cranial nerve.
The lateral rectus muscle, which is innervated by the abducens nerve, turns the eye laterally (abducts the eye), as do both oblique muscles. As easy way to remember the innervations is the formula LR6SO4, meaning that the lateral rectus is innervated by cranial nerve VI (abducens) and the superior oblique is innervated by cranial nerve IV (trochlear); all the other muscles are innervated by cranial nerve III (oculomotor).
The conjunctiva is a thin, vascular, transparent mucous membrane that lines the lids of the anterior portion of the globe. The palpebral portion covers the inner surface of the lids, whereas the bulbar portion covers the sclera up to the limbus, which is the corneal-scleral junction. The conjunctiva contains many small blood vessels, which, when dilated, produce the appearance of a “red” eye. There is little nervous innervation to the conjunctiva.
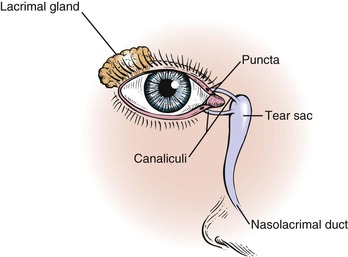
The lacrimal apparatus consists of the lacrimal gland, accessory tear glands, canaliculi, tear sac, and nasolacrimal duct. These are shown in Figure 7-5.
The lacrimal gland produces watery tears and is located above and slightly lateral to the globe. Secretion occurs mostly as reflex tearing or crying. Tears drain through the puncta on the lids and into the superior and inferior canaliculi. These canaliculi join and enter the tear sac, located at the medial canthus of the eye. The nasolacrimal duct drains tears from the sac to the nose. Of the lacrimal apparatus, only the puncta are visible on routine examination.
The sclera is the white, fibrous, outer coat of the globe visible just beneath the conjunctiva. The extraocular muscles insert onto the sclera.
The cornea is a smooth, transparent, avascular tissue that covers the iris and joins with the sclera and conjunctival reflection at the limbus. The cornea functions as a protective window, allowing light to pass into the eye. The cornea is richly innervated by the trigeminal, or fifth cranial, nerve and is therefore exquisitely sensitive to touch and pain.
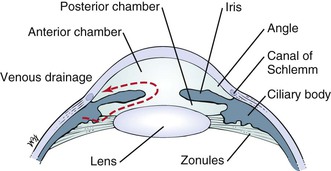
The anterior chamber, or space between the cornea anteriorly and the iris posteriorly, is filled with clear aqueous humor. Aqueous humor is produced by the ciliary body in the posterior chamber, the area behind the iris and in front of the lens. Aqueous humor circulates from the posterior chamber through the pupil into the anterior chamber and is filtered out through the canal of Schlemm, from where it eventually enters the venous system. Pressure within the eye is regulated by this filtration. The angle is formed by the juncture of the cornea and the iris at the limbus. A section through the eye at this level is shown in Figure 7-6.
The iris is the circular, colored portion of the eye. The small, round aperture in the middle of the iris is the pupil. Because it is optically empty in the healthy eye, it appears black. The pupil functions much like the aperture of a camera, controlling the amount of light that enters the eye.
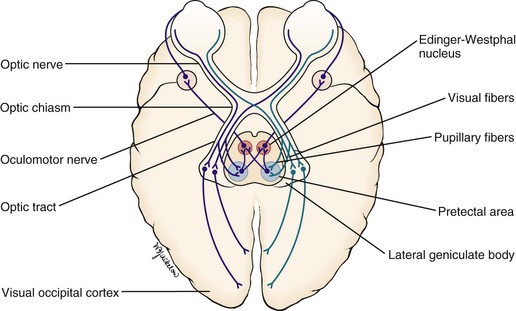
When a light is shined on one eye, both pupils constrict consensually. This constriction is the pupillary light reflex. To understand this reflex, a brief review of the neuroanatomy is in order. Figure 7-7 illustrates the pathways of the pupillary light reflex.
The optic, or second cranial, nerves are composed of 80% visual and 20% afferent pupillary fibers. The optic nerves leave both retinas and travel a short course to where they join each other. This joining is the optic chiasm. At the optic chiasm, the nasal fibers cross and join the uncrossed fibers of the other side, forming the optic tract. The visual fibers continue in the optic tract to the lateral geniculate body, where synapses occur, the axons of which terminate in the primary visual cortex of the occipital lobe. The afferent pupillary fibers bypass the geniculate body and end in the superior colliculus and pretectal area of the midbrain.
Light impulses to the eye cause the retina to transmit nerve impulses to the optic nerve, the optic tract, the midbrain, and the visual cortex of the occipital lobes. This is the afferent limb of the light reflex. In the midbrain, the pupillary fibers diverge and are relayed by crossed fibers to the opposite Edinger-Westphal nucleus of the oculomotor, or third cranial, nerve. Some fibers remain on the same side. The third cranial nerve is the efferent limb, which goes via the ciliary body to the sphincter muscle of the iris to cause it to contract. The direct effect is the constriction of the pupil of the eye on which the light is shined (the ipsilateral eye). The consensual effect is the simultaneous constriction of the opposite pupil (the contralateral eye).
The near reflex occurs when the subject looks at a nearby target. The three parts of the near reflex are accommodation, convergence, and pupillary constriction. Accommodation is defined as the near focusing of the eye, which is effected by increasing the power of the lens by contraction of the ciliary muscle, innervated by the third cranial nerve.
There is also autonomic innervation of the eyes. The iris is supplied by sympathetic and parasympathetic fibers. When the sympathetic fibers are stimulated, the pupil dilates, and the eyelid elevates. Think of the cat stalking its prey, pupils dilated, ready to pounce in the dark. The cat needs all the light it can get. The reflex is purely sympathetic. When the parasympathetic fibers in the oculomotor nerve are stimulated, pupillary constriction occurs. When we sleep, our bodies are in a parasympathetic mode and our pupils are constricted.
The lens sits directly behind the iris. It is a biconvex, avascular, colorless structure that changes its shape to focus the image on the retina. The shape is changed by the ciliary body muscles contracting. They in turn are connected to the zonules of the lens, thereby changing its shape.
The vitreous humor is the transparent, avascular gel that is located behind the lens and in front of the retina. It occupies 80% of the volume of the eye. This clear matrix is made up of collagen, hyaluronic acid, and water. It is bounded by the posterior lens capsule anteriorly and the retina posteriorly.
The choroid is the middle, vascular layer of the globe between the sclera and the retina. It acts as a source of nourishment, as well as a heat sink, serving to remove the extreme heat produced by the light energy entering the eye. Bruch’s membrane separates the choroid from the retina. Superficial to Bruch’s membrane (closer to the retina) is the retinal pigment epithelium (RPE). The RPE is a monolayer of cells between Bruch’s membrane and the retina. Some of the important functions of the RPE are to absorb light passing through the retina and to regenerate the visual pigments.
The retina is the innermost layer, or “camera film,” of the eye. The retina is attached firmly to the underlying choroid at the optic nerve posteriorly and at the ora serrata anteriorly. Between these two points, the retina is in contact with the choroid, but it is not attached to it. The ora serrata is the junction of the retina and ciliary body. The retina is only 0.4 mm in thickness and is thinnest in the region of the macula. Histologically, the retina is made up of 10 distinct layers. Basically, the retina senses light through the rods and cones in its outer layer (closest to the RPE); performs initial signal processing in its middle layer; and encodes and transmits the data in its inner layer, the nerve fiber layer. The nerve fiber layer is directly under the inner limiting membrane of the retina, the layer closest to the vitreous. These nerve fibers course along the inner portion of the retina and aggregate to form the optic nerve. On leaving the eye, the nerve fibers become myelinated.
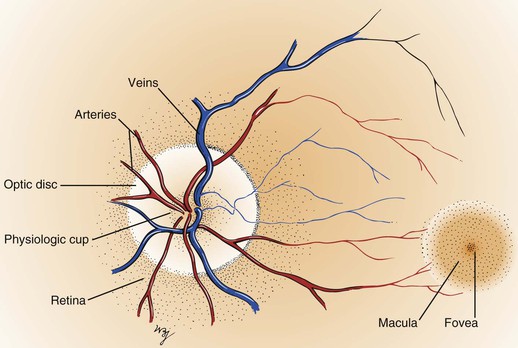
Within the retina are several important structures: the optic disc, the retinal vessels, and the macula. Figure 7-8 illustrates the retina of the left eye.
The optic disc is located at the nasal aspect of the posterior pole of the retina. This is the head of the optic nerve, from where the nerve fibers of the retina exit the eye. The optic disc is 1.5 mm in diameter and is ovoid. It is lighter than the surrounding retina and appears yellowish-pink. The disc margins are sharp with some normal blurring of the nasal portion. African-American patients may have pigmentation at the margins. The physiologic cup is the center of the disc, where the retinal vessels penetrate. This small depression normally occupies approximately 30% of the disc diameter.
The retinal vessels emerge from the disc and arborize on the retinal surface. The arteries are brighter red and thinner than the veins. An artery-to-vein ratio of 2 : 3 is normal.
The macula is a small, round area, approximately the size of the disc, located 3.5 mm temporal to and 0.5 mm inferior to the disc. The macula is easily seen because it is devoid of retinal vessels. In the center of the macula is the fovea, a depressed area composed only of cones. Cones provide detailed vision and color perception.
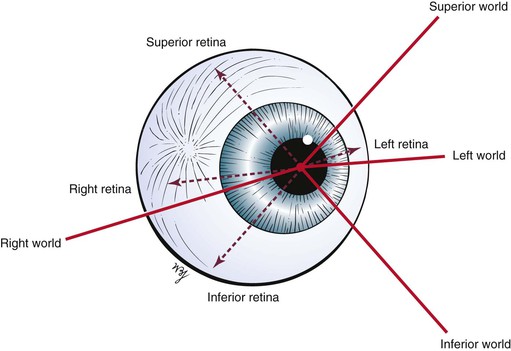
The remaining areas of the retina contain mostly rods, which compose the other neurosensory element of the retina. The rods are responsible for motion detection and night vision. It should be remembered that the image on the retina is upside down and reversed left to right: The right world is projected on the left half of the retina, and the left world is projected on the right half of the retina. An image in the superior world strikes the inferior part of the retina, and an inferiorly positioned image strikes the superior part. This concept is illustrated in Figure 7-9.
At birth, there is little pigment in the iris, which is why many infants are born with blue eyes. By 6 months of age, the pigmentation is complete. The lens is more spherical at birth than in later life. Most infants are born hyperopic (farsighted). By 3 months after birth, the medullation process of the optic nerve is completed. As the child grows, hyperopia increases until the age of 8 years and then gradually decreases. After age 8, myopia (nearsightedness) appears to increase.
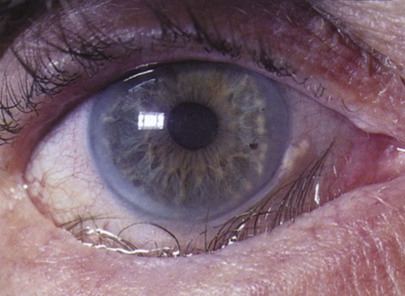
With advancing age, there is the gradual loss of elasticity of the skin around the eyes. The cornea may show an infiltration of cholesterol deposits around the limbus, which is known as an arcus senilis. The lens consistency changes from plastic to rigid, making it progressively more difficult to change its shape to focus on near objects. This condition is presbyopia. The lens may undergo changes resulting from metabolic disorders that cause its opacification; this condition is called a cataract. The vitreous humor may develop condensations, called floaters. The retinal arteries may develop atherosclerosis, with resultant retinal ischemia or infarction.
Review of Specific Symptoms
The major symptoms of eye disease are the following:
Loss of Vision
When a patient complains of loss of vision, the following two questions must be asked:
It is extremely important to ascertain the acuteness of the loss of vision and the presence or absence of pain. Sudden, painless loss of vision may result from a retinal vascular occlusion or a retinal detachment. Sudden, painful loss of vision occurs in attacks of acute narrow-angle glaucoma. Gradual, painless loss of vision commonly occurs in chronic simple glaucoma and cataracts.
Eye Pain
Eye pain may result from a variety of causes. Ask the patient the following questions:
“Did the pain come on suddenly?”
“Does the light bother your eye?”
“Do you have pain when you blink?”
“Do you have the sensation of something in the eye?”
“Do you have pain on movement of the eye?”
“Do you have pain over the brow on the same side?”
Pain may be experienced as “burning,” “aching,” “throbbing,” “tenderness,” or pain behind the eye. Each of these descriptions may have a range of causes. It is important to determine whether the patient has the sensation of a foreign body in the eye. Pain in the eye while blinking occurs in corneal abrasions and with foreign bodies in the eye. Photophobia is eye pain associated with light, as seen in inflammations of the uveal tract (i.e., iris, ciliary body, or choroid). Inflammations of the conjunctiva, conjunctivitis, produce a gritty sensation. Diseases of the cornea are associated with significant pain because the cornea is so richly innervated. Headaches and eye pain are common in acute narrow-angle glaucoma. Pain on motion of the eye occurs in optic neuritis. Eye pain associated with brow or temporal pain may be an indication of temporal arteritis (see Chapter 22, The Geriatric Patient). Contact lens wearers may have corneal irritation and may complain of eye pain.
Diplopia
Diplopia, or double vision, is a common complaint. Diplopia results from a faulty alignment of the eyes. Normally, when the eyes fixate on an object, the object is seen clearly, despite the fact that the two retinal images are not exactly superimposed. These slightly different images, however, are fused by the brain; it is this fusion that produces binocular vision, or the perception of depth. When the eyes are misaligned, the two images fall on different parts of the retinas, with only one falling normally on the fovea. The field of vision of the deviated eye is different, so that its image is not projected on its fovea; therefore, this second image is different and not superimposable. The patient may close one eye to relieve this distressing situation. A compensatory head posture may be used by the patient to relieve the double vision (see Fig. 7-152). Elevation or depression of the patient’s chin is used to overcome a vertical deviation. Tilting of the head is often used to counteract the torsional and vertical deviation. Suggested questions for the patient with diplopia are listed in Chapter 18, The Nervous System.
Tearing and Dryness
Excessive tearing of the eyes is a common complaint. Abnormal tearing may be caused either by overproduction of tears or by an obstruction of outflow. The eye depends on the flow of tears to provide constant moisture and lubrication to maintain vision and comfort. Tears are a combination of water, for moisture; oils, for lubrication; mucus, for even spreading of the tears; and antibodies and special proteins, for resistance to infection. These components are secreted by special glands located around the eye. Thus tears provide lubrication, reduce the risk of eye infection, wash away foreign matter in the eye, and keep the surface of the eyes smooth and clear.
When there is an imbalance in this tear system, a person may experience dry eyes. Nearly fifty-five million Americans 50 years of age and older are estimated to have dry eye, with only 17% having been diagnosed with the condition. Most patients with dry eyes are women. In the United States, it was estimated in 2000 that 14.4% of the population report symptoms of dry eyes. The prevalence increases with age: 8.4% in individuals younger than the age of 60 years and 19% in individuals older than the age of 60 years. In 2014, it is expected that there will be 20% more Medicare patients with dry eyes, and by 2010, it is anticipated that there will be 100% more Medicare patients.
The most common form of dry eyes is due to an inadequate amount of the water layer of tears. This condition, called keratoconjunctivitis sicca, is also referred to as the dry eye syndrome.
Patients suffering from the dry eye syndrome often experience:
• Dryness
• Eye pain
• Stinging or burning sensation
• Stringy discharge from the eye
• Sand in the eye; a gritty feeling
• Itching
• Redness
There are many causes of the dry eye syndrome. These include:
Gender: Women are more likely to develop dry eyes because of hormonal changes and menopause.
Environmental conditions: Exposure to pollutants such as smoke, exhaust, and smog, and arid and windy conditions can increase tear evaporation, resulting in dry eye symptoms. Failure to blink regularly, such as when staring at a computer screen for long periods, can also contribute to the dry eye syndrome. Excessive alcohol consumption is also associated with the dry eye syndrome.
Other factors: Long-term use of contact lenses can be a factor in the development of dry eyes. Refractive eye surgeries, such as laser-assisted in situ keratomileusis (LASIK),2 can cause decreased tear production and dry eyes.
Sometimes, a person with a dry eye will have excess tears running down the cheeks, which may seem confusing. This happens when the eye isn’t getting enough lubrication. The eye sends a distress signal through the nervous system for more lubrication. In response, the eye is flooded with tears to try to compensate for the underlying dryness. However, these tears are mostly watery and do not have the lubricating qualities or the rich composition of normal tears. They wash debris away, but they do not coat the eye surface properly.
Discharge
Discharge from the eye can be watery, mucoid, or purulent. A watery or mucoid discharge is often associated with allergic or viral conditions, whereas a purulent discharge occurs in association with bacterial infections. Most of the time, eye discharge is harmless. However, sometimes it can be an indication of a more serious condition. Pollen, gusty winds, dry eyes or an eyelash can cause an irritation that will lead to the discharge as well. Other, more serious causes of eye discharge can be associated with medical conditions such as conjunctivitis and corneal ulcers.
Redness
The symptom of the red eye is very common. The interviewer should ask the following questions:
“Have you had any injury to the eye?”
“Does anyone else in the family have a red eye?”
“Have you had any recent coughing spells? Vomiting?”
“Have you had any associated eye pain?”
“Does light bother your eyes?”
“Is there any associated discharge?”
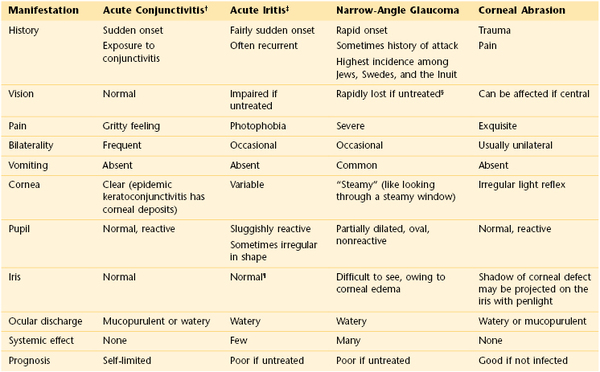
The eye may appear bloodshot. Redness may result from trauma, infection, allergy, or sudden increased pressure in the eye. Severe coughing spells or recurrent vomiting may cause a patient to have a subconjunctival hemorrhage. A family member or friend with viral conjunctivitis may be the source of a patient’s red eye. The combination of eye pain and red eye may indicate acute narrow-angle glaucoma, or an inflammatory condition known as scleritis or episcleritis. (Table 7-2 summarizes the differential diagnosis for the red eye.) Uveitis, inflammation of the uveal tract, which is associated with a red eye, can manifest with light sensitivity. Patients wearing contact lenses may suffer from corneal irritation and may have eye redness.
General Suggestions
It is important to determine the medications that a patient is taking because many drugs have deleterious effects on the eye. Some antimalarial, antituberculous, antiglaucoma, and anti-inflammatory drugs can cause eye disorders. A thorough family history reveals familial disease tendencies such as glaucoma, cataracts, retinal degeneration, strabismus, or corneal dystrophies.
There are many specific symptoms related to eye disease. The common visual, nonvisual but painful, and nonvisual and painless symptoms and some possible causes are listed in Tables 7-3, 7-4, and 7-5, respectively.
Table 7–3
Common Visual Eye Symptoms and Disease States
| Visual Symptom | Possible Causes |
| Loss of vision | Optic neuritis |
| Detached retina | |
| Retinal hemorrhage | |
| Central retinal vascular occlusion | |
| Central nervous system disease | |
| Spots | No pathologic significance* |
| Flashes | Migraine |
| Retinal detachment | |
| Posterior vitreous detachment | |
| Loss of visual field or presence of shadows or curtains | Retinal detachment |
| Retinal hemorrhage | |
| Glare, photophobia | Iritis (inflammation of the iris) |
| Meningitis (inflammation of the meninges) | |
| Distortion of vision | Retinal detachment |
| Macular edema | |
| Difficulty seeing in dim light | Myopia |
| Vitamin A deficiency | |
| Retinal degeneration | |
| Colored halos around lights | Acute narrow-angle glaucoma |
| Opacities in lens or cornea | |
| Colored vision changes | Cataracts |
| Drugs (digitalis increases yellow vision) | |
| Double vision | Extraocular muscle paresis or paralysis |
* May precede a retinal detachment or may be associated with ingestion of fertility drugs.
Table 7–4
Common Nonvisual, Painful Eye Symptoms and Disease States
| Nonvisual, Painful Symptom | Possible Causes |
| Foreign body sensation | Foreign body |
| Corneal abrasion | |
| Burning sensation | Uncorrected refractive error |
| Conjunctivitis | |
| Sjögren’s syndrome | |
| Throbbing, aching | Acute iritis (inflammation of the iris) |
| Sinusitis (inflammation of the sinuses) | |
| Tenderness | Eyelid inflammation |
| Conjunctivitis | |
| Iritis | |
| Headache | Refractive errors |
| Migraine | |
| Sinusitis | |
| Drawing sensation | Uncorrected refractive errors |
Table 7–5
Common Nonvisual, Painless Eye Symptoms and Disease States
| Nonvisual, Painful Symptom | Possible Causes |
| Itching | Dry eyes |
| Eye fatigue | |
| Allergies | |
| Tearing | Emotional states |
| Hypersecretion of tears | |
| Blockage of drainage | |
| Dryness | Sjögren’s syndrome |
| Decreased secretion as a result of aging | |
| Sandiness, grittiness | Conjunctivitis |
| Fullness of eyes | Proptosis (bulging of the eyeball) |
| Aging-related changes in the lids | |
| Twitching | Fibrillation of orbicularis oculi |
| Eyelid heaviness | Fatigue |
| Eyelid edema | |
| Dizziness | Refractive error |
| Cerebellar disease | |
| Vestibular disease | |
| Excessive blinking | Local irritation |
| Facial tic | |
| Eyelids sticking together | Inflammatory disease of eyelids or conjunctivae |
Effect of Blindness on the Patient
The loss of sight is a terrifying experience. The sighted person lives mostly in a visual and auditory world illuminated by lights and colors. When blindness occurs, the person loses not only the ability to see but also their perceptual center of the world. This center must now be replaced by hearing and touch. Because light is often equated with life, the inability to see light is associated with death. The newly blinded patient must take a new place in society. He or she can no longer read ordinary books, can no longer receive visual stimuli, and is unable to appreciate the world of visual communication. This can result in a reactive depression. The clinician must show genuine care for blind patients and try to understand their feelings of discouragement and despair.
The person who is blind from birth or early childhood has little or no conception of the visual world. Having never been able to see, this patient has no visual frame of reference.
On occasion, a blind individual recovers sight as a result of a surgical procedure later in life. Many difficulties may arise owing to the reorganization of the patient’s perception. His or her frame of reference has been shifted from touch to sight. Surprisingly, many of these patients become depressed after attaining vision. Facial expressions mean nothing because only with experience can people understand them. The following quote from a case history by Gregory and Wallace illustrates the response of such an individual:
He suffered one of the greatest hardships (blindness) and yet he lived with energy and enthusiasm. When his handicap was apparently swept away, as by a miracle, he lost his peace and self-respect.
Similarly, the patient with normal vision may develop psychosomatic eye problems as a result of anxiety. Loss of vision also can accompany panic disorders. Such individuals can have either partial or complete vision loss in one or both eyes. Supportive care of the primary problem usually results in the return of vision.
In multiple sclerosis, one of the presenting symptoms may be the sudden onset of blindness caused by optic neuritis. This blindness in most cases reverses completely, in approximately 6 weeks. This is a very frightening experience for a typically young patient.
Physical Examination
The equipment necessary for the examination of the eye is as follows: an ophthalmoscope, a penlight, a pocket visual acuity card, and a 3 × 5 inch (7.6 × 12.7 cm) card.
The physical examination of the eye includes the following:
Visual Acuity
Visual acuity is expressed as a ratio, such as 20/20. The first number is the distance at which the patient reads the chart. The second number is the distance at which a person with normal vision can read the same line of the chart. Another way of describing visual acuity is as follows: if a person has 20/20 vision, that person has 20/20 or 100% vision because 20 divided by 20 equals 1 or 100% of normal vision. The abbreviation OD refers to the right eye; OS refers to the left eye; OU refers to both eyes.3
Using the Standard Snellen Chart
If a standard Snellen eye chart is available, the patient should stand 20 feet from the chart. If the patient wears glasses for distance, he or she should wear them for the examination. The patient is asked to cover one eye with the palm4 or an index card and read the smallest line possible. If the best he or she can see is the 20/200 line, the patient’s vision in that eye is 20/200; this means that at 20 feet, the patient can see what a person with normal vision can see at 200 feet. This can also be explained as 10% of normal vision in that eye. If a patient at 20 feet cannot see the 20/200 line, he or she is moved closer until the letters are recognized. If the patient can read these letters at 5 feet, the patient’s visual acuity in that eye is 5/200.
Using a Pocket Visual Acuity Card
If the standard Snellen chart is not available, a pocket visual acuity card is helpful. This is viewed at 14 inches (35.6 cm). The patient is again asked to read the smallest line possible. If neither eye chart is available, any printed material may be used. The examiner should remember that most patients older than 40 years require reading glasses. Although visual acuity cannot be quantified, the examiner can certainly determine whether the patient has any vision. In such a case, the patient is asked to cover an eye and read the smallest line possible on a given printed page.
Evaluation of Patients with Poor Vision
Patients with poor vision who are unable to read any lines of print should be tested for finger-counting ability. This crude measurement of visual acuity is obtained by the examiner’s holding up fingers in front of one of the patient’s eyes with the other eye covered. The patient is then asked how many fingers are seen. If the patient is still unable to see, the next step would be to evaluate whether he or she has any light perception. This is performed by covering one eye and directing a light at the eye in question. The examiner asks the patient whether he or she can see when the light is on and off. No light perception is the term used when a person cannot perceive light.
Evaluation of Patients Who Cannot Read
For individuals who cannot read, such as young children or illiterate patients, the use of the letter “E” in different sizes and directions is helpful. The examiner asks the patient to point in the direction of the letter: up, down, right, left.
Visual Fields
Visual field testing is useful for determining lesions of the visual pathway. Many techniques are used for this purpose. The examiner should learn to perform the technique known as confrontation visual field testing. In this technique, the examiner compares his or her peripheral vision with that of the patient.
Assess Fields by Confrontation Testing
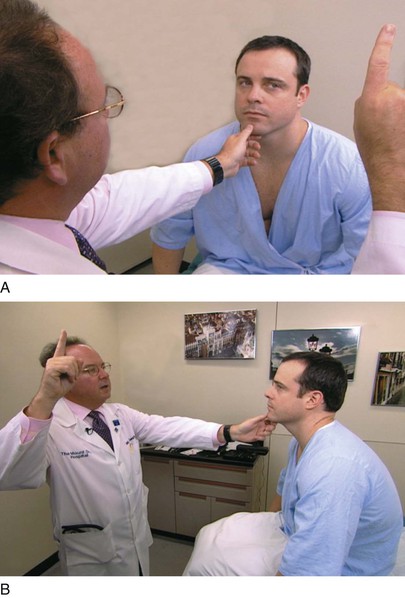
The examiner stands or sits 3 feet in front of and at eye level with the patient. The patient is asked to close the right eye while the examiner closes his or her own left eye, each fixating on the other’s nose. The examiner holds up fists with the palms facing him or her. The examiner then shows one or two fingers on each hand simultaneously and asks the patient how many fingers he or she sees. The hands are moved from the upper to the lower quadrants, and the examination is repeated. The examination is then repeated, with the other eye of the patient and that of examiner. The fingers should be seen by both patient and examiner simultaneously. To position the patient to better advantage, the hands are held up slightly closer to the examiner. This provides a wider field for the patient. If the examiner can see the fingers, the patient can see them unless he or she has a field deficit. This technique for examining the patient’s left eye is shown in Figure 7-10.
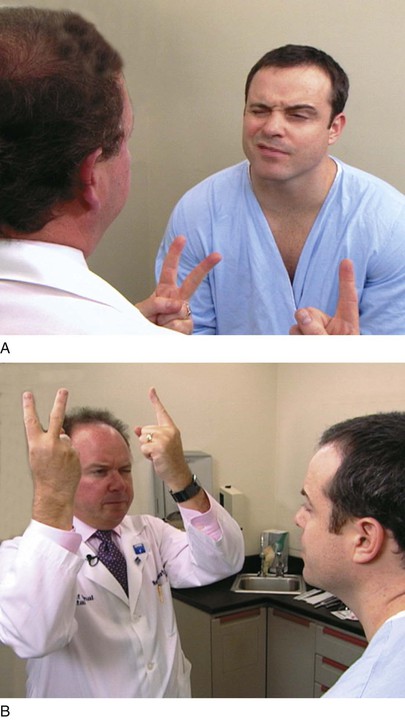
Figure 7–10 Confrontation visual field testing. A, View of the patient during examination of the lower fields of the patient’s left eye. B, Position of the examiner during examination of the upper fields of the patient’s left eye.
Because lesions along the visual pathway develop insidiously, the patient may not be aware of any changes in visual fields until late in the course of the disease. Confrontation fields, performed by the internist, may provide the first objective evidence that the patient has a lesion involving the visual pathway. An area of depressed vision is called a scotoma.
The normal central vision extends approximately 30 degrees in all directions of central fixation. The blind spot is the physiologic scotoma located approximately 15 to 20 degrees temporal to central fixation, corresponding to the optic nerve head. No sensory elements such as the rods or cones are located on the nerve head.
Assess Visual Field Abnormalities
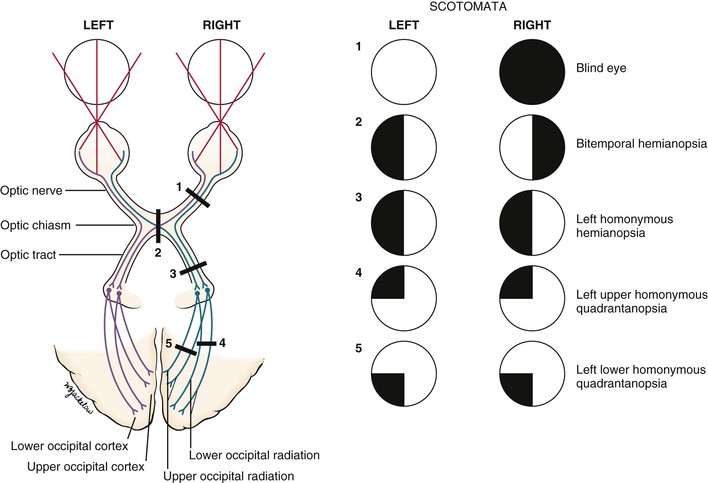
Pathologic scotomata may be appreciated on visual field testing. Scotomata may result from primary ocular disease, such as glaucoma, or from lesions in the central nervous system, such as tumors. Figure 7-11 illustrates some of the common defects.
Total loss of vision in one eye constitutes a blind eye, resulting from a disease of the eye or a lesion of its optic nerve.
Hemianopsia refers to absence of half of a visual field. A defect in both temporal fields is termed bitemporal hemianopsia. It results from a lesion involving the optic nerves at the level of the optic chiasm. Pituitary tumors are common causes.
A homonymous hemianopsia results from damage to the optic tract, optic radiation, or occipital cortex. The term homonymous indicates that the visual loss is in similar fields. A patient with a left homonymous hemianopsia is unable to see the left half of the fields of both eyes. This defect occurs with damage to the right optic tract. Homonymous hemianopsia is the most common form of field loss and occurs frequently in patients with strokes.
A quadrantanopsia is a field loss in one quadrant, also known as a “pie in the sky” lesion. A patient with a left upper homonymous quadrantanopsia has damage to the right lower optic radiations or the right lower occipital region. Tunnel vision may occur in advanced glaucoma. However, the visual fields enlarge with increasing testing distance, in contrast to hysterical blindness, in which the field size typically remains the same at all times, regardless of the testing distance.
Assess Optokinetic Nystagmus
On occasion, a patient may feign blindness. They may be trying to qualify for disability insurance or some other issue of secondary gain. Alternately, they may have psychological issues. A useful test for ruling out such malingering involves optokinetic nystagmus (OKN). OKN is the rapid alternating motion of the eyes that occurs when the eyes try to fixate on a moving target. For example, observe the eyes of a person riding a train as it enters the station. The eyes move rapidly back and forth as the person tries to fixate on a station sign. The presence of OKN indicates physiologic continuity of the optic pathways from the retina to the occipital cortex. OKN may be elicited in the examining room by having the patient fixate on the numbers on a tape measure while you rapidly pull out the tape. Because OKN is involuntary, a positive response5 provides excellent verification that the patient is feigning blindness.
Ocular Movements
Ocular movements are effected by the contraction and relaxation of the extraocular muscles. This results in simultaneous movement of the eyes up or down or from side to side, as well as in convergence.
Assess Eye Alignment
Alignment of the eyes is seen by observing the location of reflected light on the cornea. The penlight should be held directly in front of the patient. If the patient is looking directly at the penlight, the light reflex should be in the center of each pupil. If the light falls in the pupillary center of one eye but is displaced away from the pupillary center in the other eye, deviation of the eye may exist.
The condition of a deviated, or crossed, eye is called strabismus, or tropia. Strabismus is the nonalignment of the eyes in such a way that the object being observed is not projected simultaneously on the fovea of each eye. Esotropia is deviation of an eye nasally; exotropia is deviation of an eye temporally; hypertropia is deviation upward. An alternating tropia is the term used to describe the condition in which either eye deviates. Figure 7-12 shows a patient with a left exotropia.
Perform the Cover Test
The cover test is useful for determining whether the eyes are aligned (if a deviated eye is present). The patient is instructed to look at a distant target. One eye is covered with a 3 × 5 inch (7.6 × 12.7 cm) card. The examiner should observe the uncovered eye. If the uncovered eye moves to take up fixation of the distant point, that eye was not straight before the other eye was covered. If the eye did not move, it was straight. The test is then repeated with the other eye.
Evaluate the Six Diagnostic Cardinal Positions of Gaze
An important cause of a deviated eye is a paretic (weak), or paralyzed, extraocular muscle or muscles. Paralysis of these muscles is detected by examination of the six diagnostic positions of gaze.
Because the rotational actions of the oblique and vertical rectus muscles cannot be easily assessed, the eye must be moved into six diagnostic gaze positions that best isolate the vertical actions of these muscles to test their innervations. The oblique muscles are tested in adduction to maximize their vertical action. In contrast, the vertical rectus muscles are tested in abduction; in these positions, the superior rectus acts now as a pure elevator and the inferior rectus as a pure depressor. These diagnostic cardinal positions of gaze are the testing positions for the muscles, and the abduction and adduction testing movements are different from the normal actions of the muscles as indicated in Table 7-1.
Hold the patient’s chin steady with your left hand, and ask the patient to follow your right hand as it traces a large H in the air. Hold your right index finger approximately 15 to 18 inches (38 to 46 cm) from the patient’s nose. From the midline, move your finger approximately 12 inches (30 cm) to the patient’s left and pause; then up approximately 8 inches (20 cm) and pause, as shown in Figure 7-13; down approximately 16 inches (40 cm) and pause; up approximately 8 inches (20 cm); and then slowly back to the midline. Switch your hands, now holding the patient’s chin with your right hand. Cross the midline and repeat the finger movements on the other side. These are the six diagnostic cardinal positions of gaze. Observe the movement of both eyes, which should follow the finger smoothly. Look for the parallel movements of the eyes in all directions.
On occasion, when looking to the extreme side, the eyes develop a rhythmic motion called end-point nystagmus. There is a quick motion in the direction of gaze, which is followed by a slow return. This test differentiates end-point nystagmus from pathologic nystagmus, in which the quick movement is always in the same direction, regardless of gaze.
If the eye and eyelid do not move together, lid lag is present.
The six diagnostic cardinal positions of gaze, with the related muscles, are illustrated in Figure 7-14, and Table 7-6 summarizes the abnormalities in ocular motility that are caused by paretic muscles.
Table 7–6
Paretic Muscles Causing Abnormal Ocular Motility
| Paretic Muscle | Position to Which Eye Will Not Turn |
| Medial rectus | Nasal |
| Inferior oblique | Up and nasal |
| Superior oblique | Down and nasal |
| Lateral rectus | Temporal |
| Superior rectus | Up and temporal |
| Inferior rectus | Down and temporal |
The images projected on the retina may be interpreted by the brain in one of three ways: fusion, diplopia, or suppression. Fusion and diplopia have already been discussed. In children, strabismus leads to diplopia, which leads to confusion and then suppression of the image and finally to amblyopia. Amblyopia is the loss of visual acuity secondary to suppression. Amblyopia is reversible until the retinas are fully developed, at approximately the age of 7 years. Amblyopia is a phenomenon that occurs only in children. An adult who acquires strabismus secondary to a stroke, for example, cannot suppress the deviated eye’s image and will experience diplopia, which is intolerable.
Evaluate the Pupillary Light Reflex
Ask the patient to look in the distance while you shine a bright light in the patient’s eye. The light source should come from the side, with the patient’s nose acting as a barrier to light to the other eye. Observe both the direct and consensual pupillary responses. Then repeat the test on the other eye.
The swinging light test is a modification for testing the pupillary light reflex. This test reveals differences in the response to afferent stimuli of the two eyes. The patient fixates on a distant target while the examiner rapidly swings a light from one eye to the other, observing for constriction of the pupils. In some conditions, there is a paradoxical dilatation of the pupil on which the light is shined. This condition, called a Marcus Gunn defect or relative afferent pupillary defect (RAPD), is associated with an afferent limb defect in the eye being illuminated.
The most extreme example of an eye displaying the Marcus Gunn, or RAPD, phenomenon is a blind eye. When light is shined into the blind eye, there is neither a direct nor a consensual response. When the light is moved to the other eye, there is both a direct and a consensual response because both afferent and efferent pathways are normal. When the light is swung back to the blind eye, no impulses are received by the retina (afferent), and the pupil of the blind eye no longer remains constricted; it therefore dilates. There are different degrees of severity of Marcus Gunn pupils, depending on the involvement of the optic nerve.
Evaluate the Near Reflex
The near reflex is tested by having the patient look first at some distant target and then at a target placed approximately 5 inches (13 cm) away from his or her nose. When the patient focuses on the near target, the eyes should converge, and the pupils should constrict.
External and Internal Eye Structures
The examination of the external and internal eye structures includes the following:
Inspect the Orbits and Eyelids
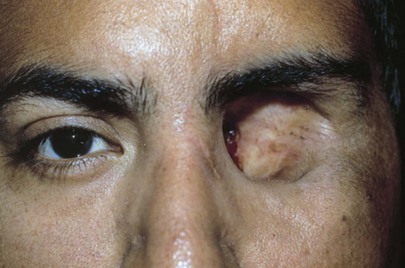
Are the globes present in the orbits? An enucleation of his left eye is pictured in Figure 7-15. The removal of the entire globe from the orbit may be caused by trauma, surgery (as in the case of a ruptured globe), or self-induced injury by a psychotic person. Rarely one may see a case of congenital anophthalmos.
Examine the eyelids for evidence of drooping, infection, erythema, swelling, crusting, masses, or other abnormalities. Have the patient open and close the eyelids. The motion should be smooth and symmetric. Do the eyes close completely?
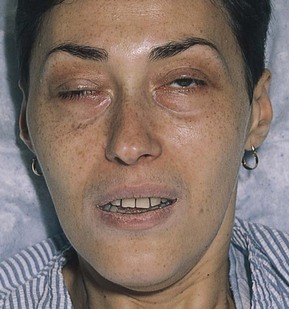
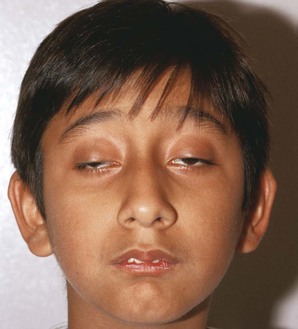
Note the position of the eyelids. When the eye is open, the upper eyelid normally covers only the upper margin of the iris. When the eye is closed, the eyelids should approximate each other completely. The space between the upper and lower lids is the palpebral fissure. Drooping of the eyelid is known as blepharoptosis, or ptosis. Figure 7-16 depicts marked bilateral ptosis, causing narrowing of the palpebral fissure that resulted from a muscle-weakening disorder, myasthenia gravis.
Figure 7-17 shows a patient with Kearns-Sayre syndrome, also known as chronic progressive external ophthalmoplegia. In this autosomal-dominant condition, there is a slowly progressive, symmetric ptosis and symmetric external ophthalmoplegia (weakness of the external eye muscles). The syndrome also is associated with retinal pigmentary degeneration and cardiac conduction defects such as complete heart block. Affected patients are frequently of short stature and may be deaf. In the patient shown in Figure 7-17, notice the arching of the brows in an effort to lift the eyelids to reduce the ptosis.
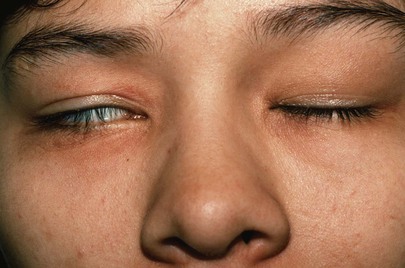
Lagophthalmos is a condition, pictured in Figure 7-18, in which there is an inability to close the eyelids completely. It is seen in thyroid disease secondary to orbital infiltration caused by inflammation, as a result of autonomic stimulation, or as a consequence of ocular surgery. The name comes from the Greek word lagos, meaning “hare,” an animal believed to sleep with its eyes open.
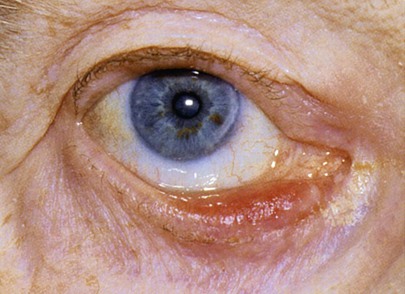
Figure 7-19 shows a patient with an entropion. An entropion is a turning inward of the lid margin in such a way that the eyelashes abrade the cornea and globe. An ectropion is a turning outward of the eyelid margin. An ectropion is pictured in Figure 7-20. Both entropions and ectropions may be seen as involutional changes associated with aging and may be associated with the dry eye syndrome.
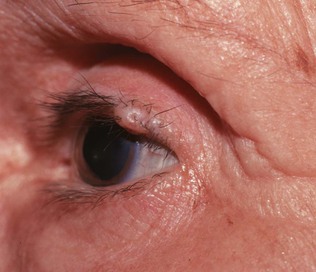
A common benign lesion of the eyelid is a marginal intradermal nevus, shown in Figure 7-21. Such lesions are well differentiated, and hairs commonly grow from them. One of the associated problems is that these hairs may scratch the cornea, causing corneal abrasions like those caused by entropions.
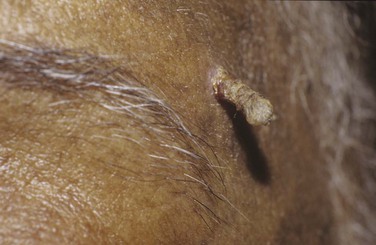
A cutaneous horn is a clinical diagnosis referring to a conical projection above the surface of the skin that resembles a miniature horn. The base of the horn may be flat, nodular, or crateriform. The horn is composed of compacted keratin. Cutaneous horns usually arise on sun-exposed skin but can occur even in sun-protected areas. More than half of all cutaneous horns are benign. Malignancy at the base of the horn, however, is present in up to 20% of cases, with squamous cell carcinoma being the most common type. No clinical features reliably distinguish between benign and malignant lesions. Tenderness at the base and lesions of larger size favor malignancy. Figure 7-22A shows a patient with a cutaneous horn on the medial aspect of the left upper eyelid. Figure 7-22B shows a close-up of the cutaneous horn. Figure 7-23 shows another patient with a cutaneous horn above her left eyebrow.
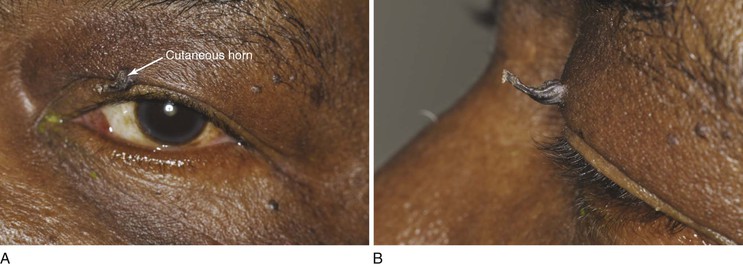
Figure 7–22 Cutaneous horn. A, Patient’s left eye with horn on medial aspect of upper eyelid. B, Close-up of lesion.
Molluscum contagiosum is a viral infection, caused by a member of the poxvirus family, that causes raised, pearl-like, umbilicated (dimpled) papules on the skin. In most people, the growths range from about the size of a pinhead to as large as a pencil eraser (2 to 5 mm in diameter). This is a common infection in children and occurs when a child comes into direct contact with a lesion. It is frequently seen on the face, neck, armpit, arms, and hands but may occur anywhere on the body except the palms and soles. The virus can spread through contact with contaminated objects, such as towels, clothing, or toys. The virus also spreads by sexual contact. Early lesions on the genitalia6 may be mistaken for herpes or warts but, unlike herpes, these lesions are painless. Persons with an impaired immune system, such as patients with acquired immune deficiency syndrome (AIDS), may have a significantly worse case of molluscum contagiosum. Figure 7-24 shows a patient with molluscum contagiosum around the eye. Note the umbilicated lesions in this figure as well as in Figure 15-37.
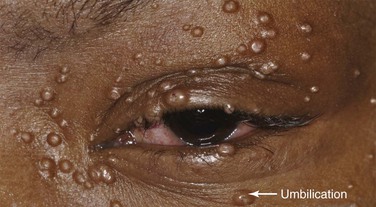
Figure 7–24 Molluscum contagiosum. Note the umbilicated (dimpled) lesions; one example is indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree