On completion of this chapter the reader will be able to: • Distinguish between the various categories of anemia. • Describe the prevention of and care of the child with iron-deficiency anemia. • Compare sickle cell anemia and β-thalassemia major in relation to pathophysiology and nursing care. • Describe the mechanisms of inheritance and nursing care of the child with hemophilia. • Relate the pathophysiology and clinical manifestations of leukemia. • Demonstrate an understanding of the rationale of therapies for neoplastic disease. • Outline a care plan for the child with neoplastic disease and the family. • Contrast the pathophysiology and management of the immunodeficiency disorders. • List nursing precautions and responsibilities during blood transfusion. • Describe the types of hematopoietic stem cell transplants. http://evolve.elsevier.com/wong/essentials Animations—Hemophilia A; Platelets and Blood Clotting; Sickle Cell Anemia Case Studies—Acute Lymphoblastic Leukemia; Idiopathic Thrombocytopenic Purpura; Iron Deficiency Anemia; Sickle Cell Anemia Nursing Care Plans—The Child with Anemia; The Child with Cancer; The Child with Hemophilia; The Child with Sickle Cell Disease Several tests can be performed to assess hematologic function, including additional procedures to identify the cause of the dysfunction. The following discussion is limited to a description of the most common and one of the most valuable tests, the complete blood cell count (CBC). Other procedures, such as those related to iron, coagulation, and immune status, are discussed throughout the chapter as appropriate. The nurse should be familiar with the significance of the findings from the CBC (Table 26-1) and be aware of normal values for age, which are listed in Appendix B. TABLE 26-1 TESTS PERFORMED AS PART OF A COMPLETE BLOOD COUNT *See Appendix B for normal values according to age. Anemias are classified in relation to (1) etiology or physiology, manifested by erythrocyte or Hgb depletion, and (2) morphology, the characteristic changes in RBC size, shape, or color (Box 26-1). Although the morphologic classification is more useful in terms of laboratory evaluation of anemia, the etiologic approach provides direction for planning nursing care. For example, anemia with reduced Hgb concentration may be caused by a dietary depletion of iron, and the principal intervention is replenishing iron stores. The classification of anemias is found in Fig. 26-1. In general, anemia may be suspected based on findings on the history and physical examination, such as a lack of energy, easy fatigability, and pallor, but unless the anemia is severe, the first clue to the disorder may be alterations in the CBC, such as decreased RBCs, and decreased Hgb and hematocrit (Hct) levels (see Fig. 26-1). Although anemia is sometimes defined as an Hgb level below 10 or 11 g/dl, this arbitrary cutoff is inappropriate for all children because Hgb levels normally vary with age (see Table 26-1 and Appendix B). Stool examination for occult (microscopic) blood (Hemoccult test) can identify chronic intestinal bleeding that results from a primary or secondary lactase deficiency. It is also important to understand the significance of various blood tests (see Table 26-1). Usually, several blood tests are ordered, but because they are generally done sequentially rather than at one time, the child is subjected to multiple finger or heel punctures or venipunctures. Laboratory technicians frequently are not aware of the trauma that repeated punctures represent to a child. However, these invasive procedures need not be painful (see Blood Specimens, Chapter 22). For example, the topical application of EMLA (an eutectic mix of lidocaine and prilocaine) or 4% lidocaine (Ela-Max or LMX) before needle punctures can eliminate pain (see Pain Management, Chapter 7). Therefore, the nurse is responsible for preparing the child and family for the tests by: • Explaining the significance of each test, particularly why the tests are not all done at one time • Encouraging parents or another supportive person to be with the child during the procedure • Allowing the child to play with the equipment on a doll or participate in the actual procedure (e.g., by cleansing the finger with an alcohol swab) Anemia caused by an inadequate supply of dietary iron is the most prevalent nutritional disorder in the United States and the most preventable mineral disturbance. The prevalence of iron-deficiency anemia has decreased during infancy in the United States, probably in part because of families’ participation in the Women, Infants, and Children (WIC) program, which provides iron-fortified formula for the first year of life and routine screening of Hgb levels during early childhood (Baker, Greer, and Committee on Nutrition American Academy of Pediatrics [AAP], 2010; Cusick, Mei, Freedman, and others, 2008). Preterm infants are especially at risk because of their reduced fetal iron supply. Children 12 to 36 months of age are at risk for anemia as a result of primarily cow milk intake and not eating an adequate amount of iron-containing food (Andrews, Ullrich, and Fleming, 2009; Baker, Greer, and Committee on Nutrition AAP, 2010; Richardson, 2007). Adolescents are also at risk because of their rapid growth rate combined with poor eating habits, menses, obesity, or strenuous activities. In formula-fed infants, the most convenient and best sources of supplemental iron are iron-fortified commercial formula and iron-fortified infant cereal. Iron-fortified formula provides a relatively constant and predictable amount of iron and is not associated with an increased incidence of gastrointestinal (GI) symptoms, such as colic, diarrhea, or constipation. Infants younger than 12 months of age should not be given fresh cow’s milk because it may increase the risk of GI blood loss occurring from exposure to a heat-labile protein in cow’s milk or cow’s milk–induced GI mucosal damage resulting from a lack of cytochrome iron (heme protein) (Glader, 2007; Richardson, 2007). If GI bleeding is suspected, the child’s stool should be guaiac tested on at least four or five occasions to identify any intermittent blood loss. If the Hgb level fails to rise after 1 month of oral therapy, it is important to assess for persistent bleeding, iron malabsorption, noncompliance, improper iron administration, or other causes of the anemia. Parenteral (IV or intramuscular [IM]) iron administration is safe and effective but painful, expensive, and occasionally associated with regional lymphadenopathy, transient arthralgias or serious allergic reaction (Andrews, Ullrich, and Fleming, 2009; Glader, 2007; McKenzie, 2004). Therefore, parenteral iron is reserved for children who have iron malabsorption or chronic hemoglobinuria. Transfusions are indicated for the most severe anemia and in cases of serious infection, cardiac dysfunction, or surgical emergency when anesthesia is required. Packed RBCs (2–3 ml/kg), not whole blood, are used to minimize the chance of circulatory overload. Supplemental oxygen is administered when tissue hypoxia is severe. The prognosis for a child with this condition is very good. However, some evidence indicates that if the iron-deficiency anemia is severe and longstanding, cognitive, behavioral, and motor impairment may result (Andrews, Ullrich, and Fleming, 2009; Lokeshwar, Mehta, Mehta, and others, 2011; McCann and Ames, 2007). If parenteral iron preparations are prescribed, iron dextran must be injected deeply into a large muscle mass using the Z-track method. The injection site is not massaged after injection to minimize skin staining and irritation. Because no more than 1 ml should be given in one site, the IV route should be considered to avoid multiple injections. Careful observation is required because of the risk of adverse reactions, such as anaphylaxis, with IV administration. A test dose is recommended before routine use. Recently, a new IV iron preparation (ferumoxytol) was approved in the United States that shows promise in complete replacement of iron with little toxicity (Auerbach, 2011). A primary nursing objective is to prevent nutritional anemia through family education. Because breast milk is a low iron source, the nurse must reinforce the importance of administering iron supplementation to exclusively breastfed infants by 4 months of age (Baker, Greer, and Committee on Nutrition AAP, 2010; Lokeshwar, Mehta, Mehta, and others, 2011). The AAP recommends that preterm, marginally low and low–birth-weight infants, or infants with inadequate iron stores at birth receive iron supplements at approximately 2 months of age (Berglund, Westrup, and Domellof, 2010). In formula-fed infants, the nurse discusses with parents the importance of using iron-fortified formula and of introducing solid foods at the appropriate age during the first year of life. Traditionally, cereals are one of the first semisolid foods to be introduced into the infant’s diet at approximately 6 months of age (Baker, Greer, and Committee on Nutrition AAP, 2010; Glader, 2007; Lokeshwar, Mehta, Mehta, and others, 2011). The best solid-food source of iron is commercial iron-fortified cereals. It may be difficult at first to teach the infant to accept foods other than milk. The same principles are applied as those for introducing new foods (see Nutrition, Chapter 10), especially feeding the solid food before the milk. Predominantly milk-fed infants rebel against solid foods, and parents are cautioned about this and the need to be firm in not relinquishing control to the child. It may require intense problem solving on the part of both the family and the nurse to overcome the child’s resistance. Diet education of teenagers is especially difficult, especially because teenage girls are particularly prone to following weight-reduction diets. Emphasizing the effect of anemia on appearance (pallor) and energy level (difficulty maintaining popular activities) may be useful. (See Mineral Imbalances, Chapter 11.)
The Child with Hematologic or Immunologic Dysfunction
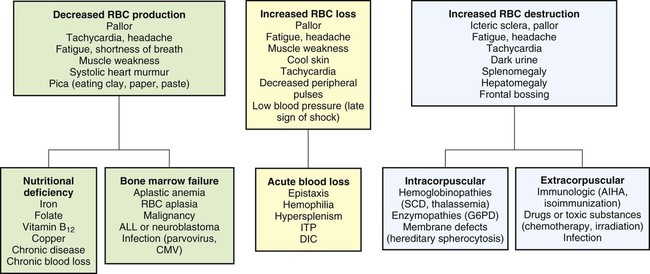
Hematologic and Immunologic Dysfunction
TEST (AVERAGE VALUE)*
DESCRIPTION, COMMENTS
RBC count (4.5–5.5 million/mm3)
Number of RBCs/mm3 of blood
Indirectly estimates Hgb content of blood
Reflects function of bone marrow
Hgb determination (11.5–15.5 g/dl)
Amount of Hgb (g)/dl of whole blood
Total blood Hgb primarily depends on number of circulating RBCs but also on amount of Hgb in each cell
Hct (35%–45%)
Percent volume of packed RBCs in whole blood
Indirectly measures Hgb content
Is approximately three times Hgb content
RBC indices
MCV (77–95 fl)
Average or mean volume (size) of a single RBC
MCV values are expressed as femtoliters (fl) or cubic microns (mm3)
MCH (25–33 pg/cell)
Average or mean quantity (weight) of Hgb in a single RBC
MCH values are expressed as picograms (pg) or micromicrograms (mmcg)
Whereas MCV and MCH depend on accurate counts of RBCs, MCHC does not; therefore, MCHC is often more reliable
All indices depend on average cell measurements and do not show individual RBC variations (anisocytosis)
MCHC (31%–37% Hgb [g]/dl RBC)
Average concentration of Hgb in a single RBC
MCHC values are expressed as percent Hgb (g)/cell or Hgb (g)/dl RBC
RBC volume distribution width (13.4% ± 1.2%)
Average size of RBCs
Differentiates some types of anemia
Reticulocyte count (0.5%–1.5% erythrocytes)
Percent reticulocytes in RBCs
Index of production of mature RBCs by bone marrow
Decreased count indicates depressed bone marrow function
Increased count indicates erythrogenesis in response to some stimulus
When reticulocyte count is extremely high, other forms of immature RBCs (normoblasts, even erythroblasts) may be present
Indirectly estimates hypochromic anemia
Usually elevated in patients with chronic hemolytic anemia
WBC count (4.5–13.5 × 103 cells/mm3)
Number of WBCs/mm3 of blood
Total number of WBCs less important than differential count
Differential WBC count
Inspection and quantification of WBC types present in peripheral blood
Values are expressed as percentages; to obtain absolute number of any type of WBC, multiply its respective percentage by total number of WBCs
Neutrophils (polys) (54%–62%) (3–5.8 × 103 cells/mm3)
Primary defense in bacterial infection; capable of phagocytizing and killing bacteria
Bands (3%–5%) (0.15–0.4 × 103 cells/mm3)
Immature neutrophil
Increased numbers in bacterial infection
Also capable of phagocytosis and killing
Eosinophils (1%–3%) (0.05–0.25 × 103 cells/mm3)
Named for their staining characteristics with eosin dye
Increased in allergic disorders, parasitic diseases, certain neoplasms, and other diseases
Basophils (0.075%) (0.015–0.030 × 103 cells/mm3)
Named for their characteristic basophilic stippling
Contain histamine, heparin, and serotonin; believed to cause increased blood flow to injured tissues while preventing excessive clotting
Lymphocytes (25%–33%) (1.5–3.0 × 103 cells/mm3)
Involved in development of antibody and delayed hypersensitivity
Monocytes (3%–7%)
Large phagocytic cells that are involved in early stage of inflammatory reaction
ANC (>1000/mm3)
Percent neutrophils/bands times WBC count
Indicates body’s capability to handle bacterial infections
Platelet count (150–400 × 103/mm3)
Number of platelets/mm3 of blood
Cellular fragments that are necessary for clotting to occur
Stained peripheral blood smear
Visual estimation of amount of Hgb in RBCs and overall size, shape, and structure of RBCs
Various staining properties of RBC structures may be evidence of immature forms of erythrocytes
Shows variation in size and shape of RBCs: microcytic, macrocytic, poikilocytic (variable shapes)
Red Blood Cell Disorders
Anemia
Classification
Diagnostic Evaluation
Nursing Care Management
![]() Case Study—The Child with Anemia
Case Study—The Child with Anemia
Prepare the Child and Family for Laboratory Tests
Iron-Deficiency Anemia
Therapeutic Management
Prognosis
Nursing Care Management
Diet
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Child with Hematologic or Immunologic Dysfunction
Get Clinical Tree app for offline access