The Child with Cancer
Learning Objectives
After studying this chapter, you should be able to:
• List common clinical manifestations of childhood cancer.
• Discuss the treatment modalities used in the treatment of children with cancer.
• Demonstrate an understanding of the nursing care associated with caring for a child with cancer.
![]()
http://evolve.elsevier.com/McKinney/mat-ch
Clinical Reference
Review of Cancer
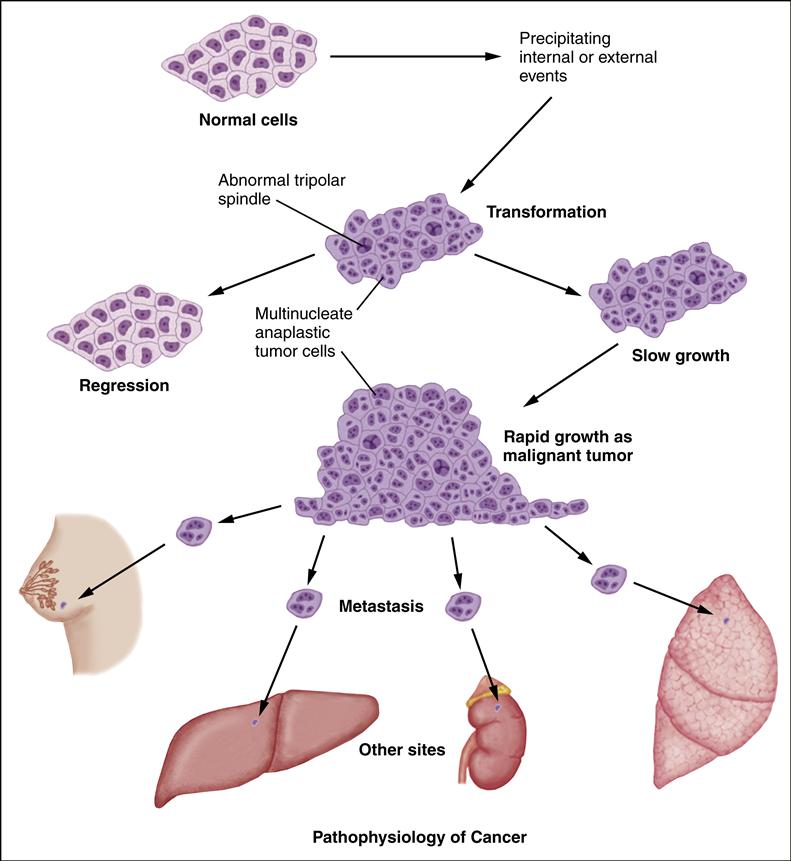
A neoplasm is any tumor that arises from new, abnormal cell growth. A tumor may be either benign or malignant. The distinguishing feature of cancer is its ability to invade surrounding tissue and spread to distant sites. Cancer cells spread in one of two ways: (1) by invasion, in which cells grow in unrestricted, disorderly fashion at the site of origin; and (2) by metastasis, in which the cells grow in sites other than the site of the primary cancer. The cancerous cells grow progressively. The cells have lost the ability to perform their intended functions because changes in the cell’s deoxyribonucleic acid (DNA) cause “wrong” information to be transmitted. As the cancerous cells continue to proliferate, they crowd out normal cells and compress vascular structures and vital organs, which results in symptoms.
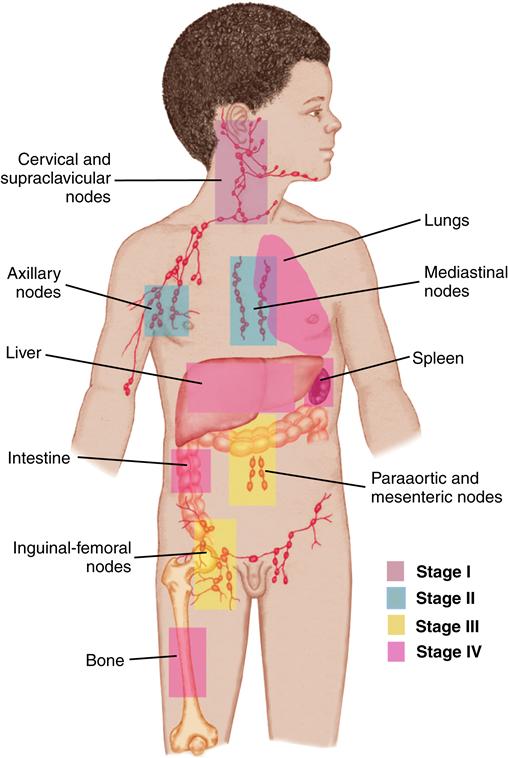
Tumor staging is based on the results of diagnostic studies and, in some cases, surgical examination. Staging describes the extent of disease locally, regionally, and systemically and guides the therapy for most solid tumors. Each tumor has its own specific system of staging, which assists in determining treatment and prognosis.
The cause of most childhood cancers is unknown. One possible underlying cause of cancer is genetic. Alterations in normal DNA occur that predispose the child to the development of cancer. A small percentage of cancers are associated with an inherited predisposition related to chromosomal abnormalities (Asselin, 2011). A second, more controversial hypothesis contends that cancer develops as a result of failure of the immune system to distinguish between normal and abnormal cells. Inactivation of tumor suppressor genes is also thought to be implicated. Known carcinogens, such as radiation, physical irritation, and chemical irritants, contribute to the development of cancer.
The cardinal signs of cancer in children differ from those seen in adults. Most adult cancers are carcinomas, and more screening tools are available to assist with their early detection. The difficulty in diagnosing cancer in children is that symptoms resemble those of common childhood illnesses. Children are often not brought for medical care until obvious signs and symptoms are present. Primary care providers are understandably reluctant to think about cancer as the cause of the child’s illness.
DIAGNOSTIC TESTS AND PROCEDURES FOR CANCER
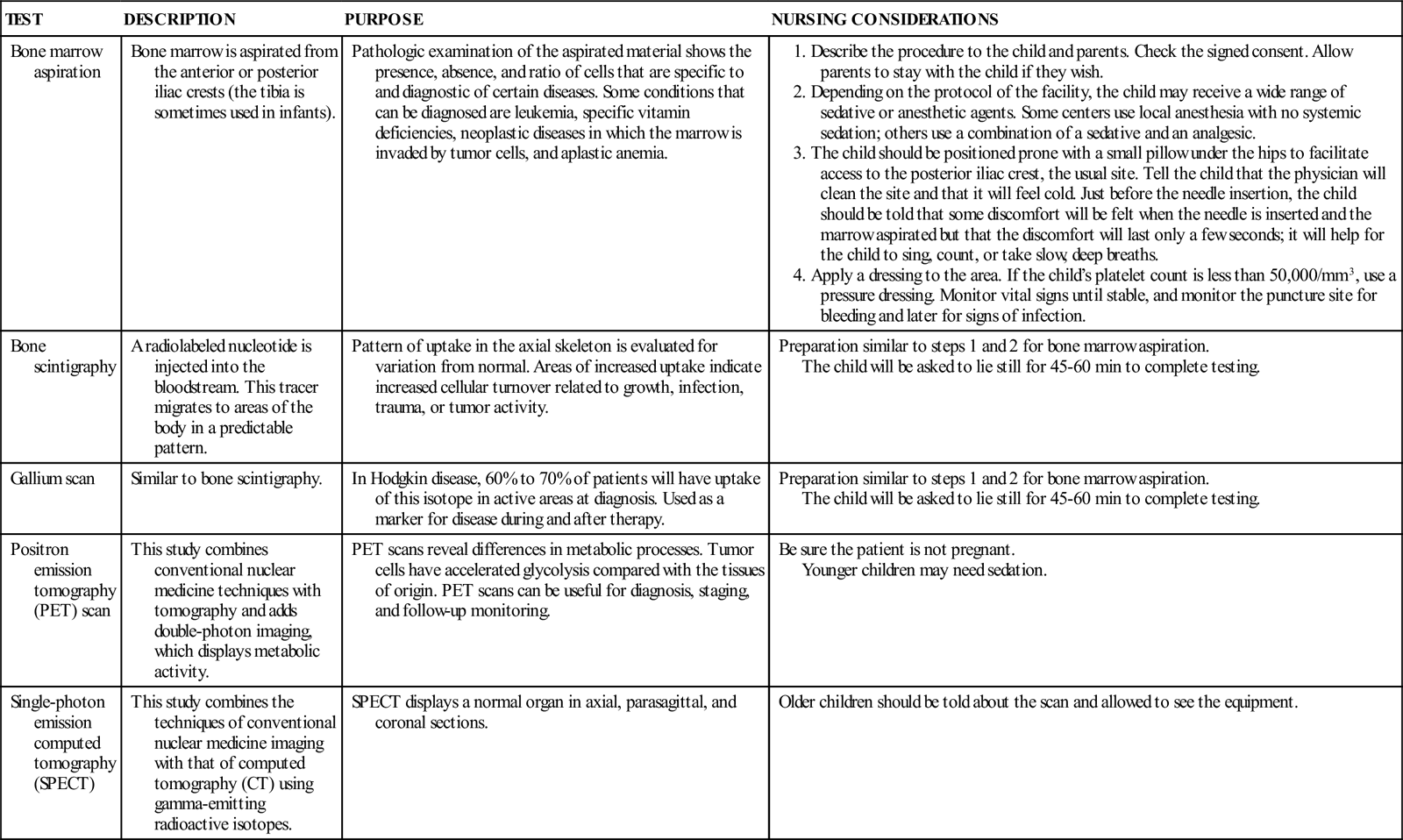
See Chapter 52 for information about other diagnostics tests (CT scan, lumbar puncture, magnetic resonance imaging [MRI]).
The Child With Cancer
Cancer in children is often difficult to diagnose, and health care providers must be aware of the clinical manifestations that should raise the suspicion of cancer. The signs and symptoms depend on the type of tumor, the location of the tumor within the body, the extent of the disease, and the child’s age. Testing, diagnosis, and initiation of therapy may occur within a very short period. The diagnosis of cancer can be devastating to both the child and the family. The nurse becomes the informational lifeline for the child and the family as they go through the treatment process.
Incidence
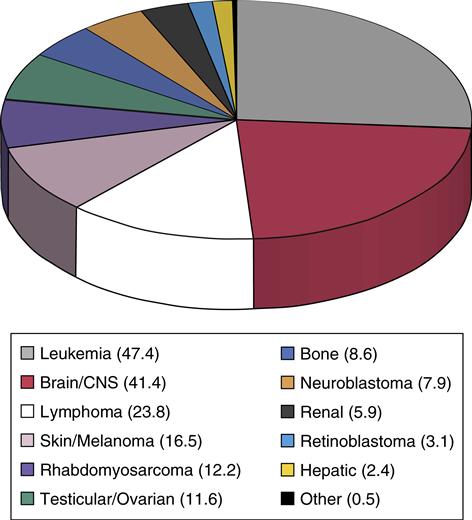
Cancer is uncommon in children; nevertheless, pediatric cancer is the second leading cause of death in childhood, after unintentional injuries, and is the leading cause of death from disease. Childhood cancer represents only approximately 1% of all new cancers diagnosed annually in both children and adults in the United States. The most common childhood cancers are leukemias, brain tumors, and lymphomas (Asselin, 2011) (Figure 48-1). Treatment challenges include minimizing treatment-related side effects while maintaining the child’s normal growth and development.
Childhood Cancer and Its Treatment
Children with cancer are treated in a multidisciplinary setting. Pediatric oncology nurses play a prominent role in the care of children with cancer and their families. They support and educate the children and their families as they move through a stressful process. Pediatric oncology nurses are challenged to maintain a high level of technical competence and an ability to provide the psychological support required by the child and family. Working with children with cancer can be an emotional experience. The nurse in this setting must have a support system and be aware of personal limitations and therapeutic relationship boundaries (Gerow, Conejo, Alonzo, et al., 2010).
A great deal of research has been done over the past 30 years to improve the outcomes for children with cancer. Current survival rates are attributed to cooperative, systematic research through the Children’s Oncology Group (COG) and the International Society for Pediatric Oncology. Each group meets twice a year to develop new research protocols and monitor the progress of current protocols; subgroups meet as needed throughout the year. Research protocols direct when drugs are to be given, how frequently, and in what dosages, and which diagnostic and follow-up studies are to be performed. Research has shown that children have better outcomes if they are treated by a scientifically derived protocol.
Because of advances in treatment, approximately 8 out of 10 children treated for cancer will survive 5 years or longer after their diagnosis (American Cancer Society, 2012b). The marked improvement in childhood cancer survival rates has placed renewed emphasis on the importance of identifying the long-term sequelae of cancer treatment in children and initiating timely intervention (American Cancer Society, 2012b).
Even with apparently successful treatment of cancer in children, the disease may recur. A recurrence may occur during therapy, shortly after therapy has been completed or years later. A second tumor may represent a new (or second) malignancy. Recurrence represents a resurgence of the initial disease, whereas a second cancer is a likely result of the initial treatment. For example, some children with acute lymphocytic leukemia (ALL) develop acute myeloid leukemia (AML) after therapy is complete. Brain tumors may develop in a small number of children with ALL who were treated with radiation to their central nervous system (CNS).
Therapeutic Management
Chemotherapy, surgery, and radiation therapy are the primary treatment modalities for children with cancer. Hematopoietic stem cell transplantation (HSCT), steroid therapy, and biologic response modifiers are reserved for a specific subpopulation of children with cancer.
Chemotherapy
Chemotherapy is the use of drugs (antineoplastic agents) to kill cancer cells. Different drugs have different side effect profiles and modes of action. Combinations of drugs known individually to be active against the specific disease are used. Tumors possess the ability to develop resistance to chemotherapy agents, so a variety of active drugs are frequently used. Chemotherapy may be given orally, intravenously, intramuscularly, subcutaneously, or intrathecally (through the spinal column). Depending on the protocol, a child may be hospitalized for chemotherapy, receive it on an outpatient basis, or be treated at home.
The side effects of chemotherapeutic agents represent challenges to caregivers. Chemotherapy nonselectively kills rapidly dividing cells. In addition to cancerous cells, the cells most often affected include cells of the hematopoietic system, gastrointestinal (GI) tract, and integumentary system (Box 48-1).
The bone marrow cells are one of the rapidly proliferating tissues adversely affected by many chemotherapy agents. Bone marrow production may become suppressed, resulting in neutropenia, anemia, and thrombocytopenia. The nadir—the time of the greatest bone marrow suppression when blood counts will be the lowest—generally occurs 7 to 14 days after chemotherapy administration, depending on the specific agent used. The greatest concern during the period of bone marrow suppression is infection.
Neutropenia places the child with cancer at risk for the development of opportunistic infections. Opportunistic infections are caused by nonpathogenic bacteria, viruses, and fungi that, because of compromised immunity, may invade and cause infection. Bacteria, generally present on the skin and within the intestinal tract, may invade the bloodstream through a break in the skin or mucous membranes, leading to a life-threatening infection. In the presence of markedly decreased white blood cells (WBCs), the usual inflammatory responses (erythema, edema, swelling) indicative of an infection is not present. Fever is frequently the only indication of infection. Health care providers and families must remain acutely aware of elevated body temperature and breaks in the skin during periods of neutropenia.
The GI tract is affected in a number of ways. Chemotherapy represents a noxious stimulus that triggers nausea and vomiting. The treatment of nausea and vomiting was revolutionized in 1992 with the release of a class of nonsedating antiemetic drugs called 5-HT3 serotonin antagonists. These drugs include ondansetron (Zofran), granisetron (Kytril), and dolasetron (Anzemet). 5-HT3 serotonin antagonists prevent serotonin from binding to vagus nerve receptors, consequently interrupting neurologic signals to the vomiting center of the brain. They have been more effective in combating chemotherapy-induced nausea and vomiting than earlier antiemetics.
Another nonsedating antiemetic drug has further improved the control of chemotherapy-induced nausea and vomiting. Aprepitant (Emend) belongs to a class of drugs known as substance P antagonists. Aprepitant works by binding to neurokinin 1 receptors in the central and peripheral nervous systems, thereby preventing activation of these receptors by substance P that would trigger vomiting. Aprepitant is used in conjunction with 5-HT3 serotonin antagonists and has proven to be very effective at preventing chemotherapy-induced nausea and vomiting.
Anorexia is associated with nausea and a change in taste experienced by some people in response to certain chemotherapeutic agents. Anorexia can lead to malnourishment resulting in weight loss and poor linear growth. Although antiemetics can be effective at preventing nausea and vomiting, they are not able to prevent alterations in taste that can occur with chemotherapy administration. This alteration in taste, often accompanied by an increased sensitivity to odors, contributes to anorexia.
Certain chemotherapeutic agents cause sloughing of the mucosal tissue of the GI tract, leading to the development of mucositis, both oral (stomatitis) and perianal, and esophagitis. These conditions can be painful and can contribute to poor nutrition. Bacteria and yeasts, present as part of the normal digestive process in the mouth and intestinal tract, may cross the open skin or mucous membrane and be absorbed into the bloodstream. The presence of breaks in the integument may lead to bacterial infections of the blood, particularly alpha-hemolytic streptococcus.
Decreased activity, pain medication, and poor oral intake may contribute to the development of constipation. Certain chemotherapeutic agents (such as vincristine) may also contribute to constipation. Passage of hard stool may cause abrasion of the delicate mucous membrane of the rectum. The stool is loaded with microorganisms as part of the digestive process. Again, the presence of breaks in the integument may lead to bacterial infections of the blood.
Hair loss (alopecia) has a tremendous psychological effect, especially on school-age children and adolescents. Some chemotherapeutic agents do not produce hair loss, but most do. Children should be reassured that their hair will grow back after the completion of therapy. Accommodations should be made to ease the child’s transition to alopecia, including the use of wigs, hats, or scarves. Although young children may respond casually to hair loss, teenagers, particularly girls, often struggle with this dramatic change in their body image.
Changes to the appearance of the skin and nails are common while receiving chemotherapy. These changes include hyperpigmentation of the skin, especially of the hands. Striae, or stretch marks, are also common with treatment regimens that contain high-dose steroids. The nails may become brittle and appear either darkened or with whitish inclusions and/or scarring. These changes, with the exception of striae, typically resolve on completion of the chemotherapy regimen. In addition, many of the medications (such as methotrexate and trimethoprim-sulfamethoxazole) can lead to hypersensitivity to sunlight. The child should avoid excessive sun exposure by the use of long sleeves, hats, and sunscreen.
Treatment-related fatigue, common in adult cancer patients, is poorly reported in the child and adolescent populations, although an increasing body of research has identified and described fatigue in adolescents with cancer (Erickson, 2010). Other side effects are specific to the agent being used, as well as the dose.
Nurses administering chemotherapeutic agents should have evidence of special chemotherapy training by the institution in which they work. The Association of Pediatric Hematology/Oncology Nurses (APHON) (2011) has developed a Pediatric Chemotherapy and Biotherapy Provider Program to help standardize nursing education regarding administration of pediatric chemotherapy and biotherapy. The program consists of a provider course and an instructor course. Nursing responsibilities and precautions related to chemotherapy administration are detailed in Box 48-2.
Surgery
Surgery is frequently part of cancer therapy for children. The surgery may be limited to a biopsy or may involve the removal of a solid tumor mass. The purpose of a biopsy is to obtain a small piece of the tumor for microscopic examination. Examination of the tissue by a pathologist confirms the tumor type and influences therapy decisions. Surgery may also be used for debulking or resecting a solid tumor mass. In some diseases, the tumor cannot be resected at the beginning of therapy. After the child has received a few rounds of chemotherapy, the mass may decrease in size and a less extensive surgical procedure can then be performed (see Chapter 37 for a discussion of preoperative care).
A central venous catheter (CVC) is frequently placed during an initial surgical procedure to facilitate chemotherapy administration. A CVC is a central line that provides easy access to the venous system; the proximal tip of the catheter ends in the large vein just above the heart, the superior vena cava. Three types of CVCs are available. In an external catheter, the distal portion exits the skin and a tiny polyester cuff is “tunneled” under the exit site where the skin will adhere and hold the catheter in place. In an implanted venous access device (IVAD) or Port-A-Cath, the distal portion ends in a well or reservoir, which is placed in the subcutaneous tissue of the anterior chest wall. A percutaneously (peripherally) inserted central catheter (PICC) is usually placed in an interventional radiology unit. It is inserted in the brachial vein at or near the antecubital fossa using a technique that initially is similar to placement of a peripheral intravenous (IV) catheter. The PICC is then advanced up the veins of the arm until the tip ends in the superior vena cava.
Preparing the child and family for surgery includes providing information about preparation for surgery (bowel preps, intake [nothing-by-mouth {NPO}] restrictions) and education regarding the postoperative expectations such as pain control, wound healing, signs and symptoms of bleeding. Because the risk of infection is higher for those receiving chemotherapy, the signs and symptoms of wound infection are important and may be subtle in an immunosuppressed child with a lower than usual WBC count. Those symptoms include warmth, redness, tenderness, and drainage. In addition, it is important for the nurse to check the most recent blood counts before surgery to ensure that the surgical timing is appropriate in relation to the last chemotherapy administration.
Radiation Therapy
Radiation may be given to cure or eradicate disease or given in low doses as a palliative therapy to prevent further growth of a tumor. To eradicate microscopic disease and promote bone marrow suppression, total body irradiation is given before some stem cell transplants. Radiation may be given in fractionated doses, in which the daily dose is split into smaller doses given more frequently to minimize side effects and increase tumor kill by decreasing time for cell repair between doses.
Preparing the child and family for radiation involves education about the process in addition to the side effects. Some institutions provide an introductory tour (often called a simulation) of the radiation facility so the child may experience the room and surroundings before therapy begins. During the tour, children should be shown the window or monitor through which they will be observed while undergoing radiation alone in the
room. Some children need to be sedated for radiation treatments; others can be coached to lie still with the help of child life specialists and parents. The child must lie still for what seems like long periods because the radiation oncologist must carefully control the depth and peripheral margins of the radiation site.
During the simulation, computed tomography (CT) scans and x-rays are performed to identify the site where the radiation therapy will be delivered. The child may receive skin markings with an indelible marker to help guide the radiation oncologists during each treatment. The markings are often covered with a transparent dressing. These markings (or tattoos) should be protected from inadvertent removal.
The side effects of radiation are dose and treatment site specific. As with chemotherapy, side effects are a result of radiation’s effect on healthy, rapidly dividing cells. The side effects usually appear 7 to 10 days after the initiation of therapy. Most often, acute side effects usually dissipate a few days or weeks after the end of radiation therapy. Common side effects include fatigue, skin damage, hair loss, nausea and vomiting, and low blood counts. Children receiving cranial radiation are particularly affected by fatigue and an increased need for sleep during and shortly after completion of a course of radiation. Skin damage can include changes in pigmentation (darkening), redness, peeling, and increased sensitivity. Extra care must be taken to avoid excessive skin exposure to heat, sunlight, friction (such as rubbing with a towel or washcloth), and creams or moisturizers. Only topical creams and moisturizers prescribed by the radiation oncologist should be applied to the radiated skin.
The decision regarding radiation dose, frequency, and location depends on the purpose of the radiation and the disease process being treated. In general, radiotherapy is used more cautiously during childhood because children’s developing tissues and organs are more vulnerable to radiation’s adverse effects (Bleyer & Ritchey, 2011).
Radiation therapy slows the growth of tumors and kills rapidly dividing cells nonselectively. Unfortunately, in a developing child, normal cell development may not be complete when radiation exposure occurs. Radiation therapy to developing brain tissue may alter cognitive potential. In children younger than 3 years, the effect of radiation therapy can be cognitively devastating. Bone growth is altered if radiation therapy is delivered to areas of growth potential, such as facial bones, spine, or growth plates in long bones. The result many years later may be skeletal malformations and failure to achieve anticipated growth.
Radiation exposure has been linked to the development of certain types of cancer, and radiation exposure to treat cancer may lead to the development of a second malignancy. Between 3% and 10% of children treated for cancer will have a second malignancy develop. A subset of these secondary malignancies will be linked to the exposure to radiation as a primary treatment (National Cancer Institute, 2010).
Hematopoietic Stem Cell Transplantation
In recent years, the use of hematopoietic stem cell transplantation (HSCT) has become accepted therapy for the treatment of several hematologic and oncologic disorders. Transplantation allows extremely high doses of chemotherapy (with or without radiation) to be given without regard for bone marrow recovery because hematopoiesis will be restored through transplantation. Stem cells are harvested from bone marrow, peripheral blood, and umbilical cord blood. HSCT is often used interchangeably with bone marrow transplant (BMT) in the clinical setting even when referring to stem cells from cord or peripheral blood.
BMT uses bone marrow to reconstitute the immunologic function of the child after high-dose chemotherapy. Stem cell transplantation uses a unique immature cell present in the peripheral circulation to restore immunologic function in a similar manner. Stem cells are able to differentiate into any type of hematologic cell.
The healthy bone marrow cells or stem cells are infused into the bloodstream and migrate to the marrow space to replenish the child’s immunologic function. The decision regarding the source of marrow or stem cells depends on the disease process being treated and the availability of an appropriate donor source.
Recent advances in the understanding of histocompatibility and advances in supportive care have improved outcomes in allogeneic (matched related or unrelated donor) transplants. The child’s own harvested stem cells (an autologous transplant) using peripheral blood can be the source of stem cells in certain instances. This allows for aggressive chemotherapy that leads to almost total bone marrow ablation. Peripheral blood stem cells (PBSCs) are then given back to “rescue” and restore hematopoietic function of the child’s bone marrow.
Umbilical cord blood is another source of transplanted stem cells. Because of the ability to “bank” or store umbilical cord blood, this source is becoming more significant. Cord blood from infants is easily harvested and banked. The donor undergoes no risk during harvesting of the cord blood, and the graft is thought to be more immunologically “tolerant” than stem cells from older donors. A national or international search for a matched, unrelated donor can be done through the National Marrow Donor Program (NMDP).
In preparation for a transplant, the child begins a regimen of chemotherapy with or without radiation (called conditioning). The goal of conditioning is to eradicate any disease from the body with high-dose chemotherapy and radiation therapy. WBC, red blood cell (RBC), and platelet counts begin to drop as the chemotherapy and radiation exert their effects on the bone marrow. When the conditioning phase is over, the child receives the donor marrow or stem cells by IV infusion.
Once the marrow is infused, nursing care focuses on preventing profoundly immunosuppressed children from developing life-threatening infections and on minimizing treatment-related side effects. Parents and the child anxiously wait for the day when the complete blood cell counts begin to show signs of marrow engraftment. The production of WBCs, RBCs, and platelets from the transplantation of normal cells is evidence that the marrow has engrafted, or been accepted by the body.
Common complications in the days and weeks after HSCT include mucositis, diarrhea, fevers, and nosebleeds. Children receive aggressive nutritional support because most will have substantial difficulty taking foods and fluids orally as a result of severe mucositis and GI discomfort and diarrhea.
The major problem associated with allogeneic transplants is graft-versus-host disease (GVHD). GVHD is caused when the infused immunocompetent donor bone marrow recognizes the recipient’s tissue as foreign and attacks the child’s body affecting numerous organ systems. Children can exhibit a wide variety of symptoms associated with GVHD, such as mild to severely elevated liver enzyme levels, mild to copious diarrhea, and maculopapular skin reactions ranging from rashes to full skin desquamation. Antirejection drugs such as prednisone, cyclosporine, and tacrolimus are given to prevent GVHD from occurring or lessen its severity.
Transplantation is currently standard therapy for children in first remission with Philadelphia chromosome–positive ALL (a genetically specific type of ALL with a 90% relapse rate), AML, stage IV neuroblastoma, severe aplastic anemia, severe combined immunodeficiency syndrome, and certain other hematologic disorders (see Chapter 47). Transplantation is also used to treat children with certain solid tumors, Hodgkin disease, and non-Hodgkin lymphoma that are resistant to conventional chemotherapy and radiation, and to treat children who experience relapses (Velardi & Locatelli, 2011).
Steroid Therapy
High-dose and/or long-term steroid therapy is a mainstay of treatment for children with leukemia, as an adjunct for control of nausea and vomiting, and for children with brain tumors complicated by increased intracranial pressure. High-dose steroids (dexamethasone and prednisone) cause a myriad of side effects that include increased appetite, fluid retention, weight gain, hypertension, insulin-dependent diabetes (usually reversible once steroid therapy is stopped), emotional lability (mood changes), sleep disturbances, changes in appearance (abdominal striae, cushingoid features), and immunosuppression (Hinds, Hockenberry, Gattuso, et al., 2007; Taketomo, Hodding, & Kraus, 2010).
It is important to educate the child and caregivers about these side effects as they can be quite frightening (sleep disturbances such as vivid dreaming) and disruptive (mood shifts from angry to sad to happy in very short periods of time). In addition, the family should be prepared for changes in the child’s diet such as cravings for high salt-containing foods that can contribute to increased fluid retention, weight gain, and hypertension. The family should be aware of the need to notify the health care team if the child has increased thirst and/or voiding at night, which can be an indication of hyperglycemia. The physical changes associated with steroid use can create body image disturbances for children. These changes (cushingoid features) include puffy cheeks; abdominal weight gain; striae (stretch marks); increased acne; and a flushed, shiny appearance of the skin.
The child and caregivers should be reassured that these symptoms typically resolve over a period of weeks once the steroid therapy has stopped.
Biologic Agents
Recent additions to cancer therapy are the biologic response modifiers. Biologic response modifiers are naturally occurring substances found in small quantities in the body that influence immune system functions (e.g., colony-stimulating factors [CSFs]).
Used to enhance cell recovery, different CSFs work on different types of blood cells to reduce the time and severity of bone marrow suppression. Granulocyte colony-stimulating factors (GCSFs) stimulate WBC recovery. GCSFs may reduce the length of time a child has neutropenia by stimulating production of neutrophils, a type of granulocyte. Other CSFs may promote recovery of platelets or RBCs and subsequently reduce the need for blood products.
Over the past few years, a number of immune-modulating agents have been examined in the laboratory and some have translated into clinically beneficial treatment modalities. Certain “targeted” monoclonal antibodies are being used for very specific types of neoplasms and often are used in conjunction with other modalities, such as radiation and chemotherapy. They provide the advantage of effective treatment with less toxicity to normal tissue than other cancer modalities (Bleyer & Ritchey, 2011).
Complementary and Alternative Medical (CAM) Therapies
Complementary therapies are proven therapies (based on research) or therapies that are not yet scientifically proven but are deemed not to be harmful as adjunctive treatment. Alternative therapies are those designed to replace conventional therapy in the treatment of individuals with cancer. The use of CAM therapies, although often harmless and potentially beneficial for the child and family, can negatively affect the efficacy of therapy. Therefore, it is important to determine if children are receiving CAM therapies. One approach is to build a supportive, open rapport with these children and their families. However, not all families will disclose their use of CAM therapies without direct, but supportive, questioning. Most pediatric oncology centers provide an informational notebook or handbook to the family at the time of diagnosis. One technique for eliciting discussion regarding the use of CAM therapies is to include a description of their use and to emphasize the importance of discussing this issue with the child’s health care providers.
When assisting families in their thinking about complementary and alternative therapies, health professionals should ask them to consider the following questions (National Center for Complementary and Alternative Medicine, 2010):
• What are the benefits and risks associated with the therapy?
• Do the benefits outweigh the risks?
• What side effects can be expected?
• What are the costs, and will the therapy be covered by insurance?
• What training and other qualifications does the practitioner have?
• Are there scientific articles or references about using the treatment?
• Could the therapy interfere with or delay conventional treatments?
• How long will the treatment last, and how often will it be assessed?
• Will it be necessary to buy equipment or supplies?
• Are there any conditions for which this treatment should not be used?
Family-centered care is delivering care with the intent of developing mutually beneficial partnerships between health care providers, families, and patients. The first family-centered approach regarding the use of CAM therapies includes the goal of preserving the dignity of patients and their families. The nurse can achieve this goal by validating the feelings of the child and family regarding their desire to achieve a cure for the child’s cancer. The nurse first seeks to understand what CAM therapy the family has chosen and why the family has made the choice. The next family-centered care approach is information sharing. The nurse provides factual information and useful resources to families regarding CAM therapies and cancer. The ultimate decision regarding the use of CAM therapies rests with the family in consultation with the health care team. Nurses collaborate with the family in care planning that includes physician-approved CAM therapies.
Leukemia
As the first disseminated cancer shown to be curable, the approaches to caring for children with childhood leukemia set the standard for principles of pediatric cancer diagnosis, prognosis, and treatment (Tubergen, Bleyer, & Ritchey, 2011). Leukemia is the most common form of cancer in children younger than 15 years of age. The cause of disease is an abnormal proliferation of immature WBCs (blasts), which compete with normal cells for space and nutrients. Bone marrow production of other cells is suppressed, so very low numbers of RBCs (anemia) and platelets (thrombocytopenia) may be seen at diagnosis. Considerable progress in treatment has been achieved through years of research. Leukemia was uniformly fatal in the 1960s. Today, children diagnosed with the most common form of leukemia, acute lymphocytic leukemia (ALL), can almost always achieve remission, with a 5-year disease-free survival rate approaching 85% (Campana & Pui, 2008).
Etiology
The cause of childhood leukemia is unknown. Geographic distribution varies around the world, with leukemia being uncommon in developing countries but more common in industrialized countries. This variation may be correlated with under diagnosis in developing countries or exposure to agents may be implicated in the development of leukemia in industrialized countries.
Genetic factors appear to play a significant role in the development of leukemia. When karyotyped, the leukemic cells in most children with the disease reveal chromosomal abnormalities. Some of these chromosomal abnormalities have become prognostic factors for the disease (Tubergen et al., 2011). Because of the genetic basis of this disease, identical twins have a significantly greater chance of sharing chromosomal abnormalities that later lead to disease. So, the risk of a second twin acquiring the disease after the first has been diagnosed in infancy is significantly higher than that of the general population (Tubergen et al., 2011). The fraternal twin of a child who has had ALL has a two to four times higher likelihood of developing the disease than other children. Children with Down syndrome have a 10% to 20% greater risk of developing leukemia than the general population (Campana & Pui, 2008). Other less common preexisting chromosomal abnormalities, such as Fanconi anemia and neurofibromatosis, have been correlated with the development of leukemia.
Exposure to ionizing radiation and certain chemical toxins has been shown to increase the risk of leukemia development. Leukemia was well documented in both the child and adult survivors of the atomic bomb detonations in Japan during World War II. Chemical exposure to alkylating agents, a drug class used to treat cancer, has been shown to increase the risk of developing acute myeloid leukemia (AML).
Large epidemiologic studies are ongoing to examine links to pesticide exposure, electromagnetic fields, parental smoking, parental alcohol use, and parental exposures to occupational chemicals. Thus far, relationships between these exposures and leukemia have not been demonstrated.
Incidence
Leukemias represent approximately 31% of all cancers in children younger than 14 years in the United States, with the highest percentage of children diagnosed with leukemia having ALL (>75%) (Leukemia and Lymphoma Society, 2010). ALL is more common in boys, and the peak incidence occurs between ages 2 and 3 years of age (Tubergen et al., 2011). With improvements in treatment, mortality in children younger than 15 years old has decreased markedly, and nearly 90% survive beyond 5 years after diagnosis (Leukemia and Lymphoma Society, 2010).
Manifestations
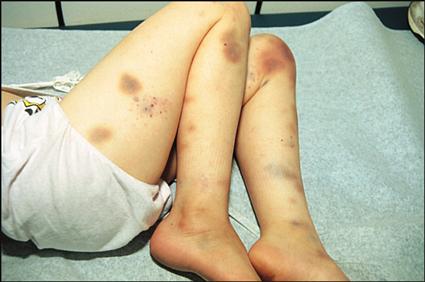
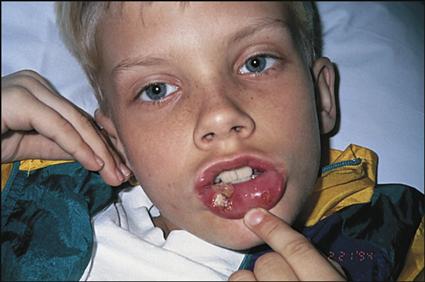
Clinical manifestations of leukemia include fever, pallor, excessive bruising, bone or joint pain (usually leg or knee pain), lymphadenopathy, malaise, hepatosplenomegaly, abnormal WBC counts (either lower or higher than normal for age), and mild to profound anemia and thrombocytopenia. The severity of the clinical manifestations varies with the cell type of leukemia and the length of time before diagnosis.
Diagnostic Evaluation
The diagnosis can be strongly suspected from a history of the clinical manifestations and an initial complete blood count (CBC). The confirmatory test for leukemia is microscopic examination of bone marrow obtained by bone marrow aspiration and/or biopsy. A bone marrow aspirate usually provides sufficient material to establish the diagnosis of ALL. A lumbar puncture is also performed to look for leukemic blast cells in the spinal fluid, which are indicative of CNS involvement.
Flow cytometry, the analysis of the bone marrow cells using a laser beam, is another test that is commonly performed on the initial bone marrow sample. Flow cytometry provides a rapid diagnosis by characterizing the type of leukemia within hours. In addition, a portion of the initial bone marrow sample is sent for cytogenetic analysis to determine the chromosomal changes that may have occurred in the leukemic blast cells. These results are typically available in 2 to 3 weeks and therefore are not useful for making induction treatment decisions. However, this chromosomal information can be used to determine the intensity of the child’s consolidation and maintenance therapy based on known prognostic indicators.
Therapeutic Management
Combination chemotherapy is the preferred treatment for leukemia. The particular drugs used and their dose, route, and
scheduling depend on the protocol that will be used for that specific type of leukemia. Children are placed into prognostic categories with specifically tailored therapies. Treatment of ALL is divided into phases: induction, consolidation, and maintenance. The aim of the first month of chemotherapy treatment, or induction, is to induce remission. Remission is the reduction of immature blast cells in the bone marrow to less than 5%. Approximately 98% of children with ALL achieve remission within 1 month (Tubergen et al., 2011).
Before induction, the child is treated for presenting signs, which may include sepsis, anemia, hemorrhage, and metabolic abnormalities. Serum electrolyte levels are determined to ensure metabolic stability before chemotherapy is initiated. An elevated uric acid level, indicating rapid cell turnover, can be expected if the WBC count is very high. As WBCs break down in reaction to chemotherapy, they release uric acid. Uric acid has poor water solubility and can compromise kidney function (tumor lysis syndrome). When the WBC count is extremely high, allopurinol and IV fluids with sodium bicarbonate are given to decrease the serum uric acid level and alkalinize the urine before chemotherapy starts. Parenteral urate oxidase may be given in situations when lysing of the tumor by chemotherapy is expected to be significant. This recombinant enzyme oxidizes uric acid into a water soluble product that can be excreted (Taketomo et al., 2010). During induction, the hospitalized child receives the first doses of chemotherapy while the response to the drugs is assessed. Remission can be verified within the first 28 days after the initiation of chemotherapy by sequential bone marrow aspirates and lumbar punctures. If a significant number of blast cells are still present, a new and stronger drug regimen is given. The presence of more than 5% blasts in the marrow at day 28 is an ominous sign indicative of a poorer prognosis.
Once the child is medically stable, most chemotherapy treatment for ALL is given on an outpatient basis. Children are usually healthy and able to return to school and engage in most age-appropriate activities.
Consolidation is the phase of therapy for ALL that follows induction and remission. The goal of consolidation therapy is to maintain remission and prevent disease in extramedullary “sanctuary sites” such as the testes and CNS where systemic therapy is not easily delivered. Intrathecal chemotherapy is given prophylactically to prevent relapse in the CNS. If the testes are involved, radiation therapy is administered.