The Child with a Neurologic Alteration
Learning Objectives
After studying this chapter, you should be able to:
• Describe the embryologic development of the nervous system.
• Describe the anatomy and physiology of the nervous system.
• Describe the normal compensatory mechanisms that keep intracranial pressure within a constant range.
• Identify the neurologic differences among the infant, child, and adult.
• Be able to perform a neurologic assessment of a child and record findings.
• List the measures used to keep a child safe during a seizure.
• List the measures used to prevent or treat cerebral edema.
• Differentiate between abnormal flexion and extension posturing and discuss the significance of each.
![]()
http://evolve.elsevier.com/McKinney/mat-ch
Clinical Reference
Review of the Central Nervous System
Embryologic Development
The nervous system is one of the first systems to form in utero. A neural tube remains hollow throughout development and eventually becomes the central nervous system. By the 4th week of gestation, the neural tube has closed at the anterior end to form the brain and at the posterior end to form the spinal cord.
During the second month of gestation, the brain becomes the prominent body structure. It grows rapidly and continues to grow until approximately the fifth year of life. Two periods of rapid brain cell growth appear to occur during gestation. Between the 15th and 20th weeks of gestation, the number of neurons increases significantly. At 30 weeks, the number of neurons increases again, continuing through 1 year of age. Appropriate prenatal care during periods of rapid neuronal increase can prevent developmental neurologic deficits.
The Myelin Sheath
Myelin is the fatty substance that surrounds the nerves of both the central and the peripheral nervous systems. The myelin begins to form at approximately the 16th week of gestation. Myelin insulates the nerves and helps conduct electrical impulses. Coordination of fine and gross motor skills progresses with the deposition of the myelin sheath. Nerve fibers can conduct impulses in the absence of myelin; however, the impulses travel more slowly. Gross motor skills develop before fine motor skills as coordination and control advance throughout childhood. The myelin sheath can be destroyed by disease, drugs, and the aging process.
The Neural System
The neural system develops multiple connections among the areas of the brain that control specific functions, including vision, hearing, motor function, sensation, coordination, and speech. Each function is under the control of a specific area of the brain. The right half, or hemisphere, of the brain controls the left side of the body and is concerned with the social aspects of perception, intuition, and experience. The left hemisphere controls the right side of the body and is largely concerned with language acquisition and use and logical verbal reasoning.
The neonate’s neurologic system functions at a subcortical level. Spinal cord reflexes, such as sucking and cardiorespiratory functions, are present. Cortical functions, including memory and coordination, are only partially developed.
The Axial Skeleton
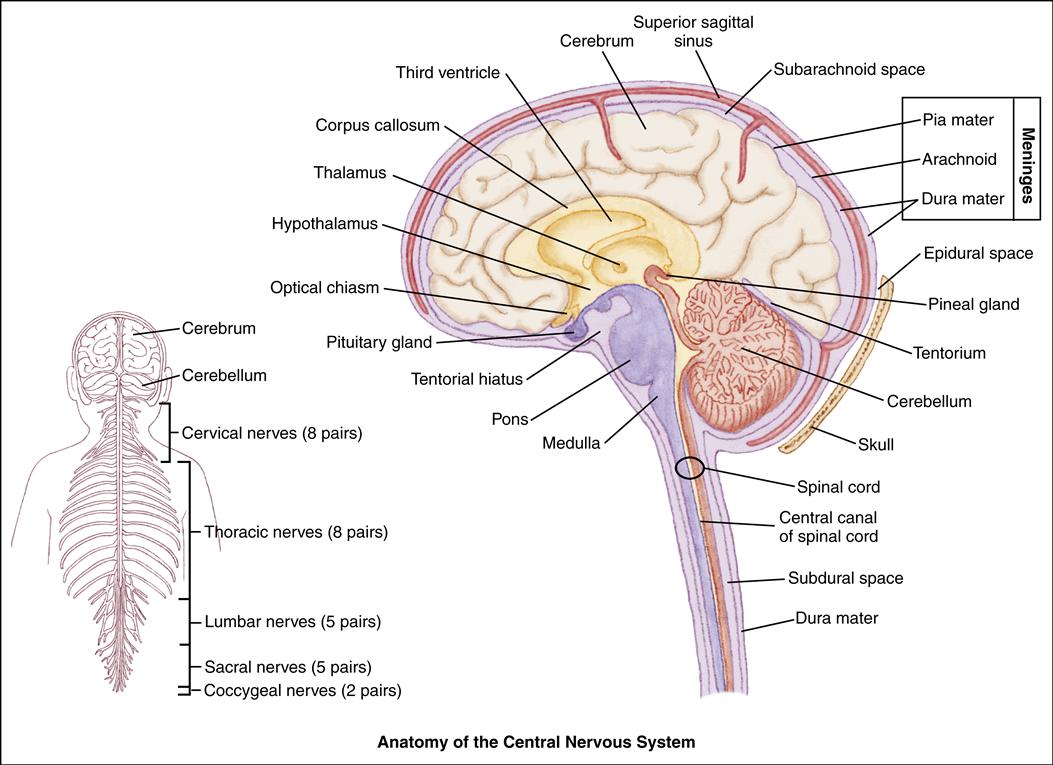
The axial skeleton protects the underlying structures of the central nervous system (CNS). For convenience of study, the bones of the skull and the vertebral column are divided into regions that form the wall of the cranial cavity and the spinal column. The frontal, occipital, temporal, and parietal bones form the cranial vault. The floor of the cranial vault is composed of three compartments, or fossae—the anterior, middle, and posterior fossae. The anterior fossa houses the frontal lobes of the brain, the middle fossa contains the upper brainstem and the pituitary gland, and the posterior fossa contains the lower brainstem. Blood vessels and cranial nerves enter and leave the skull through the foramina.
At birth, the skull plates are not fused but are separated by nonossified spaces called fontanels. The posterior fontanel usually fuses by age 2 months and the anterior fontanel by 16 to 18 months. The fontanels allow the cranium to expand in response to rapid brain growth. Before fusion of the fontanels and sutures, an increase in intracranial pressure (ICP) will produce an increase in head circumference and may result in macrocephaly.
Because brain growth is rapid during infancy, the long-term sequelae of neurologic insults that occur to infants are difficult to predict. Brain growth can be assessed by head circumference measurements. These measurements are an important part of the routine physical examination of children and should be plotted on a growth chart. Insufficient or excessive head and brain growth could indicate a potential neurologic problem. Premature closing of the fontanels or sutures can cause massive neurologic damage, and continued evaluation by the physician is needed.
The Meninges
The meninges are the membranes that surround the brain and spinal column. The outer layer is the dura mater, a fibrous connective tissue structure containing many blood vessels (Atabaki, 2007). The dura mater consists of two layers having outer and inner meningeal components. Between the periosteum of the bone and the dura mater lies the epidural space. Sheets of dura also extend downward and inward to form partitions within the cranium. The falx cerebri separates the cerebral hemispheres, and the falx cerebelli separates the cerebellar hemispheres.
The tentorium is a tentlike structure that separates the cerebellum from the occipital lobe of the cerebrum. The large gap through which the brainstem passes is the tentorial hiatus.
The middle meningeal layer is the arachnoid, a delicate, avascular, weblike, serous membrane loosely covering the brain. Between the arachnoid and the dura lies the subdural space, which contains a small amount of fluid, just sufficient to prevent adhesion of the two membranes.
The innermost layer is the pia mater. It is a delicate, transparent membrane that adheres closely to the outer surface of the brain. The pia mater is a vascular membrane, consisting of arteries and veins.
Between the pia mater and the arachnoid is the subarachnoid space, which is filled with cerebrospinal fluid (CSF). The CSF acts as a cushion to reduce the force of trauma on the brain. Endothelial cells within the brain form a semipermeable blood-brain barrier that allows some substances to pass to the brain and prevents others from entering. The blood-brain barrier provides protection for the brain.
The Brain
The three sections of the brain are the cerebrum, the cerebellum, and the brainstem. The cerebrum is the largest component, filling the upper portion of the skull. It is divided into two hemispheres, right and left, which are separated by a longitudinal fissure. The two hemispheres are joined by a band of commissural fibers called the corpus callosum. The cerebral hemispheres are further divided into lobes in relation to the cranial bones: frontal, parietal, temporal, and occipital. The cerebrum also includes part of the thalamus, the hypothalamus, the basal ganglia, and the olfactory and optic nerves.
The cerebellum is composed of white matter and gray matter. The gray matter is often referred to as the cerebral cortex. It is attached to the brainstem by paired bundles of fibers. The brainstem consists of the midbrain, the pons, the medulla, the thalamus, and the third ventricle.
The Cranial Nerves
Twelve pairs of cranial nerves arise from the brain and brainstem, each with a specific function. Testing these nerves can indicate the location and degree of CNS injury (see Chapter 33).
The Spinal Cord
The spinal cord is described as segmented into the cervical, thoracic, lumbar, and sacral regions. The spinal nerves are named for their corresponding vertebral segments.
The spinal cord transmits signals to and from the brain and responds to local sensory information through automatic motor responses called reflexes. The simplest type of spinal cord response is the reflex arc. Sensation is transmitted to the spinal cord from a sensory nerve fiber. It synapses with a motor neuron in the same cord segment, causing a muscle or tendon contraction in the corresponding motor nerve. Deep tendon reflexes are examples of the reflex arc.
Sensory innervation occurs as sensory nerves carrying body sensations enter the spinal cord on the dorsal surface. Most sensory fibers for pain and temperature ascend to the brain by lateral spinal tracts. Sensory fibers for touch and pressure ascend through anterior tracts. Almost all sensory fibers pass through the thalamus, where the perceptions of touch, pressure, and temperature are interpreted. Perceptions of texture, size, and weight are interpreted in the cortex.
Motor nerves are stimulated to respond after the brain receives a signal from a sensory nerve. The motor nerves cross over to the contralateral (opposite) side of the spinal cord from which they originate and then exit on the ventral surface of the spinal cord. The side of the body contralateral to the injured side of the brain will be the side affected by injury.
Functional differences exist between the upper and lower motor neurons. The outcome of a spinal cord injury is affected by the site of the injury. An injury between the brain and the dendrites (the nerve fibers that carry impulses toward the cell body) will render the brain incapable of signaling the muscle cells to cease responding reflexively, and the muscle will become contracted, or spastic. If the injury is to a section of the nerve between the muscle and axons (the nerve fibers that carry impulses away from the cell body), the muscles will become incapable of responding reflexively, causing them to become flaccid.
CSF ANALYSIS IN CHILDREN: NORMAL FINDINGS
| PARAMETER | NEONATE | CHILD OLDER THAN 6 MONTHS | |
| PRETERM | TERM | ||
| WBCs (per mm3) | ≤25 | ≤19 (infants ages 6-28 days) ≤9 (infants ages 29-56 days) | ≤5 |
| Protein (mg/dL) | <150 | <170 | <45 |
| Glucose (mg/dL) | >30 | >60 | >40 |
| Pressure (mm Hg) | 50-80 | 50-80 | 100-280 |
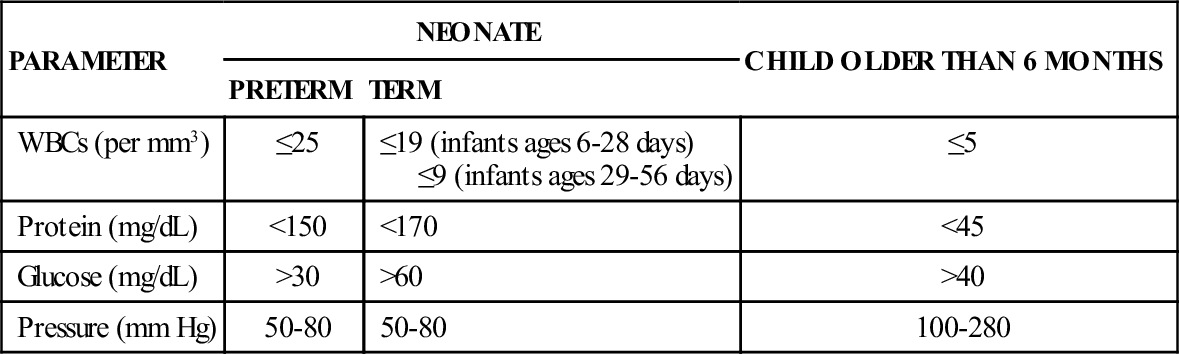
CEREBROSPINAL FLUID ANALYSIS: FINDINGS IN PATHOLOGIC CONDITIONS
| CONDITION | APPEARANCE | PRESSURE | CELLS | PROTEIN | GLUCOSE/OTHER |
| Traumatic tap | Bloody; supernatant fluid clear | Normal | Any red blood cells | 4 mg/dL rise per 5000 red cells | Not applicable |
| Acute bacterial meningitis | Cloudy to milky or yellow (xanthochromatic) | Usually elevated | Polymorphonuclear cells: ≥100/mm3 | 100-500 mg/dL | Decreased compared with blood glucose |
| Viral meningitis | Clear | Normal or increased | Zero to a few hundred per mm3, mostly leukocytes | 50-200 mg/dL | Normal |
| Encephalitis | Clear, colorless | Normal or slightly increased | Normal or increased | 50-200 mg/dL | <40 mg/dL |
| Subdural hematoma | Yellow to clear, colorless | Increased | Normal | Normal or increased | Normal |
| Diabetic coma | Clear, colorless | Decreased | Normal | Normal or slightly increased | May be 200-300 mg/dL |
| Guillain-Barré syndrome | Clear | Normal | <10 white blood cells per mm3 | More than 2× normal | Normal |
Cerebrospinal Fluid
Cerebrospinal fluid (CSF) is a clear liquid produced in the choroid plexus of the ventricles at approximately 0.3 to 0.4 mL/min. The CSF aids in protecting the brain, spinal cord, and meninges by acting as a watery cushion surrounding them to absorb the shocks to which they are exposed. CSF also functions to maintain homeostasis as it drains unwanted substances away from the brain. It is reabsorbed through the arachnoid villi into the venous sinuses. The total volume of CSF is renewed approximately three times or more each day.
Cerebral Blood Flow and Intracranial Regulation
The internal carotid arteries supply blood to all parts of the brain. Approximately 17% of cardiac output and 20% of body oxygen are transported to the brain. The brain requires approximately 10 times the oxygen used by the rest of the body.
Cerebral blood flow (CBF) is controlled by cerebral perfusion pressure (CPP), which is the difference between the mean arterial blood pressure (MBP) and ICP.
Autoregulation, or self-regulation, is a unique physiologic ability. It allows cerebral arteries to change diameter in response to changes in the CPP. The cerebral vessels can maintain a steady blood flow to the brain during alterations in blood pressure and perfusion. However, autoregulation fails when the limits of cerebrovascular dilation are reached.
Autoregulation may be impaired as a result of trauma, ischemia, or increased intracranial pressure. It is influenced significantly by changes in partial pressure of oxygen in arterial blood (PaO2) and partial pressure of carbon dioxide in arterial blood (PaCO2). An increase in PaCO2 (above 40 mm Hg) produces cerebral vasodilation and an increase in CBF. A decrease in PaCO2 (25 to 30 mm Hg) causes cerebral vasoconstriction and thus reduces blood flow to the brain. Alterations in PaO2 between 80 and 100 mm Hg have little effect on CBF, although hypoxia will dramatically increase CBF.
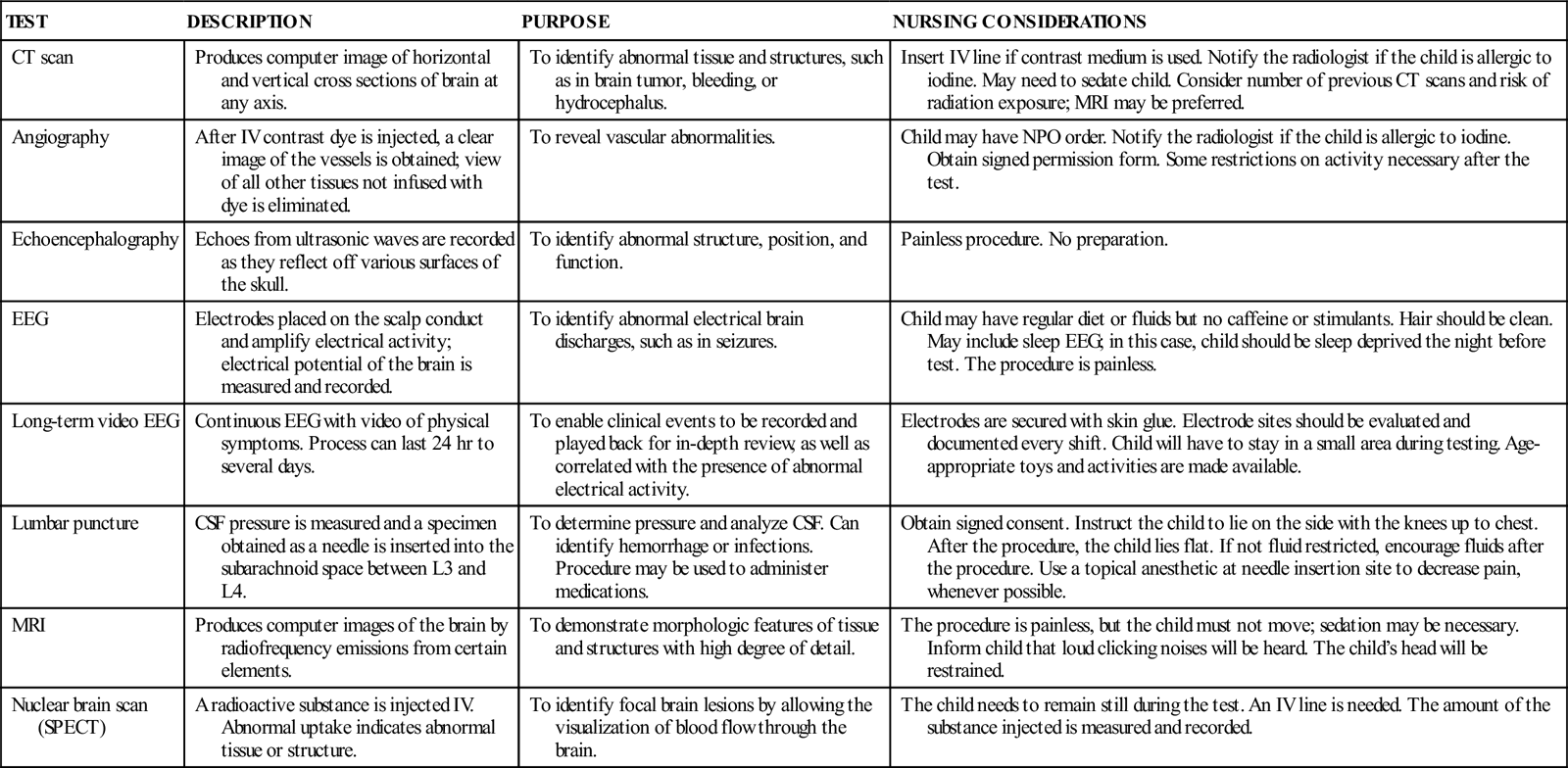
COMMON DIAGNOSTIC TESTS AND PROCEDURES FOR NEUROLOGIC DISORDERS
| TEST | DESCRIPTION | PURPOSE | NURSING CONSIDERATIONS |
| CT scan | Produces computer image of horizontal and vertical cross sections of brain at any axis. | To identify abnormal tissue and structures, such as in brain tumor, bleeding, or hydrocephalus. | Insert IV line if contrast medium is used. Notify the radiologist if the child is allergic to iodine. May need to sedate child. Consider number of previous CT scans and risk of radiation exposure; MRI may be preferred. |
| Angiography | After IV contrast dye is injected, a clear image of the vessels is obtained; view of all other tissues not infused with dye is eliminated. | To reveal vascular abnormalities. | Child may have NPO order. Notify the radiologist if the child is allergic to iodine. Obtain signed permission form. Some restrictions on activity necessary after the test. |
| Echoencephalography | Echoes from ultrasonic waves are recorded as they reflect off various surfaces of the skull. | To identify abnormal structure, position, and function. | Painless procedure. No preparation. |
| EEG | Electrodes placed on the scalp conduct and amplify electrical activity; electrical potential of the brain is measured and recorded. | To identify abnormal electrical brain discharges, such as in seizures. | Child may have regular diet or fluids but no caffeine or stimulants. Hair should be clean. May include sleep EEG; in this case, child should be sleep deprived the night before test. The procedure is painless. |
| Long-term video EEG | Continuous EEG with video of physical symptoms. Process can last 24 hr to several days. | To enable clinical events to be recorded and played back for in-depth review, as well as correlated with the presence of abnormal electrical activity. | Electrodes are secured with skin glue. Electrode sites should be evaluated and documented every shift. Child will have to stay in a small area during testing. Age-appropriate toys and activities are made available. |
| Lumbar puncture | CSF pressure is measured and a specimen obtained as a needle is inserted into the subarachnoid space between L3 and L4. | To determine pressure and analyze CSF. Can identify hemorrhage or infections. Procedure may be used to administer medications. | Obtain signed consent. Instruct the child to lie on the side with the knees up to chest. After the procedure, the child lies flat. If not fluid restricted, encourage fluids after the procedure. Use a topical anesthetic at needle insertion site to decrease pain, whenever possible. |
| MRI | Produces computer images of the brain by radiofrequency emissions from certain elements. | To demonstrate morphologic features of tissue and structures with high degree of detail. | The procedure is painless, but the child must not move; sedation may be necessary. Inform child that loud clicking noises will be heard. The child’s head will be restrained. |
| Nuclear brain scan (SPECT) | A radioactive substance is injected IV. Abnormal uptake indicates abnormal tissue or structure. | To identify focal brain lesions by allowing the visualization of blood flow through the brain. | The child needs to remain still during the test. An IV line is needed. The amount of the substance injected is measured and recorded. |
Care of the child with a neurologic problem requires knowledge of neuroanatomy, neurophysiology, and normal growth and development. The nurse plays an important role in the early recognition of pediatric neurologic problems, some of which have the potential for devastating long-term outcomes. The nurse assesses the child’s condition by comparing the child’s normal behavior with current behavior. The family is an invaluable source of information about the child’s normal behavior and how current behavior deviates from that norm. The child and the family need support and understanding because the child’s condition represents a crisis in their lives. The family’s ability to respond and influence the child’s coping mechanisms directly influences the recovery and adaptation process.
Many conditions of the neurologic system share common assessment data, diagnoses, and interventions. Principles of nursing care for the child with a neurologic system disorder can be applied to a variety of situations.
Increased Intracranial Pressure
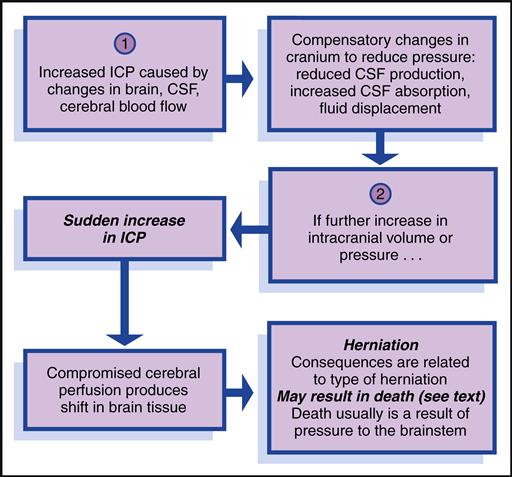
Increased intracranial pressure (ICP) reflects the pressure exerted by the blood, brain, CSF, and any other space-occupying fluid or mass. Increased ICP results from a disturbance in autoregulation and is defined as pressure sustained at 20 mm Hg or higher for 5 minutes or longer.
Etiology
Alterations in the brain can result from a space-occupying lesion, such as a brain tumor or hematoma. The brain can swell as a result of head trauma, infection, or a hypoxic episode. Overproduction of fluid, malabsorption of fluid, or a communication problem within the system can disrupt CSF dynamics. Aneurysms within the brain and acute liver failure can also lead to increased ICP.
Manifestations
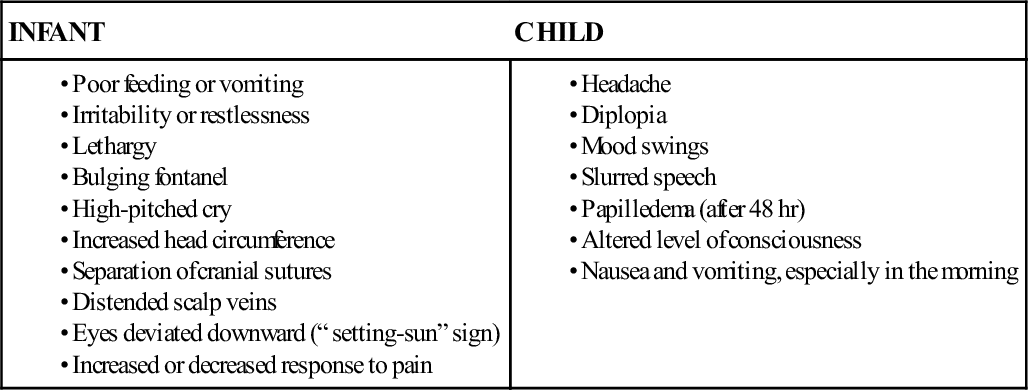
Signs and symptoms of increased ICP differ according to the child’s developmental level (Box 52-1).
Level of Consciousness
Children with increased ICP often have an altered level of consciousness. The Glasgow Coma Scale (GCS) is a standardized scale that, in a modified form, is frequently used to assess level of consciousness in infants and children. It consists of a three-part assessment: eye opening, verbal response, and motor response (Table 52-1). Each level of response is assigned a number value. When the assessment of each response is complete, the scores are totaled, providing an objective measure of the child’s level of consciousness. The total numeric scores range from 15, indicating no change in level of consciousness, to 3, indicating a deep coma and poor prognosis. A modified version of the GCS is available for children, and is a reliable tool for assessing level of consciousness and predicting the need for acute medical interventions when properly administered (Kirkham, Newton, & Whitehouse, 2008).
TABLE 52-1
GLASGOW COMA SCALE MODIFIED FOR CHILDREN
| CHILD | INFANT |
| Eyes | |
| 4 = Opens eyes spontaneously | 4 = Opens eyes spontaneously |
| 3 = Opens eyes to speech | 3 = Opens eyes to speech |
| 2 = Opens eyes to pain | 2 = Opens eyes to pain |
| 1 = No response | 1 = No response |
| ____ = Score (Eyes) | |
| Motor | |
| 6 = Obeys commands | 6 = Spontaneous movements |
| 5 = Localizes | 5 = Withdraws to touch |
| 4 = Withdraws | 4 = Withdraws to pain |
| 3 = Flexion | 3 = Flexion (decorticate) |
| 2 = Extension | 2 = Extension (decerebrate) |
| 1 = No response | 1 = No response |
| ____ = Score (Motor) | |
| Verbal | |
| 5 = Oriented | 5 = Coos and babbles |
| 4 = Confused | 4 = Irritable cry |
| 3 = Inappropriate words | 3 = Cries to pain |
| 2 = Incomprehensible words | 2 = Moans to pain |
| 1 = No response | 1 = No response |
| _____ = Score (Verbal) | |
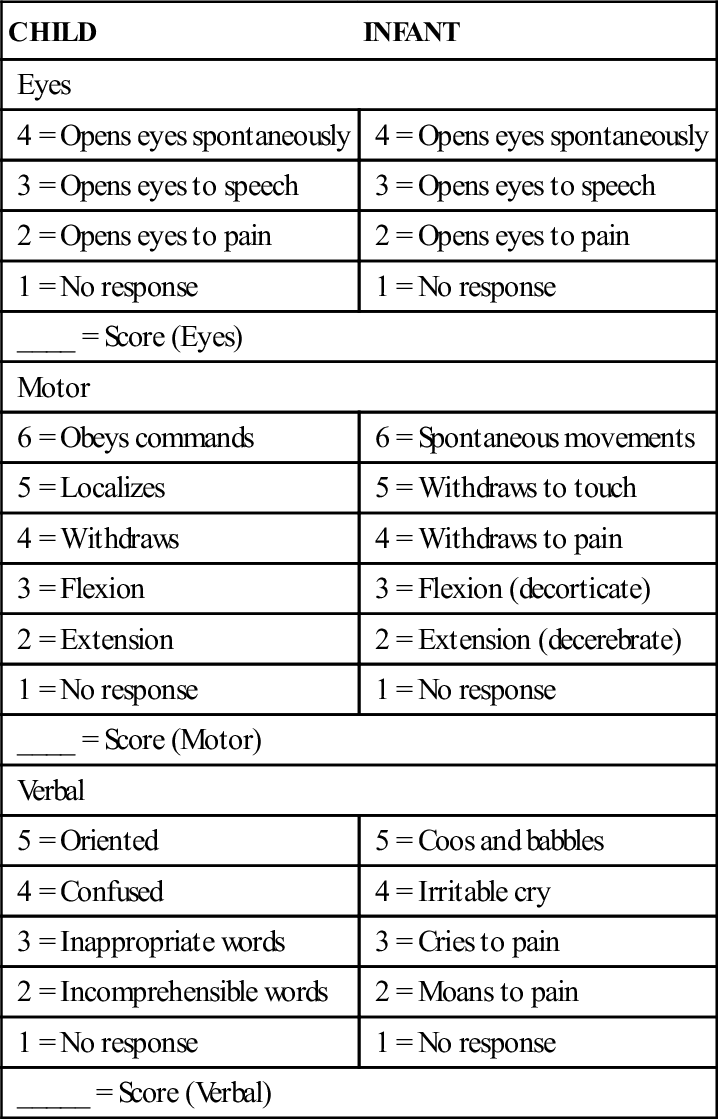
Total scores will range from 3 to 15.
Reprinted from James, H. E., Anas, N. G., & Perkin, R. M. (1985). Brain insults in infants and children. Orlando, FL: Grune & Stratton.
Behavior
Changes in the child’s normal behavior pattern may be an important early sign of increased ICP. Parents often are the first to notice a change in the child’s behavior; therefore a parent’s comment that “he isn’t acting like himself” should be taken seriously. Irritability, mild confusion, and agitation are symptoms that warrant further assessment. The child who no longer recognizes parents, cannot follow commands, or has minimal response to pain is deteriorating. Decreased responsiveness to painful stimuli is a significant sign of alteration in level of consciousness.
Pupil Evaluation
As ICP rises, compression of the third cranial nerve occurs, resulting in pupil dilation with sluggish or absent constriction in response to light. A fixed, dilated pupil is an ominous sign in an unconscious child. This suggests a herniation of the center section of the brain (also known as a transtentorial herniation). Other eye dysfunctions associated with increased ICP include ptosis and ovoid pupil. Older children may complain of blurry vision, diplopia, or decreased visual acuity.
Motor Function
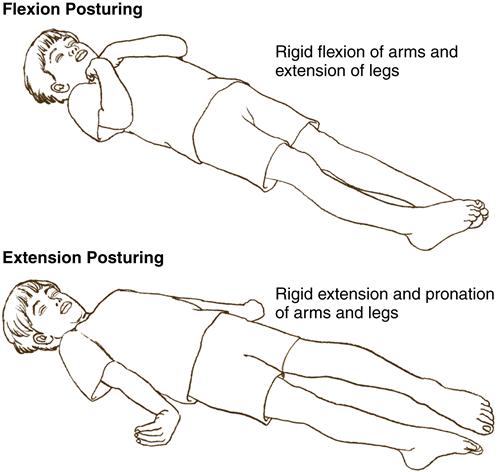
The child with increased ICP exhibits changes in motor function. Purposeful movement will decrease, and abnormal posturing may be observed. Flexion, or decorticate posturing, refers to flexion of the upper extremities (elbows, wrists) and extension of the lower extremities. Plantar flexion of the feet may also be observed. This type of posturing implies an injury to the cerebral hemispheres. Extension, or decerebrate posturing, involves extension of the upper extremities with internal rotation of the upper arm and wrist. The lower extremities will extend, with some internal rotation noted at the knees and feet. This type of posturing indicates damage to more areas of the brain, such as the diencephalon, midbrain, or pons. The progression from flexion to extension posturing usually indicates deteriorating neurologic function and warrants physician notification (Figure 52-1). Flaccid paralysis indicates further deterioration in the child’s condition.
Vital Signs
Temperature elevation may occur in children with increased ICP. Cushing’s response, which consists of an increased systolic blood pressure with widening pulse pressure, bradycardia, and a change in respiratory rate and pattern, is usually apparent just before or at the time of brainstem herniation. This usually indicates an alteration in brainstem perfusion, with the body attempting to improve cerebral blood flow by increasing blood pressure. In children, Cushing’s response is a late sign of increased ICP.
As ICP rises, the child’s baseline respiratory pattern may change, exhibiting Cheyne-Stokes respiration, central neurogenic hyperventilation, or apneustic breathing. Cheyne-Stokes respiration refers to a pattern of breathing characterized by increasing
rate and depth and then decreasing rate and depth with a pause of variable length. The cycle will be repeated again and again. Central neurogenic hyperventilation is identified by a rapid rate despite normal arterial blood gas values. This type of breathing pattern usually indicates midbrain or pontine involvement. Apneustic breathing occurs when the child demonstrates prolonged inspiration and expiration. As Cushing’s response occurs, the child will develop apnea. Late signs of increased ICP include tachycardia that leads to bradycardia, apnea, systolic hypertension, widening pulse pressure, and flexion or extension posturing.
Diagnostic Evaluation and Therapeutic Management
Diagnostic tests for increased ICP include computed tomography (CT), magnetic resonance imaging (MRI), lumbar puncture, serum and urine electrolytes, arterial blood gas determinations, a complete blood cell count, electroencephalography (EEG), and radiography. Normal blood gas levels are PaO2 greater than 80 mm Hg and PaCO2 less than 45 mm Hg in a child with normal ICP.
The management of increased ICP is multimodal and is directed toward treating its underlying cause, reducing the volume of the CSF, preserving cerebral metabolic function, and avoiding situations that increase ICP. An intraventricular catheter may be used to measure ICP, drain CSF, and/or administer medications (Box 52-2).
The head of the child’s bed should be elevated 30 degrees, and normothermia should be maintained. The child may be given an osmotic diuretic (e.g., mannitol), sedation and analgesia, and anticonvulsant medications (May, 2009). Blood glucose levels are closely monitored to maintain normal levels and prevent further increases in metabolic demands. Hypertonic intravenous solutions are considered for children with hypovolemia (May, 2009). Aggressive passive hyperventilation should
be avoided because this intervention may lead to cerebral vasoconstriction with a resultant decrease in cerebral blood flow to ischemia levels (Dumont, Visioni, Rughani, et al., 2010; Neumann et al., 2008). Corticosteroids are no longer used in the treatment of increased ICP due to traumatic brain injury (TBI). In fact, high doses of corticosteroids have been associated with increased mortality for patients with TBI (Tang & Lobel, 2009).
Spina Bifida
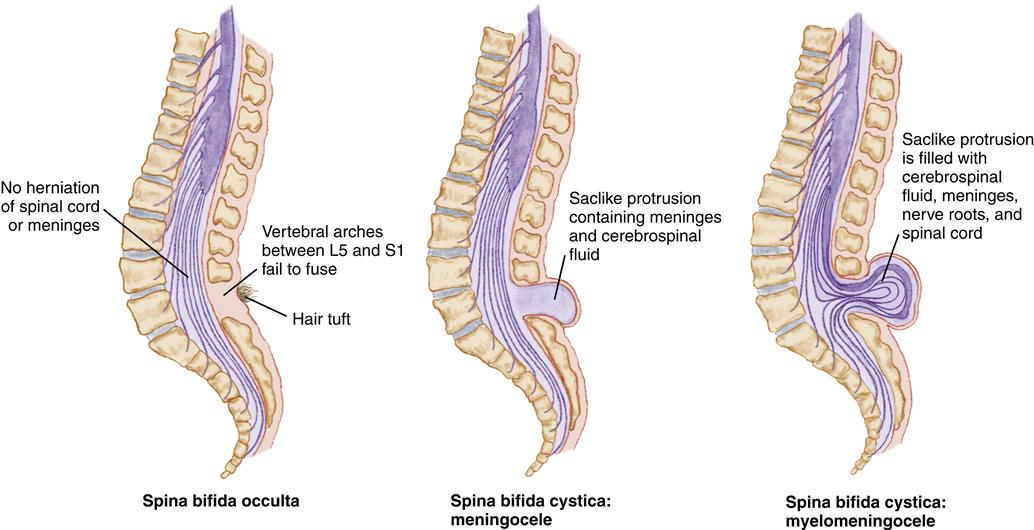
Spina bifida is a congenital neural tube defect characterized by incomplete closure of the vertebrae and neural tube during fetal development. Spina bifida is classified as spina bifida occulta and spina bifida cystica (Figure 52-2). Spina bifida occulta usually occurs between the L5 and S1 vertebrae, with failure of the vertebrae to completely fuse. The child may have no sensory or motor defects. The only clinical manifestation may be a dimple, a small tuft of hair, a hemangioma, or a lipoma in the lower lumbar or sacral area, detected accidentally on routine radiographs. Spina bifida cystica is a more extensive defect with a range of sensory and motor impairments.
Etiology and Incidence
The cause of spina bifida is unknown in most cases. Evidence suggests a possible genetic predisposition. Maternal folic acid deficiency has been strongly linked to neural tube defects. Daily consumption of 0.4 mg of folic acid by all women of childbearing age is recommended. Evidence of a viral origin has prompted research, but other than folic acid, no cause or preventive measures have been identified.
Manifestations
In addition to the appearance of the lesion, manifestations relate to the degree of deficit, which is determined by the level of the lesion (Figure 52-3).
| T12: | Flaccid lower extremities, decreased sensation, and bowel and bladder incontinence |
| L1 to L3: | Hip flexion, flail feet |
| L2 to L4: | Hip adduction |
| L3 to S2: | Hip adduction, hip extension, knee flexion |
| S3 and below: | No motor impairment |
| Sacral roots: | Plantar flexion |
Children with spina bifida are at high risk for developing latex allergies because of frequent exposure to latex during catheterizations, shunt placements, and other operations. Latex allergy is estimated to occur in approximately 73% of children with spina bifida (Spina Bifida Association, 2009a). Allergic reactions can range from mild signs and symptoms to anaphylactic shock. Children should be tested for latex allergy, and precautions should be taken from birth to decrease exposures. The nurse should check equipment and supplies for latex and select nonlatex alternatives for use.
Diagnostic Evaluation
Diagnostic tests include determining alpha-fetoprotein (AFP) levels in blood at 16 to 18 weeks of gestation. If the AFP screen is elevated, amniocentesis and fetal ultrasound are performed. After delivery, the infant may undergo a CT scan or myelography.
Therapeutic Management
Prenatal microsurgical closure of the myelomeningocele, performed at approximately 19 to 25 weeks of gestation, shows promise for reducing the severity of Chiari type II malformations and incidence of hydrocephalus (Fichter, Dornseifer, Henke, et al., 2008). Furthermore, there may be long-term orthopedic benefits associated with prenatal closure (Danzer, Gerdes, Bebbington, et al., 2009). Risks associated with prenatal surgery include premature birth, with its associated consequences, and possible fetal death. Maternal risks (e.g., abruptio placentae, preterm membrane rupture, preterm labor, wound infection, chorioamnionitis, uterine hemorrhage, loss of uterus, and damage to adjacent organs) are directly related to the hysterotomy (Kunisaki & Jennings, 2008).
Following birth, immediate surgical closure of the defect decreases the risk of infection, morbidity, and mortality. Other benefits are improved prognosis without further cord deterioration and earlier and easier physical handling and bonding between the newborn and the parents.
The child will need lifelong management of neurologic, orthopedic, and urinary problems and is best managed in a multispecialty outpatient setting. Urodynamic studies are performed early, and a bladder-emptying program is initiated, with close monitoring of the child’s urinary tract infection status. In most instances, the child will require orthopedic bracing and possibly orthopedic surgery to maximize the child’s mobility.
Hydrocephalus
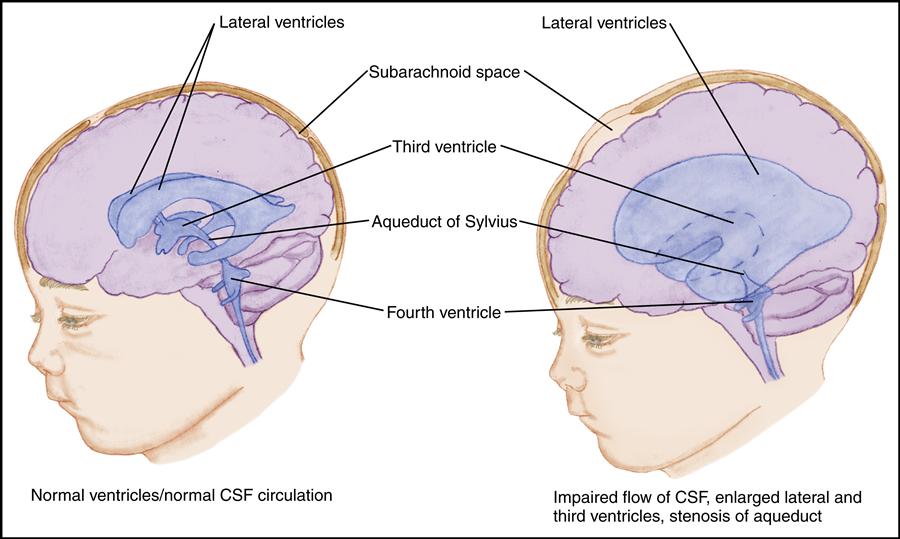
Hydrocephalus develops as a result of an imbalance between the production and absorption of CSF. As excess CSF accumulates in the ventricular system, the ventricles become dilated and the brain is compressed against the skull. This results in enlargement of the skull if the sutures are open; it results in signs and symptoms of increased ICP if the sutures are fused.
Etiology
Hydrocephalus may be congenital, acquired, or of unknown etiology. In infancy, hydrocephalus is most often congenital or related to prematurity. Congenital hydrocephalus results from developmental defects such as Arnold-Chiari malformations, congenital arachnoid cysts, congenital tumors, or aqueductal stenosis. In premature infants, neonatal meningitis or subarachnoid hemorrhage may result in hydrocephalus. Hydrocephalus is often associated with myelomeningocele. Intrauterine infection and perinatal hemorrhage cause hydrocephalus in some infants. In older children, hydrocephalus is usually acquired as a complication of meningitis, tumor, or hemorrhage.
Incidence
In general, the estimated prevalence of hydrocephalus is 1 in every 500 children in the United States (National Institute of Neurological Disorders and Stroke [NINDS], 2010b). The incidence of hydrocephalus with spina bifida is considered to be 3 to 4 in 1000 live births. Obstructive, or noncommunicating, hydrocephalus accounts for nearly all cases of hydrocephalus in children.
Manifestations and Diagnostic Evaluation
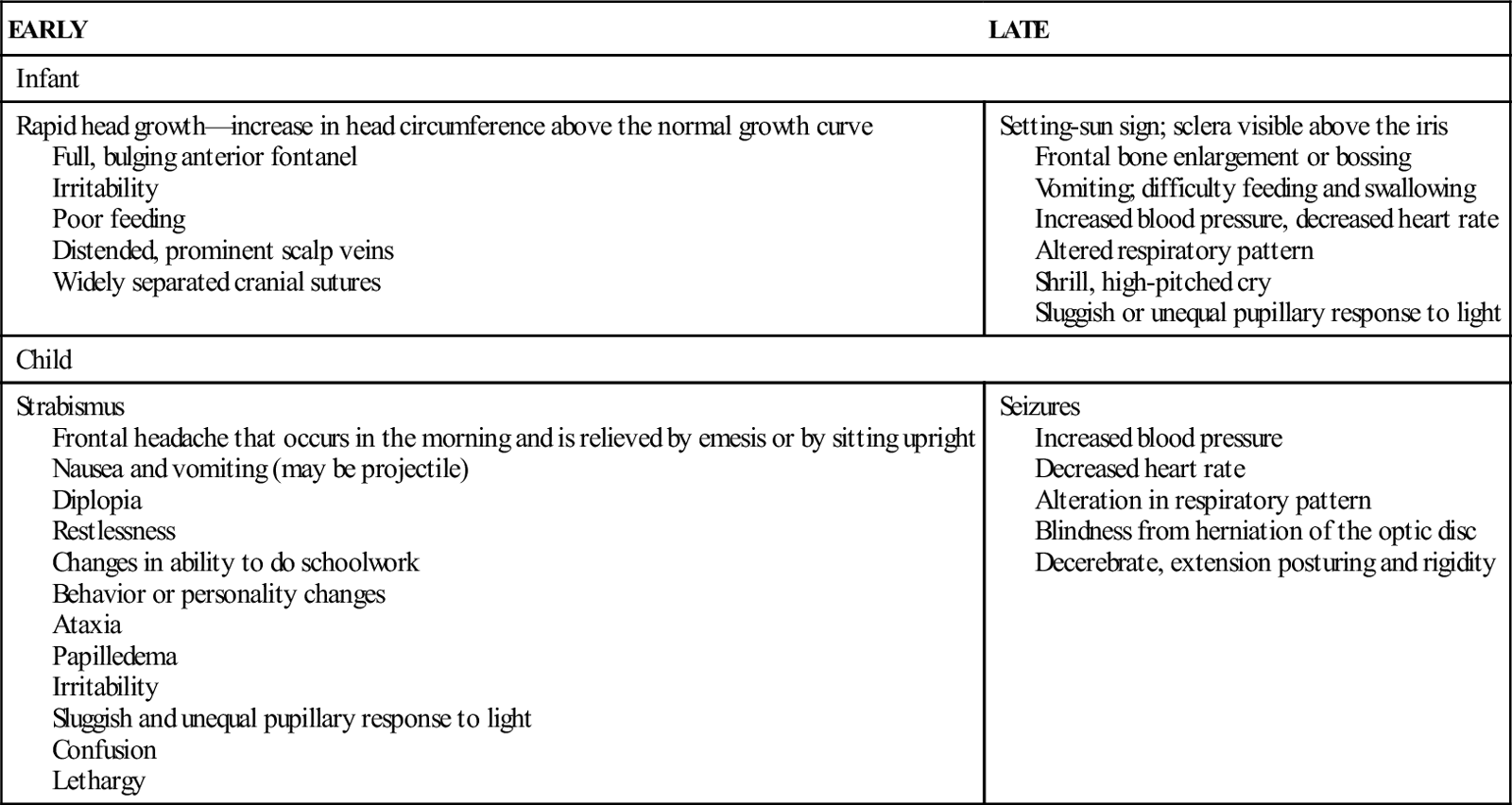
Because of anatomic differences between infants and children, manifestations of hydrocephalus differ according to developmental stage (Table 52-2).
TABLE 52-2
EARLY AND LATE MANIFESTATIONS OF HYDROCEPHALUS
| EARLY | LATE |
| Infant | |
| Rapid head growth—increase in head circumference above the normal growth curve Full, bulging anterior fontanel Irritability Poor feeding Distended, prominent scalp veins Widely separated cranial sutures | Setting-sun sign; sclera visible above the iris Frontal bone enlargement or bossing Vomiting; difficulty feeding and swallowing Increased blood pressure, decreased heart rate Altered respiratory pattern Shrill, high-pitched cry Sluggish or unequal pupillary response to light |
| Child | |
| Strabismus Frontal headache that occurs in the morning and is relieved by emesis or by sitting upright Nausea and vomiting (may be projectile) Diplopia Restlessness Changes in ability to do schoolwork Behavior or personality changes Ataxia Papilledema Irritability Sluggish and unequal pupillary response to light Confusion Lethargy | Seizures Increased blood pressure Decreased heart rate Alteration in respiratory pattern Blindness from herniation of the optic disc Decerebrate, extension posturing and rigidity |
Diagnostic tests for hydrocephalus include serial measurements of head circumference, CT, MRI, ultrasonography, and lumbar puncture with pressure monitoring.
Therapeutic Management
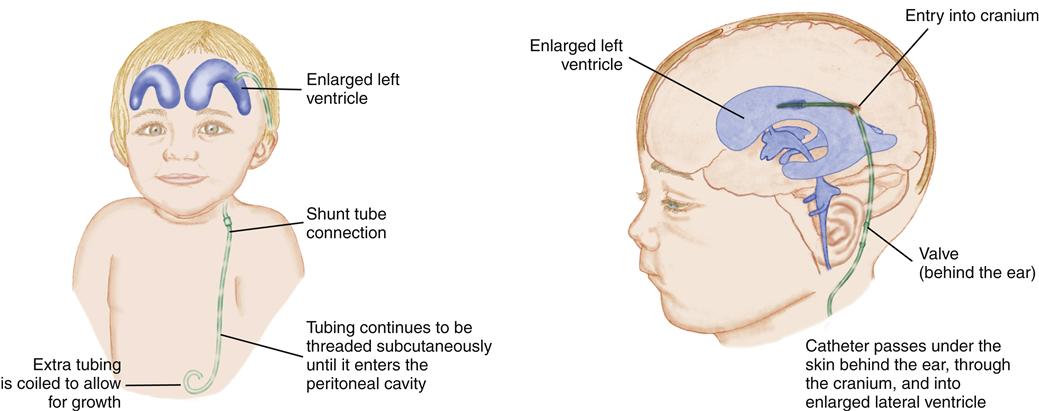
Therapy is aimed at preventing further CSF accumulation and reducing disability and death. The objective is to bypass the blockage and drain the fluid from the ventricles to an area where it may be reabsorbed into the circulation. A ventriculoperitoneal shunt, a tube leading from the ventricles out of the skull and passing under the skin to the peritoneal cavity, accomplishes this (Figure 52-4). An alternative shunt, the ventriculoatrial shunt, which is used in older children, drains the fluid from the ventricles to the right atrium of the heart.
The shunt may need to be revised as the child grows. Long-term follow-up is essential. The child may exhibit mild learning challenges and may have accelerated pubertal development (Spina Bifida Association, 2009b).
A surgical procedure, endoscopic third ventriculostomy, facilitates the rerouting of CSF around the obstructed ventricular system (Bhatia, Tahir, & Chandler, 2009). This technique has become increasingly popular over the past 20 years. For this procedure, the surgeon creates a small burr hole in the skull through which an endoscope is passed. The third ventricle is visualized and a small opening is created in its floor. This allows the CSF to bypass the fourth ventricle and return to circulation, where it is reabsorbed. The procedure is 50% to 80% successful in children older than 2 years. Other surgical techniques for treating hydrocephalus may be preferred in some children, particularly those under age 2, because surgical outcomes from the third ventriculostomy approach are poor for this age-group (Ogiwara, Dipatri, Alden, et al., 2010).
Cerebral Palsy
Cerebral palsy is a chronic, nonprogressive disorder of posture and movement (O’Shea, 2008). It is characterized by difficulty in controlling the muscles because of an abnormality in the extrapyramidal or pyramidal motor system (motor cortex, basal ganglia, cerebellum). Co-morbidities such as cognitive, hearing, speech, and visual impairments, as well as seizures, are common but vary widely from one affected child to another (Straub & Obrzut, 2009).
Etiology and Incidence
The damage to the motor system can occur prenatally, perinatally, or postnatally (Box 52-3). Cerebral palsy (CP) is one of the most common chronic neurologic impairments in children, and more that 500,000 Americans are affected. The rate of cerebral palsy is 1.1 per 1000 live births. Problems associated with prematurity and low birth weight are related to the occurrence of CP. Aggressive neonatal intensive care, administration of surfactant to mature infant lungs, and administering steroids to mothers prior to delivery has improved survival rates to as high as 85% for a 26-week-old premature infant, and also resulted in an increased prevalence of CP (Marsal, 2009). Infants with the lowest birth weights (less than 1000 g) may be at increased risk for CP because of intracerebral hemorrhage or periventricular leukomalacia (Fukuda, Yokoi, Kitajima, et al., 2009). In general, however, children diagnosed with CP are born at term after a normal labor and delivery (Shankaran, 2008).