The Child with a Fluid and Electrolyte Alteration
LEARNING OBJECTIVES
After studying this chapter, you should be able to:
• Identify the regulatory mechanisms that maintain fluid and electrolyte balance in the body.
• Describe dehydration and acid-base imbalance.
• Differentiate among the various types of acid-base disturbances.
• Describe the processes and nursing care of a child with diarrhea or vomiting.
• Integrate assessment findings with nursing implementation to determine the success of therapy.
• Describe nursing interventions to prevent fluid and electrolyte imbalances.
![]()
http://evolve.elsevier.com/McKinney/mat-ch
Clinical Reference
Review of Fluid and Electrolyte Imbalances in Children
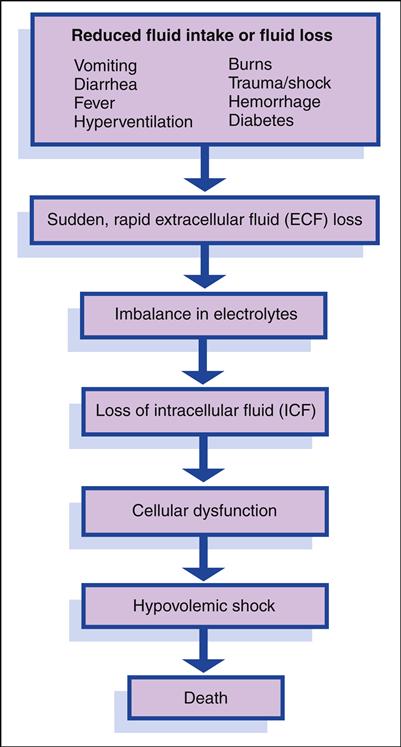
Characteristics unique to children affect their fluid and electrolyte balance. Infants and young children are more vulnerable than adults to changes in fluid and electrolyte balance. Under normal conditions, the amount of fluid ingested during a day should equal the amount of fluid lost through sensible water loss (e.g., urine output) and insensible water loss (through the respiratory tract and skin). Insensible water loss per unit of body weight is significantly higher in infants and children. The faster respiratory rates of infants and young children also result in higher evaporative water losses. Any condition that prevents normal oral fluid intake (e.g., vomiting) or results in fluid losses (e.g., diarrhea, hyperventilation, burns, hemorrhage) is especially significant because it depletes the body’s store of water and electrolytes much more rapidly in infants and young children than in adults.
Body water is located in two major compartments: within the cell, in the intracellular compartment; and outside the cell, in the extracellular compartment. These two compartments are separated by the cell membrane, across which body fluid is continually exchanged. Extracellular fluid (ECF) is located in several places: in interstitial spaces (surrounding the cells [e.g., lymph fluid]), intravascularly (within the blood vessels or plasma), and transcellularly (e.g., cerebrospinal fluid, pericardial fluid, pleural fluid, synovial fluid, sweat, digestive secretions). A child is more likely to lose ECF than intracellular fluid (ICF). ECF is lost first when fluid loss occurs (e.g., through illness, trauma, fever). The intracellular compartment is more difficult to dehydrate.
In the neonate, approximately 40% of body water is located in the extracellular compartment compared with 20% in the adolescent and adult. In the infant, half of the ECF may be exchanged compared with an adult exchange of one sixth of the ECF in a similar time. Because approximately 50% of this ECF is exchanged daily in an infant, dehydration can occur very suddenly and rapidly if fluid intake is inadequate or fluid losses are excessive. Because of the infant’s higher metabolic rate, the rate of water turnover is rapid. Depletion of ECF, often caused by gastroenteritis, is one of the most common problems among infants and young children. In adults and older children, because a greater proportion of fluid is located in the intracellular compartment, severe fluid depletion does not occur as
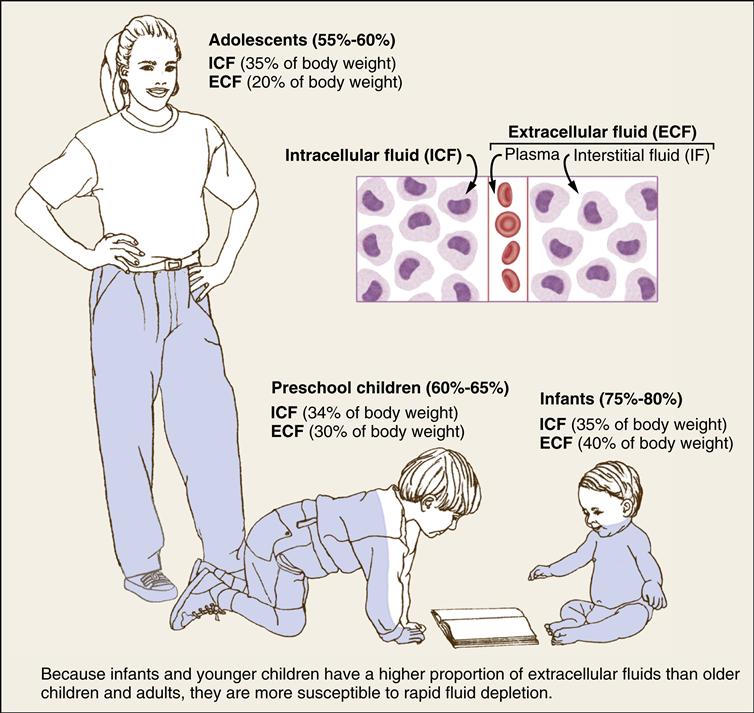
rapidly. Maturity in body space distribution is usually reached around age 3 years.
Body fluids are basically composed of two elements, water and solutes. Water is the primary constituent, with the infant’s weight being approximately 75% water to the adult’s 55% to 60%. In general, the volume of total body water to total body weight decreases with increasing age. An inverse relationship exists between total body water and total body fat. Compared with adults, neonates, particularly premature infants, have a lower proportion of fat.
Solutes are composed of both electrolytes and nonelectrolytes. Most of the body’s solutes are electrolytes, primarily sodium (Na+), potassium (K+), chloride (Cl−), calcium (Ca2+), and magnesium (Mg2+). The primary electrolyte of the ECF is sodium; potassium and magnesium are the primary electrolytes in the ICF. The extracellular compartment contains more sodium and chloride during infancy, which increases the vulnerability of infants to electrolyte imbalances. Changes in the concentration of these electrolytes may result in cellular dysfunction and illness. Problems of fluid and electrolyte balance involve both water and electrolytes; thus treatment includes replacement of both, calculated according to serum electrolyte laboratory values.
Alterations in Acid-Base Balance in Children
Alterations in acid-base balance can affect cellular metabolism and enzymatic processes. The body’s ability to regulate this status is crucial. Children can have acid-base imbalance as a result of many pathologic conditions. The pH, or measure of acidity or alkalinity of body fluids, is regulated within a narrow range (normal blood pH is 7.35 to 7.45). Maintenance of serum pH within normal limits is crucial to maintaining cellular function, enzyme activity, and neuromuscular membrane potentials. Chemical buffers, the respiratory system, and the kidneys work together to keep the blood pH within normal range. Acid is constantly produced as a byproduct of metabolism. The body attempts to maintain blood pH within normal limits by reducing the buildup of acid. Chemical and cellular buffer systems minimize the effect of alterations in blood pH by neutralizing excess acids and bases that accumulate in body fluids. Two of the most significant buffers are bicarbonate and proteins. Bicarbonate, the most important buffer for plasma and interstitial fluids, is responsible for most ECF buffering and can exert its effects relatively quickly (within minutes).
When alterations in pH become too much for the buffer systems to handle, compensatory mechanisms in the respiratory and renal systems are activated. The respiratory system works rapidly to compensate for acid-base disturbances. If the blood pH drops below normal (causing acidosis), the respiratory rate and depth will increase, removing carbon dioxide and raising blood pH. Conversely, in the presence of alkalosis, the respiratory rate and depth decrease, thus lowering blood pH.
Kidneys regulate bicarbonate and remove hydrogen ions from the blood. If the blood is too alkaline, the kidneys conserve hydrogen ions, thus lowering blood pH. In the presence of acidosis, the kidneys excrete hydrogen ions and conserve bicarbonate, raising blood pH. Renal compensatory processes work more slowly than respiratory mechanisms—usually within 1 to 2 days. If compensatory mechanisms are ineffective, acid-base imbalances occur. When a dysfunction results in decreased hydrogen ion concentration in the blood, the arterial pH increases (causing alkalosis). When a dysfunction results in an increase in hydrogen ions, the arterial pH decreases (causing acidosis).
OVERVIEW OF FLUID AND ELECTROLYTE DISORDERS
| DISORDER | PRECIPITATING EVENTS | CLINICAL MANIFESTATIONS |
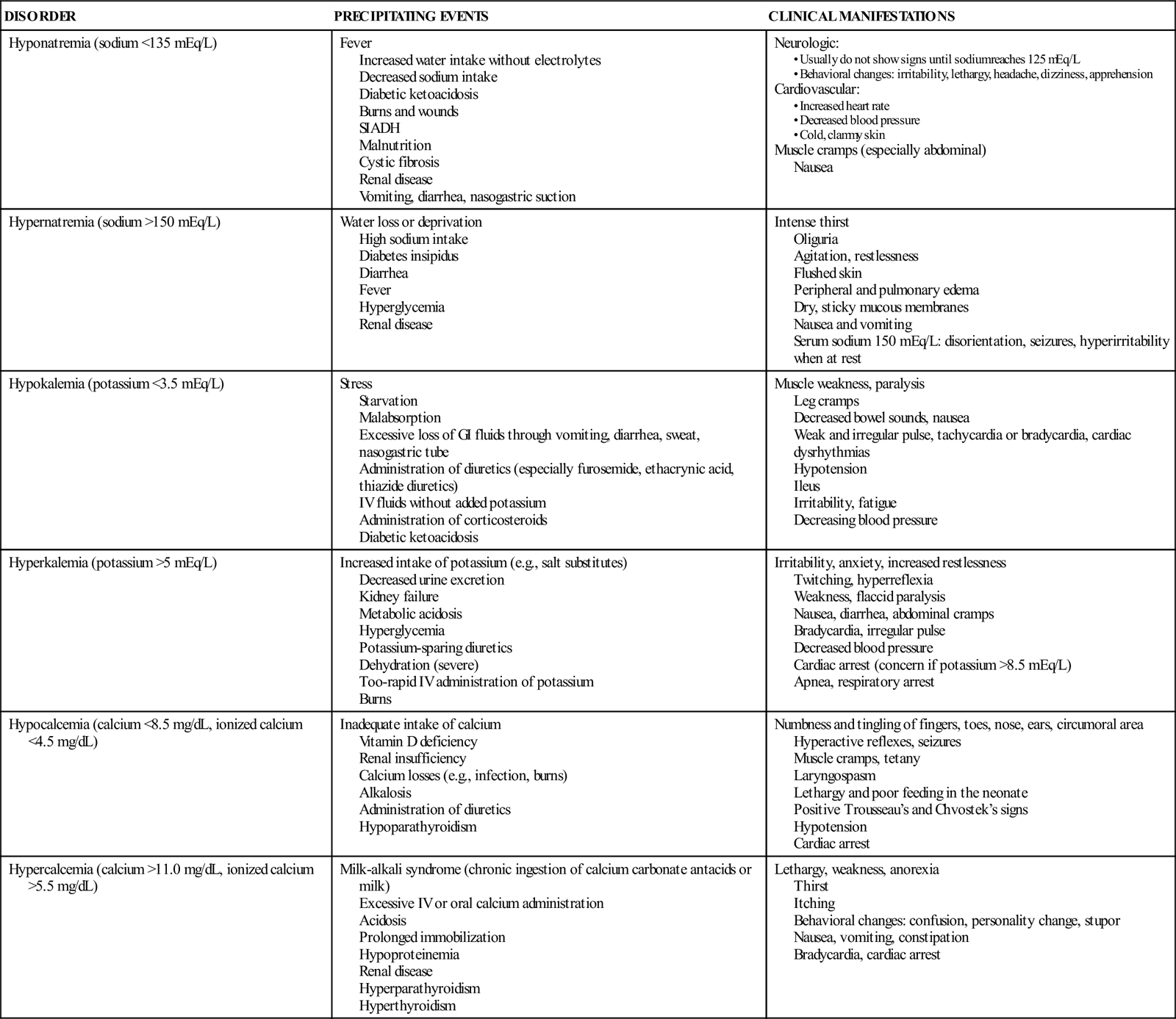
| Hyponatremia (sodium <135 mEq/L) | Fever Increased water intake without electrolytes Decreased sodium intake Diabetic ketoacidosis Burns and wounds SIADH Malnutrition Cystic fibrosis Renal disease Vomiting, diarrhea, nasogastric suction | Neurologic: Cardiovascular: Muscle cramps (especially abdominal) Nausea |
| Hypernatremia (sodium >150 mEq/L) | Water loss or deprivation High sodium intake Diabetes insipidus Diarrhea Fever Hyperglycemia Renal disease | Intense thirst Oliguria Agitation, restlessness Flushed skin Peripheral and pulmonary edema Dry, sticky mucous membranes Nausea and vomiting Serum sodium 150 mEq/L: disorientation, seizures, hyperirritability when at rest |
| Hypokalemia (potassium <3.5 mEq/L) | Stress Starvation Malabsorption Excessive loss of GI fluids through vomiting, diarrhea, sweat, nasogastric tube Administration of diuretics (especially furosemide, ethacrynic acid, thiazide diuretics) IV fluids without added potassium Administration of corticosteroids Diabetic ketoacidosis | Muscle weakness, paralysis Leg cramps Decreased bowel sounds, nausea Weak and irregular pulse, tachycardia or bradycardia, cardiac dysrhythmias Hypotension Ileus Irritability, fatigue Decreasing blood pressure |
| Hyperkalemia (potassium >5 mEq/L) | Increased intake of potassium (e.g., salt substitutes) Decreased urine excretion Kidney failure Metabolic acidosis Hyperglycemia Potassium-sparing diuretics Dehydration (severe) Too-rapid IV administration of potassium Burns | Irritability, anxiety, increased restlessness Twitching, hyperreflexia Weakness, flaccid paralysis Nausea, diarrhea, abdominal cramps Bradycardia, irregular pulse Decreased blood pressure Cardiac arrest (concern if potassium >8.5 mEq/L) Apnea, respiratory arrest |
| Hypocalcemia (calcium <8.5 mg/dL, ionized calcium <4.5 mg/dL) | Inadequate intake of calcium Vitamin D deficiency Renal insufficiency Calcium losses (e.g., infection, burns) Alkalosis Administration of diuretics Hypoparathyroidism | Numbness and tingling of fingers, toes, nose, ears, circumoral area Hyperactive reflexes, seizures Muscle cramps, tetany Laryngospasm Lethargy and poor feeding in the neonate Positive Trousseau’s and Chvostek’s signs Hypotension Cardiac arrest |
| Hypercalcemia (calcium >11.0 mg/dL, ionized calcium >5.5 mg/dL) | Milk-alkali syndrome (chronic ingestion of calcium carbonate antacids or milk) Excessive IV or oral calcium administration Acidosis Prolonged immobilization Hypoproteinemia Renal disease Hyperparathyroidism Hyperthyroidism | Lethargy, weakness, anorexia Thirst Itching Behavioral changes: confusion, personality change, stupor Nausea, vomiting, constipation Bradycardia, cardiac arrest |
ASSESSMENT OF FLUID DISTURBANCES
| PARAMETER | FLUID VOLUME DEFICIT | FLUID VOLUME EXCESS |
| Weight | Loss: Percentage suggests degree of dehydration | Gain: Related to retention of interstitial and vascular fluid volume |
| Heart rate/pulse | Rapid, weak, thready | Rapid, bounding |
| Respirations | Normal | Moist breath sounds, dyspnea |
| Blood pressure | Normal to decreased (late sign of impending shock) | Increased |
| Skin and mucous membranes | Pale, cool, poor turgor, prolonged capillary refill (>2 sec), dry mucous membranes | Edema |
| Salivation or tearing | Decreased to absent | Normal |
| Sensorium changes | Thirst, irritability, lethargy; stupor or coma if associated metabolic acidosis | Fatigue |
Data from Porth, C. (2011). Essentials of pathophysiology (3rd ed.). Philadelphia: Wolters Kluwer.
COMMON LABORATORY AND DIAGNOSTIC TESTS FOR FLUID AND ELECTROLYTE IMBALANCE∗
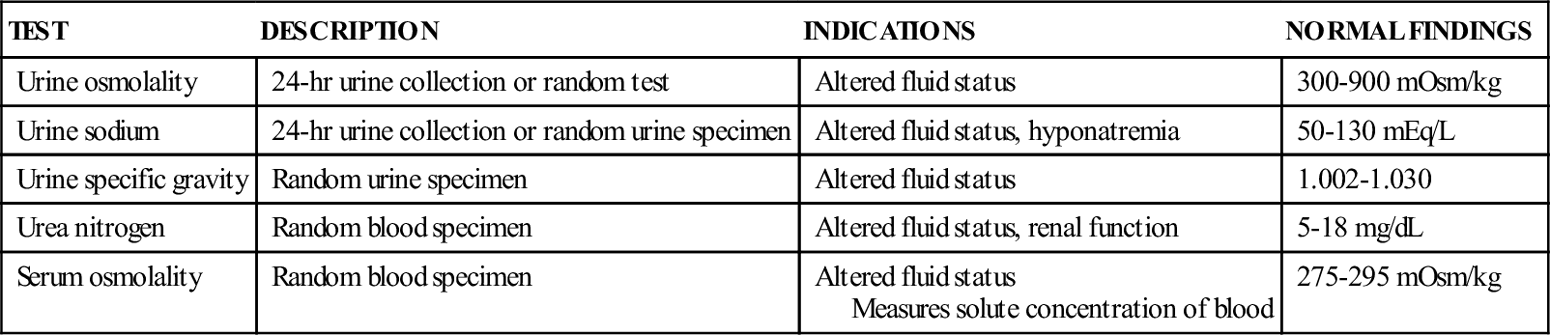
| TEST | DESCRIPTION | INDICATIONS | NORMAL FINDINGS |
| Urine osmolality | 24-hr urine collection or random test | Altered fluid status | 300-900 mOsm/kg |
| Urine sodium | 24-hr urine collection or random urine specimen | Altered fluid status, hyponatremia | 50-130 mEq/L |
| Urine specific gravity | Random urine specimen | Altered fluid status | 1.002-1.030 |
| Urea nitrogen | Random blood specimen | Altered fluid status, renal function | 5-18 mg/dL |
| Serum osmolality | Random blood specimen | Altered fluid status Measures solute concentration of blood | 275-295 mOsm/kg |
∗None of these studies have any specific nursing considerations, although the nurse may be required to collect and transport the specimen; there is no advance preparation for collection.
SELECTED LABORATORY VALUES FOR ACID-BASE DISTURBANCES
| TEST | METABOLIC ACIDOSIS | METABOLIC ALKALOSIS | RESPIRATORY ACIDOSIS | RESPIRATORY ALKALOSIS |
| ABG: pH | <7.35 | >7.45 | <7.35 | >7.45 |
| PaCO2 (mm Hg) | <40 | >45 | >45 | <35 |
| PaO2 (mm Hg) | WNL or slightly decreased | Decreased | Decreased | Decreased |
| HCO3− (mEq/L) | <22 | >26 | WNL or slightly increased | Decreased |
| K+ (mEq/L) | >4.0 | Decreased | WNL | Slightly decreased |
| Na+ (mEq/L) | Varies according to condition | Decreased | WNL | Slightly decreased |
| Cl− (mEq/L) | Usually increased | Decreased | WNL | Slightly decreased |
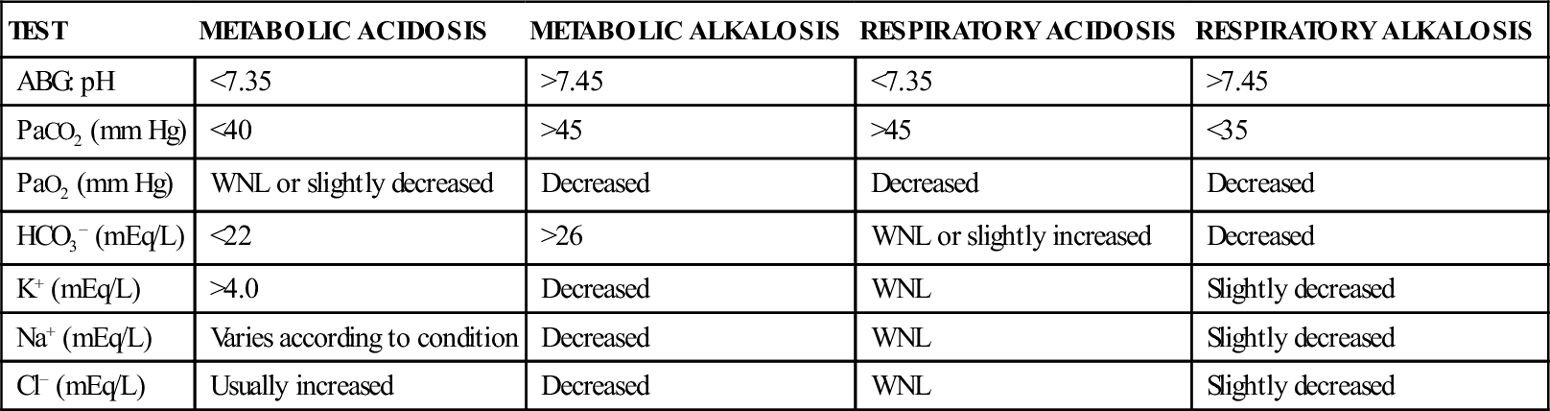
ACID-BASE DISTURBANCES: PRINCIPAL CAUSES, CLINICAL MANIFESTATIONS, AND TREATMENT
| CONDITION | PRINCIPAL CAUSES | COMPENSATORY MECHANISMS∗ | CLINICAL MANIFESTATIONS | PRINCIPAL TREATMENT METHODS |
| Metabolic acidosis | Ketoacidosis (DKA, alcohol-induced ketoacidosis) Increasing metabolic rates from fever, RDS, seizures Interference with normal metabolism: ketosis, tissue hypoxia Loss of bicarbonate from diarrhea, ileostomy, or fistula drainage Acute and chronic renal failure ECF expansion and decreasing HCO3− concentration | Hyperventilation causes decreased PaCO2 | Increasing heart rate, dysrhythmias (fibrillation) Hyperventilation Kussmaul respirations Cold, clammy skin (mild to moderate acidosis) Warm, dry skin (severe acidosis) Level of consciousness changes from weakness, fatigue, and confusion to stupor and coma | Identify and treat the underlying disorder Provide NaHCO3−, K+ replacement, and mechanical ventilation as indicated |
| Metabolic alkalosis | Volume depletion related to various conditions (vomiting, pyloric stenosis, gastric drainage, and diuretics) Increased alkali intake Medical conditions (cystic fibrosis) | Hypoventilation causes increased PaCO2 | Dysrhythmias (atrioventricular with prolonged QT interval) Increasing heart rate Decreased respiratory rate and depth Change in level of consciousness from apathy and confusion to stupor Muscular weakness | Treatment depends on underlying cause; mild to moderate alkalosis usually does not require treatment Use of fluids with NaCl and KCl, along with isotonic saline solution, an H2-receptor antagonist (e.g., cimetidine) to decrease gastric hydrochloric acid, acidifying agents, and potassium-sparing diuretics (e.g., spironolactone [Aldactone], mannitol) |
| Respiratory acidosis | Pulmonary disease (BPD, RDS, asthma, cystic fibrosis, croup) Airway obstruction Chest conditions (flail chest, pneumothorax) Acute and chronic respiratory failure Neuromuscular abnormalities (Guillain-Barré syndrome, toxins, drugs, paralysis) CNS depression from sedative overdose, trauma, anesthesia | Release of HCO3− and increased renal reabsorption of HCO3− and acid excretion | Increasing heart rate Dysrhythmias with hypotension Increasing rate and depth of respirations, forceful use of accessory muscles with retraction and cyanosis Increasing intracranial pressure | Correction of ventilation problem: use of oxygen, intubation, mechanical ventilation, NaHCO3− |
| Respiratory alkalosis | Hyperventilation from CNS stimulation such as emotions, fear, hysteria, pain, salicylate poisoning Decreased lung compliance and hypoxemia from conditions such as pulmonary edema, HF, pneumonia, asthma, pulmonary emboli Pregnancy Compensation from metabolic acidosis Sepsis | Decreased renal reabsorption of HCO3− | Dizziness, paresthesias, lightheadedness, diaphoresis Dysrhythmias (changes in ST-T wave) | Mild to moderate respiratory alkalosis usually does not require specific treatment For hyperventilation-induced conditions, provide oxygen, rebreathing oxygen masks, breathing into a paper bag, psychological reassurance Institute mechanical ventilation if condition is severe Give sedatives or tranquilizers for anxiety-induced condition, acetazolamide to prevent motion sickness |
∗Important items to remember when acid-base compensation occurs:
Dehydration
Dehydration, or fluid loss in excess of fluid intake, is one of the most common causes of hospitalization in infants and children because it results from occurrences of severe gastroenteritis, which is a major cause of morbidity and mortality (Diggins, 2008). Decreased fluid intake or increased fluid loss may cause dehydration. Dehydration produces both fluid and electrolyte deficiencies. Dehydration is classified as isonatremic, hyponatremic, or hypernatremic (Table 40-1), according to the status of the serum sodium concentration. In isonatremic dehydration, the most common type of dehydration in children, water and electrolytes are lost in approximately the same proportion as they exist in the body, and serum sodium levels remain within the normal range of 138 to 145 mEq/L. In hyponatremic dehydration, the electrolyte loss is greater than the water loss, resulting in a serum sodium concentration of less than 135 mEq/L. In hypernatremic dehydration, the water loss is greater than the electrolyte loss and the serum sodium concentration is more than 150 mEq/L.
TABLE 40-1
TYPES OF DEHYDRATION: ETIOLOGY, CLINICAL MANIFESTATIONS, AND LABORATORY VALUES
| ISONATREMIC DEHYDRATION | HYPONATREMIC DEHYDRATION | HYPERNATREMIC DEHYDRATION |
| Etiology | ||
| Vomiting, diarrhea, insensible fluid loss from respiratory and integumentary systems Decreased oral intake with increased activity | Renal Losses Diuretics, hyperglycemia, nephritis, adrenal insufficiency Extrarenal Losses Vomiting, diarrhea, third spacing, burns, tube drainage Other HF, SIADH, nephrosis; administration of large amounts of electrolyte-free solutions (plain water) during illness or postoperatively | Renal Losses Osmotic diuretics, diabetes insipidus, diabetes mellitus Extrarenal Losses Vomiting, diarrhea Other Fever, increased sodium in formula, diet, or tube feeding; administration of hypertonic sodium IV fluids; burns; ineffective breastfeeding |
| Clinical Manifestations | ||
| Mild thirst Skin turgor poor Dry skin Decreased urine output Dry mucous membranes Skin temperature cold Body temperature afebrile or febrile Lethargy | Increased thirst Skin turgor very poor Skin usually clammy Decreased urine output Mucous membranes dry to slightly moist Skin temperature cold Body temperature afebrile or febrile Very lethargic, possible seizures | Thirst very increased Skin turgor fair Skin texture thickened or “doughy” Decreased urine output Mucous membranes parched Skin temperature cold or hot Body temperature afebrile or febrile Lethargic, hyperirritable with stimulation |
| Laboratory Values | ||
| Serum sodium: 138-145 mEq/L | Renal Losses Serum sodium <135 mEq/L Plasma osmolality decreased | Renal Losses Serum sodium >150 mEq/L |
| Urine Sodium usually within normal limits Specific gravity slightly elevated Osmolality usually within normal limits Volume usually within normal limits or slightly decreased | Urine sodium increased Urine specific gravity decreased Urine osmolality decreased Urine volume increased | Urine sodium increased Urine specific gravity decreased Urine osmolality decreased Urine volume increased |
| Extrarenal Losses Serum sodium <135 mEq/L Urine sodium decreased Urine specific gravity increased Urine osmolality increased Urine volume decreased | Extrarenal Losses Serum sodium >150 mEq/L Urine sodium decreased Urine specific gravity increased Urine osmolality increased Urine volume decreased | |
| Other Serum sodium <135 mEq/L Urine sodium decreased Urine specific gravity increased Urine osmolality increased Urine volume decreased | Other Serum sodium >150 mEq/L Urine sodium decreased Urine specific gravity increased Urine osmolality increased Urine volume decreased | |
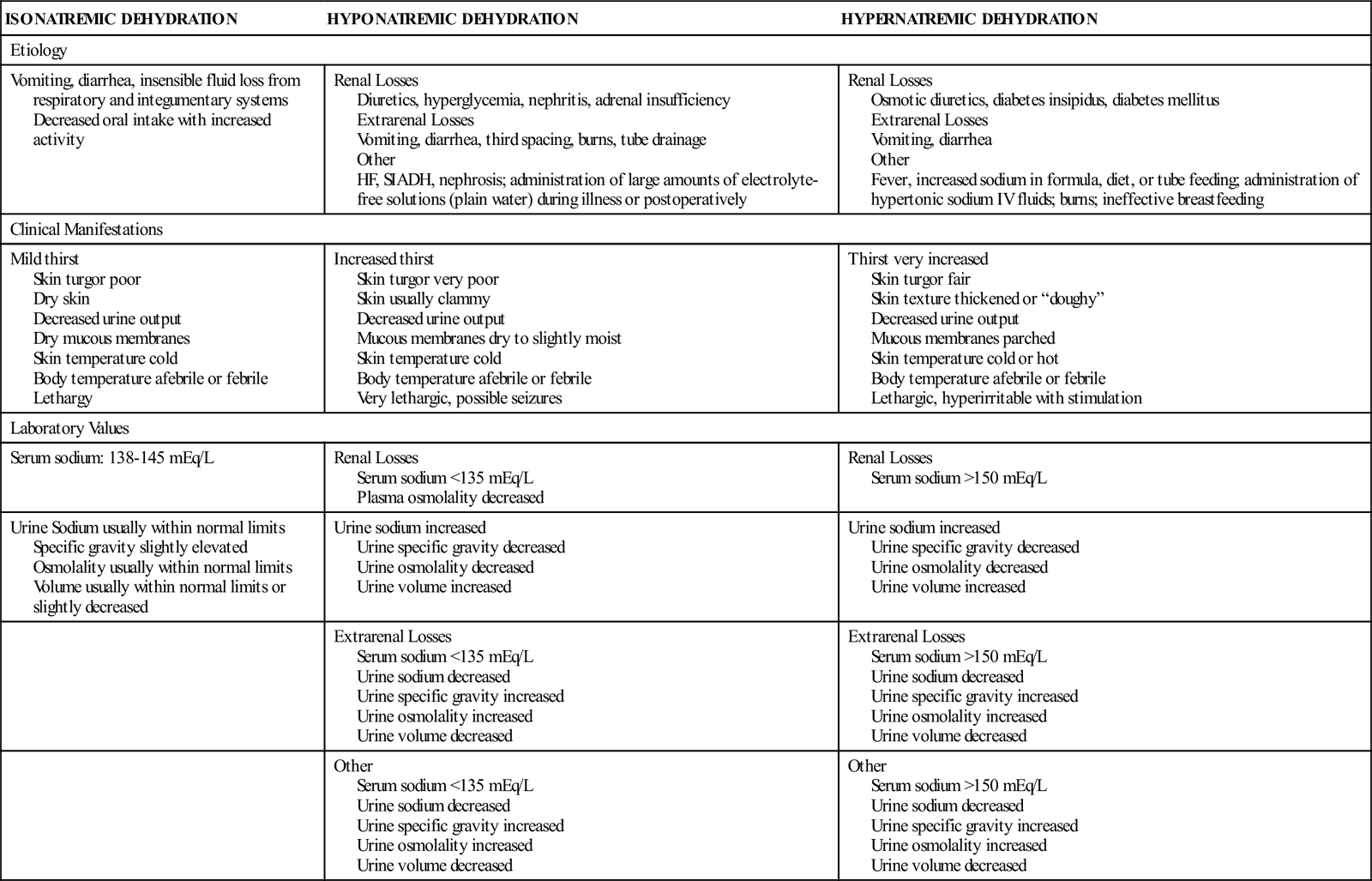
Data from Greenbaum, L. (2007). Electrolyte and acid base disorders. In R. Behrman, R. Kliegman, H. Jenson, et al. (Eds.), Nelson textbook of pediatrics (18th ed.). Philadelphia: Saunders.
Etiology and Incidence
Dehydration has many varied causes. Common alterations that may lead to dehydration reflect disturbances in the following systems:
• Gastrointestinal tract: Vomiting, diarrhea, pyloric stenosis, malabsorption
• Endocrine system: Fever, diabetes mellitus, cystic fibrosis
Any age-group can be affected, but neonates and infants, as discussed previously, are especially vulnerable to the effects of dehydration. Gastroenteritis with resulting dehydration accounts for 1.5 million deaths worldwide in children and is the second leading cause of death globally in children (Bhutta, 2011).
Manifestations
Classifications of the severity of dehydration vary according to the published source. In general, for infants and young children with isonatremic dehydration, the fluid deficit is described as mild, moderate, or severe dehydration, depending on the percentage of body weight lost (Greenbaum, 2011; Yu, Lougee, & Murno, 2010):
• Mild dehydration: Less than 5% loss of body weight
• Moderate dehydration: 5% to 10% loss of body weight
• Severe dehydration: Greater than 10% of body weight
• The Centers for Disease Control and Prevention (CDC) (2008) adds an additional category, minimal dehydration (<3% body weight loss) for treatment purposes
One milliliter of body fluid is approximately equal to 1 g of body weight, so a weight loss or gain of 1 kg (2.2 lb) in 24 hours represents a 1-L fluid loss or gain.
Older children have a lower total body water content and ECF volume than do infants and younger children. Therefore, an equivalent percentage of body weight lost from dehydration represents a more severe fluid depletion in the older child. Isonatremic dehydration in the older child is classified as mild if less than 3% of body weight is lost, moderate if 3% to 6% of body weight is lost, and severe if more than 6% of body weight is lost (Greenbaum, 2011).
The signs and symptoms associated with degree of isonatremic dehydration are listed in Table 40-2. As with impending shock, the most essential manifestations are changes in heart rate; general appearance, behavior, or sensorium; urine output; skin and mucous membrane qualities; and, in infants, fontanels. Sunken eyes and decreased tears are definitive signs of dehydration (Goldman, Friedman, & Parkin, 2008), but lack of tears is not an accurate sign in very young infants, who may not produce tears until approximately 2 to 3 months of age.
TABLE 40-2
ASSESSMENT OF THE SEVERITY OF DEHYDRATION
| CLINICAL SIGNS | MINIMAL OR NO DEHYDRATION | MILD TO MODERATE DEHYDRATION | SEVERE DEHYDRATION |
| Weight loss | <3% | <5%-10% | >10% |
| Vital signs | |||
| Pulse | Normal | Normal to increased, weak | Tachycardic, bradycardic in most severe cases; thready |
| Respiratory rate | Normal | Normal to fast | Rapid and deep |
| Blood pressure | Normal | Normal | Markedly decreased as a sign of hypovolemic shock |
| General appearance | Well, alert; drinks normally, might refuse liquids | Fatigued, restless, irritable; thirsty and eager to drink | Apathetic, lethargic, unconscious; drinks poorly or unable to drink |
| Mucous membranes | Normally moist | Dry | Parched |
| Anterior fontanel | Normal | Sunken | Markedly depressed |
| Eyes | Normal, tears present | Slightly sunken, tears decreased | Markedly sunken, tears absent |
| Capillary refill | <2 sec; extremities feel warm | Prolonged; extremities cool | Prolonged, minimal; extremities cold; mottled or cyanotic |
| Skin turgor (see Figure 40-1) | Normal | Prolonged recoil | Tenting |
| Urine output | Mildly decreased | Decreased, concentrated urine | Minimal |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree