The Child with a Cardiovascular Alteration
Learning Objectives
After studying this chapter, you should be able to:
• Describe the anatomy and physiology of the normally functioning heart.
• Discuss specific techniques used in a comprehensive cardiac assessment.
• Discuss the nursing process used for an infant or child with congestive heart failure.
• Discuss the importance of early recognition and treatment of infective endocarditis.
• Describe nursing care of a child with rheumatic fever, Kawasaki disease, or hypertension.
• Explain the effects of childhood obesity on future cardiovascular health.
![]()
http://evolve.elsevier.com/McKinney/mat-ch/
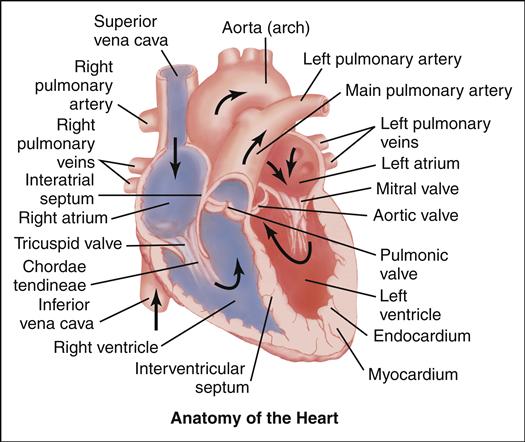
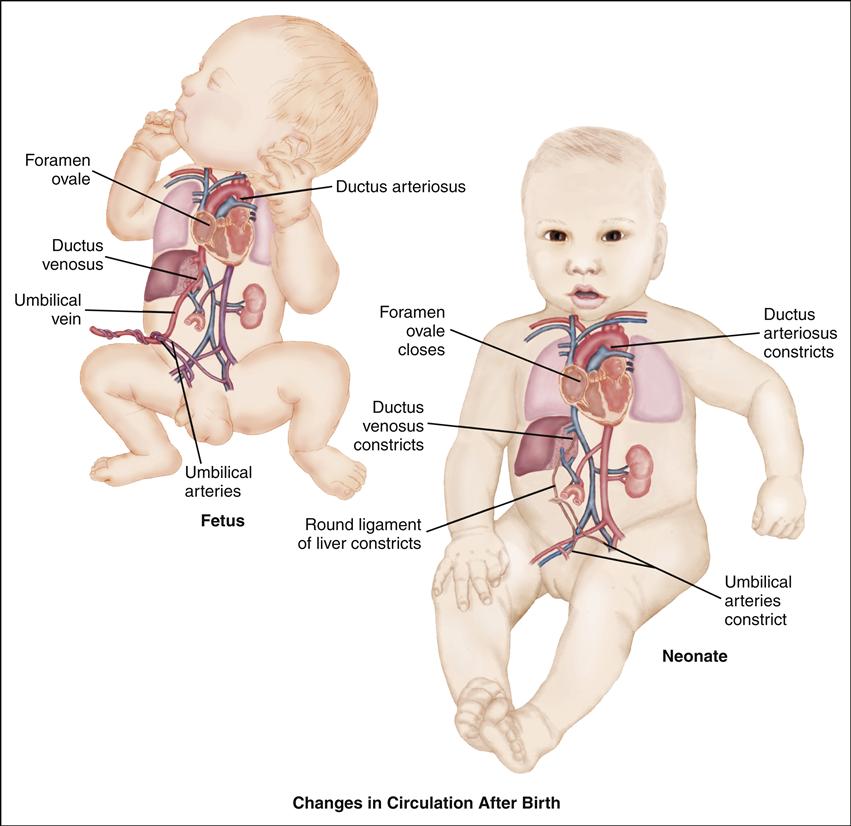
Cardiovascular alterations in children are either congenital or acquired. Congenital heart disease (CHD) denotes one or more structural abnormalities that develop before birth, although the clinical symptoms may not be present in the newborn period. Acquired heart disease, such as the cardiomyopathies, Kawasaki disease, or rheumatic fever, develops after birth and may be seen both in children with normal hearts and in those with CHD. Over the past 3 decades, there have been major advances in the diagnosis and management of CHD. Because of these advances, approximately 90% of infants born with CHD can be expected to live into adulthood. To appreciate each defect or combination of multiple defects and their effect on a child’s life, an understanding of normal cardiac anatomy and physiology, including development of the heart, circulation (including fetal, transitional, and postnatal), normal structures, and function is required.
Congenital Heart Disease
Congenital cardiac defects are some of the most frequently seen congenital defects in infants and children and have an overall incidence of 8 in every 1000 live births (American Heart Association [AHA], 2011). As interventional cardiology becomes more effective, more adults are living with congenital heart disease (CHD); the prevalence of CHD in adults is approximately 4 per 1000 (AHA, 2011).
Although the nongenetic etiology and risk factors for CHD are not fully known, maternal diabetes mellitus, maternal infection during pregnancy, maternal smoking, maternal obesity, and maternal exposure to some chemicals and pollutants have been implicated (AHA, 2011). Children with certain genetic conditions or defects have an extremely high incidence of cardiac disorders, including children with chromosome aberrations, most specifically trisomy 21 (Down syndrome), in which the incidence of CHD is approximately 50% (Park, 2008). Other congenital conditions that increase the risk for CHD include Turner syndrome in girls (single X chromosome), Klinefelter variant in boys (genetically having additional X chromosomes), and children with Marfan syndrome or with velocardiofacial syndrome (DiGeorge syndrome) (Park, 2008). A family history of CHD increases the risk.
Classification of CHD
Congenital heart defects can be classified according to structural abnormalities, functional alterations, or both (Table 46-1). Historically, defects were simply classified according to whether they were cyanotic or acyanotic. Currently, these classifications are subdivided into groups that are defined by blood flow patterns. These include presence of increased pulmonary blood flow, normal to decreased pulmonary blood flow, obstructive lesions, and miscellaneous complex lesions.
TABLE 46-1
CLASSIFICATION OF CONGENITAL HEART DISEASE
| DEFECT | UNDERLYING MECHANISM | EXAMPLES |
| Left-to-right shunting lesions (lesions that increase pulmonary blood flow) | A defect in the atrial septum or persistence of a PDA causes saturated blood to shunt through this opening. This left-to-right shunting of blood results in a volume overload in the right side of the heart and in the pulmonary artery; cardiac workload (including ventricular strain, dilation, and hypertrophy) increases to manage the additional volume (pressure overload). This abnormal increase in highly saturated blood combined with the increased fluid volume in the lungs, results in altered gas exchange. One of the major consequences of left-to-right shunting lesions is HF. Other consequences include pulmonary vascular disease, pulmonary hypertension, Eisenmenger syndrome, and frequent upper and lower respiratory infections that can progress to respiratory failure. | ASD VSD PDA AVSD, endocardial cushion defect |
| Obstructive or stenotic lesions, or lesions that decrease cardiac outflow | Stenosis, the narrowing or constriction of an opening, can occur in a valve or vessel constricting or obstructing blood flow through the area. Pressure rises in the area behind the obstruction; blood flow distal to the obstruction may be decreased or absent. Stenotic lesions can occur in the right or left side of the heart; obstruction on the left side of the heart decreases the amount of available blood for systemic perfusion. Physiologic effects of stenotic lesions include increased cardiac workload and ventricular strain, with clinical consequences of HF, decreased cardiac output, and pump failure. | Pulmonary stenosis Aortic stenosis Coarctation of the aorta |
| Cyanotic lesions with decreased pulmonary blood flow | These lesions arise from an error in fetal development that results in hypoplasia (incomplete development), malalignment, or obstruction on the right side of the heart, and decreased amount of blood volume to the lungs. Pulmonary blood flow may rely on having a PDA. There is abnormally decreased oxygen saturation in the left side of the heart. Physiologically, the child manifests hypoxemia, increased cardiac workload, and ventricular strain. The hypoxemia results in cyanosis, and even with oxygen administration, saturations do not approximate normal. Other clinical findings may include upper respiratory infection, severely limited pulmonary blood flow, and marked exercise intolerance. | Tetralogy of Fallot Tricuspid valve abnormalities Pulmonary atresia with intact ventricular septum |
| Cyanotic lesions with increased pulmonary blood flow | When the fetal heart fails to develop into separate pulmonary and systemic circulations or when there is a reversal of circulation so that desaturated blood goes to the systemic circulation and saturated blood to the pulmonary circulation, cyanosis occurs. Sometimes classified as mixing lesions, these defects cause increased cardiac workload, ventricular strain, and decreased cardiac output. Lesions are usually discovered early in the neonatal period. The infant might appear ruddy or cyanotic, with increased respiratory effort, or, if systemic circulation is compromised, may be dusky or gray and in cardiogenic shock (see Chapter 34). To support life, these complex cardiac defects may require intervention that allows for mixing of arterial and venous blood. | Truncus arteriosus Hypoplastic left heart syndrome∗ Transposition of the great arteries |
∗Also classified as a lesion that decreases cardiac outflow.
Clinical signs of congenital cardiac defects can manifest any time during the newborn period, infancy, or early childhood. The degree of symptoms, indications for medical, surgical, or transcatheter interventions, and chronicity of the condition depend on the diagnosis.
Shunting: Saturation Considerations
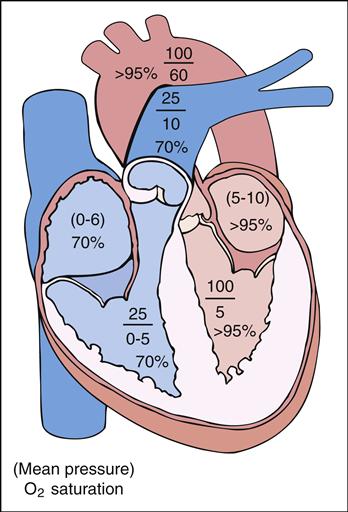
Abnormal blood flow from one part of the circulatory system to another is called a shunt. A shunt occurs when (1) there is an abnormal opening or connection between the cardiac chambers or great arteries, (2) the pressure is higher on one side of the heart compared with the other (pressure gradient), and (3) the oxygen saturation is increased or decreased in the normally desaturated or fully saturated blood. It is important to remember that the venous or right side is usually a low-pressure, desaturated (average 70%) system and the arterial or left side is usually a high-pressure, fully saturated (95% to 100%) system. The combination of pressure differences and the size of the abnormal opening determine the extent of shunting.
Blood Flow Considerations
Generally, the amount of blood flow to the lungs through the pulmonary artery is the same as the amount of blood flow to the systemic circulation through the aorta. This ratio of pulmonary to systemic blood flow is described as the pulmonary-to-systemic ratio (QP/QS ratio) and is usually 1:1. Patients with CHD may have normal, increased, or decreased QP/QS blood flow ratios. Understanding the principles of shunting and normal saturations in each of the heart’s chambers helps clarify the blood flow direction in CHD.
Physiologic Consequences of CHD in Children
There are two primary physiologic consequences of CHD in children. These include heart failure and cyanosis.
Heart Failure
The definition of heart failure (HF) is the heart’s inability to circulate blood to maintain sufficient cardiac output to meet the metabolic demands of the body. Despite the causes of HF, the heart initially responds to the need for increased cardiac output by increasing the heart rate. Over time the heart may become enlarged (cardiomegaly), causing the muscle walls of the heart to grow weak and inefficient. This can reduce blood volume in the body, which causes the body’s arteries to constrict and force the heart to work even harder. This poor function causes congestion in the body and/or lung tissues (pulmonary edema).
Etiology
The etiology of HF in neonates, infants, and children differs from that for adults. In infants and children, HF is most often related to an underlying congenital cardiac defect that causes volume or pressure overload, but may also develop from many other etiologies, including acquired heart disease. Examples of acquired heart disease include cardiomyopathies, dysrhythmias, infections (e.g., endocarditis, myocarditis), and tumors. Damage to the heart also can occur as a result of inborn metabolic disorders and exposure to certain drugs, and toxins (Park, 2008). Many times the symptoms can be treated by drugs. If there is an anatomic defect, correction of the cause either by surgery or transcatheter intervention is often needed.
Defects that lead to volume overload and cause symptoms of HF in infants and children include left-to-right shunts (e.g., atrial septal defects [ASDs], ventricular septal defects [VSDs], common AV canal defect [CAVC], and patent ductus arteriosus [PDA]). These defects allow excess blood to pass from the left side of the heart to the right, causing the heart to work harder to pump this extra volume to the lungs.
Defects that lead to pressure overload and cause symptoms of HF mainly include left-side heart obstructive lesions (e.g., critical aortic stenosis, severe aortic coarctation, congenital mitral stenosis, and hypoplastic left heart syndrome) (Park, 2008). These defects cause the heart to pump harder against an obstruction in order to get blood to the body. This extra work, over time, will cause the heart muscle to enlarge (hypertrophy) and become inefficient.
Defects that are acquired and cause symptoms of HF include primary myocardial diseases that attack the heart muscle and cause it to weaken. Dysrhythmias disrupt the delicate balance of the electrical or conduction system of the heart; cause the heart rate to be slow, fast, or erratic; and, over time, lead to poor cardiac output. The incidence of HF in infants and children is difficult to estimate because of improvements in surgical options.
Manifestations
The earliest clinical manifestations of HF are often subtle. The infant may have mild tachypnea (70 to 100 breaths per minute) at rest and sometimes difficulty feeding. Parents may report that feedings take longer and the child requires frequent rest periods. This type of history describes a scenario where less nutrition is consumed while more energy is expended, resulting in fewer calories being consumed, although metabolic demands are increased. Feedings therefore provide little satisfaction, and the infant may appear hungry and irritable soon after a feeding. Over time, the infant fails to gain weight and eventually develops a condition known as failure to thrive (FTT).
Children with HF may also exhibit respiratory-related symptoms. They may complain of dyspnea (particularly on exertion) and tachypnea. They are often described as having less energy than their peers. They may exhibit diaphoresis and complain of decreased appetite. This is due to chronic abdominal pain, usually related to poor circulation and decreased perfusion to the abdominal organs. This can also lead to failure to gain weight. Other clinical manifestations of HF include an abnormal cardiac rhythm known as a gallop; periorbital and facial edema, neck vein distention (in older children), hepatomegaly, and
splenomegaly; and decreased peripheral perfusion, decreased urine output, mottling, and cyanosis or pallor.
Diagnostic Evaluation
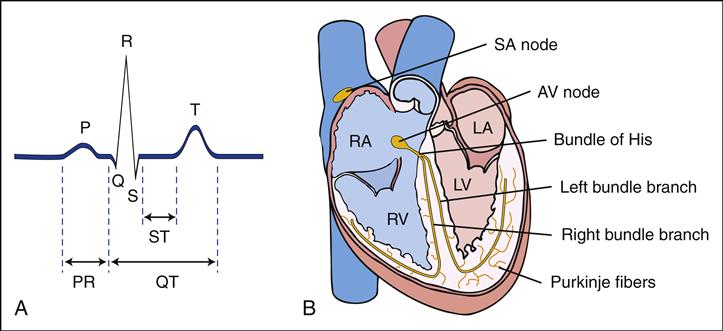
The diagnosis of HF is established on the basis of clinical history, physical examination, chest radiographic appearance, electrocardiography (ECG), and echocardiography (ECHO). Chest x-rays may reveal cardiomegaly with increased pulmonary vascular markings. These markings reflect increased interstitial pulmonary fluid. Laboratory studies that may be indicated to determine the presence of HF in children include determinations of arterial blood gas values, serum electrolyte levels, complete blood cell count, erythrocyte sedimentation rate (ESR), serum glucose and calcium levels, and urinalysis.
Therapeutic Management
Management of a child with HF involves correcting the underlying problem as soon as it is feasible to do so. The medical management of HF is directed toward decreasing cardiac workload and improving cardiac output through the manipulation of the hemodynamics and neurohormonal responses. Supplemental oxygen can be helpful for increasing oxygen saturation, but it should be used with caution in children with left-to-right shunts. Because oxygen is a vasodilator, it may increase pulmonary blood flow. Pharmacologic agents used include positive inotropes (e.g., digoxin), diuretics, and angiotensin-converting enzyme (ACE) inhibitors. In addition, optimizing nutritional intake to meet the metabolic demands and improve growth is of paramount importance. Inability to decrease symptoms and achieve weight gain is an indication for surgical intervention.
Digoxin is a cardiac glycoside that increases cardiac output and improves cardiac effectiveness by several mechanisms. It has a positive inotropic effect that strengthens the force of myocardial contractions. It has a negative chronotropic effect that slows the heart rate and, at higher doses, conduction of cardiac impulses through the AV node, allowing the ventricles more time to fill with blood. It also improves blood flow to the kidneys and enhances diuresis.
Obtain a baseline ECG before initiating digoxin. Digoxin may be administered intravenously or orally. The effectiveness of digoxin depends on achieving and maintaining a therapeutic serum drug level. A loading or digitalizing dose according to the child’s age and weight is administered in divided doses over 12 to 18 hours, and maintenance doses are given daily, usually in two divided doses. The range between therapeutic and toxic digoxin levels is narrow, with the therapeutic range 0.5 to 2.0 ng/mL (Skidmore-Roth, 2011). To avoid a falsely elevated serum digoxin level, serum digoxin levels should be measured at a minimum of 6 hours from the previous dose of digoxin. Measurement in the first 3 to 5 days after digitalizing could also yield elevated results, and so ECG changes are a more accurate sign of an elevated serum digoxin level during this time (Park, 2008). Levels are generally obtained when assessing for toxicity or medication adherence. Digoxin levels may be difficult to measure and monitor in preterm infants (Park, 2008). If a child is receiving digoxin and is having dysrhythmias, a digoxin level should be obtained. Hypokalemia and hypomagnesemia can increase the risk for digoxin toxicity. In children with altered renal function, the dose needs to be decreased.
Diuretics are administered to eliminate excess water and sodium through increased urine production, thereby reducing systemic and pulmonary congestion. Furosemide is a potent loop diuretic and is preferred for initial diuretic therapy. Another classification of diuretics, the thiazides, acts at the distal renal tubules. These can be less potent than loop diuretics. These drugs cause the kidneys to waste potassium, placing the child at risk for hypokalemia. Potassium-sparing diuretics, such as spironolactone, are weak diuretics. This class of diuretics is often given with loop diuretics or thiazides to decrease the potential for hypokalemia. Potassium supplements can also be given in tandem with diuretics to replace these losses.
Vasodilators, such as hydralazine, captopril or enalapril, may be used to relax vascular smooth muscles and reduce afterload (Park, 2008). Captopril and enalapril are called ACE inhibitors because they block the conversion of angiotensin I to angiotensin II and reduce vasoconstriction and sodium retention. In addition, ACE inhibitors also decrease norepinephrine release from the sympathetic nervous system.
Pulmonary Hypertension
Pulmonary hypertension (PAH) is elevated blood pressure in the blood vessels of the lungs. PAH is diagnosed when the mean pulmonary arterial pressure exceeds 20 mm Hg at rest (normal is 15 mm Hg at rest) (Park, 2008). The most common cause of PAH in children is CHD
(Adatia, Kothari, & Feinstein, 2010). Initially, in infants with a significant left-to-right shunting defect there is reversible pulmonary vasoconstriction and increased pulmonary blood flow that causes elevated pulmonary artery pressure. PAH occurs over time when vascular changes eventually lead to vessel wall thickening, severe irreversible vasoconstriction, and vascular obstruction. This severe condition leads to a reversal of the cardiac shunting, becoming right-to-left (called Eisenmenger syndrome), with less blood being pumped to the lungs, so that a previously acyanotic child becomes cyanotic as oxygen-poor blood gets pumped to the body. It is critical to time any surgical intervention before the development of irreversible vascular changes. This information is assessed clinically and in the cardiac catheterization laboratory. Repair of lesions with large left-to-right shunts is generally recommended in the first 3 to 6 months of life.
Children with large left-to-right shunting lesions, particularly at the ventricular level, have high pulmonary artery pressures but low pulmonary vascular resistance. Initially, HF develops. Management is directed toward treating the symptoms of HF. These may include digitalis, diuretics, warfarin, and supplemental oxygen. Additionally, the family is advised to have the child avoid strenuous exercise and high altitudes. Vasodilators may be helpful if the child demonstrates adequate response when these medications are given during cardiac catheterization (Park, 2008). Inhaled nitric oxide has been shown to be an effective pulmonary vasodilator and is used in the treatment of persistent PAH of the newborn.
PAH can also be caused by heritable (genetic mutations) or idiopathic etiologies. Idiopathic disease occurs when no underlying disease can be found. Conditions leading to PAH include alveolar hypoxia, such as pulmonary parenchymal disease or airway obstruction that leads to vasoconstriction. Pulmonary venous hypertension is seen in left heart outflow obstructive lesions and connective tissue disorders.
There are many research studies and ongoing trials for PAH-specific therapies in children, but with early surgical intervention, children with reversible PAH can have a return of normal pulmonary pressures postoperatively. Early intervention is important because, as increasing pulmonary vascular resistance caused by pulmonary vascular changes develops, children have a higher risk for surgical morbidity and mortality and for development of irreversible PAH. Management of children with idiopathic or advanced PAH remains challenging, as current pharmacologic therapies (e.g., IV epoprostenol or oral bosentan) have somewhat improved survival but have not adequately addressed disease progression (Dunbar Ivy, Rosenzweig, LeMarie, et al., 2010).
Cyanosis
Cyanotic cardiac lesions produce cyanosis when desaturated blood from the venous system enters the arterial system without passing through the lungs, commonly through an abnormal opening that allows for shunting of desaturated blood. Cyanosis, a bluish discoloration of the skin, nail beds, and mucous membranes, appears when tissues are deprived of adequate amounts of oxygen. Cyanosis becomes visible when hemoglobin that is not bound to oxygen reaches a level of approximately 5 g/dL blood and the measured oxygen saturation drops below 85%. The degree of cyanosis varies; some children will appear pale and mildly cyanotic, whereas others will be quite dusky. In anemic infants or children, desaturation will be higher before cyanosis is apparent (the lower total hemoglobin level in anemia means that a higher percentage needs to be desaturated before cyanosis is visible). Concurrently, with significant polycythemia (increased red blood cells with resulting elevated hemoglobin), children will appear cyanotic when less desaturated (the higher hemoglobin level in polycythemia means that a lower percentage needs to be desaturated before cyanosis is visible).
Cyanosis can occur when blood flow to the lungs is decreased (e.g., in severe pulmonary artery stenosis, pulmonary atresia, tetralogy of Fallot, or tricuspid atresia) or desaturated blood is pumped to the body (e.g., in total anomalous pulmonary venous return, truncus arteriosus, and hypoplastic left heart syndrome). Cyanosis intensifies with crying in children with these defects and is not alleviated by the administration of 100% oxygen.
The clinical consequences of cyanosis include polycythemia, anemia, clotting abnormalities, hypercyanotic episodes, central nervous system (CNS) injury caused by abscess or embolic events, PAH, and endocarditis. Developmental delay can be related to CNS injury, severe hypoxic events, or chronic illness.
Polycythemia is a compensatory response of the body to chronic hypoxia. The body attempts to improve tissue oxygenation by increasing the oxygen-carrying capacity of the blood—in other words, by producing additional red blood cells. Accelerated red blood cell production increases the viscosity of the blood and crowds the vascular space so there is less room for plasma and clotting factors. Children who are polycythemic are at greater risk for bruising and prolonged bleeding because of decreased specific clotting factors.
Increased blood viscosity makes the peripheral circulation sluggish and places the child at risk for CNS injury from a brain abscess or cerebrovascular accident. Depletion of iron stores may also result, and anemia may develop if iron is not available to participate in hemoglobin formation.
Dehydration can occur rapidly in cyanotic heart disease. Hyperthermia (fever or environmental), poor oral intake, vomiting, and diarrhea can cause acute dehydration. Dehydration can be life threatening for the child with cyanotic heart disease because the fluid loss can contribute to the relative viscosity of the blood. The increased viscosity can close a shunt that is necessary for pulmonary blood flow (shunt thrombosis). Once the shunt closes, there is no pulmonary blood flow, resulting in metabolic acidosis and severe hypoxemia that can lead to cardiopulmonary arrest.
Hypercyanotic Episode
A serious, clinically significant, and dramatic event seen in children with cyanotic heart disease is the hypercyanotic episode. These events are often called tet spells because they frequently occur in children with unrepaired tetralogy of Fallot. The exact cause is unknown, but it is thought that the child has acute spasm of the right ventricular outflow tract as a result of agitation or another adverse event that dramatically decreases pulmonary blood flow, causing hypoxia and metabolic acidosis (Bernstein, 2011). These episodes include rapid and deep respirations, irritability and crying, peripheral vasodilation, increased systemic venous return, increasing cyanosis that can be very severe, and a decrease in the systolic murmur, reflecting decreased pulmonary blood flow (Bernstein, 2011). As the child becomes more cyanotic, the child has increased tachypnea and hyperpnea, which increase the degree of right-to-left shunting. The incidence of hypercyanotic spells is not directly related to the degree of baseline desaturation and cyanosis.
Hypercyanotic episodes are seen most frequently in the first 2 years of life and seem to occur mainly in the morning. Often the episode is preceded by crying, feeding, or defecation. The infant becomes agitated and may eventually lose consciousness. Although usually self-limiting, the spells can progress to a vicious cycle that can be fatal if not recognized and treated. Frequent or prolonged episodes may lead to diminished cerebral oxygenation and ischemic brain injury.
Treatment of the episode includes calming the infant, placing the infant in the knee-chest position, and administering oxygen. Morphine sulfate is administered to suppress the respiratory center and decrease the degree of hyperpnea (which contributes to vasodilation) (Park, 2008). Potent medications that cause vasoconstriction (e.g., phenylephrine) may be needed to increase systemic vascular resistance, decrease the degree of right-to-left shunting, and force blood into the pulmonary system. Preventing or treating hypovolemia is also an important factor. Hypercyanotic episodes indicate the need to surgically repair or palliate the defect.
Nursing Care
The Child with Cyanosis
Assessment
The nurse must know the source of pulmonary blood flow when caring for a child with cyanotic heart disease. Infants and children who are dependent on a shunt for pulmonary blood flow are at risk for shunt thrombosis (as detailed earlier). Infants with right-to-left shunts are at increased risk for an arterial air embolus from an IV line because a small embolus can cross directly from the venous circulation to the arterial circulation through the shunt.
Evaluation of the child with cyanotic heart disease includes an assessment of baseline cyanosis and general appearance. Assess the level of activity, including irritability. Visible cyanosis is most easily seen in natural light and is evaluated by observing the skin of the central mucous membranes of the mouth and conjunctiva and the nail beds. General skin color is also assessed. Cyanotic children may be smaller than their peers and may demonstrate clubbing, thickening, and flattening of the fingertips and toes as a result of polycythemia (see Figure 45-6). Oxygen saturations should be obtained and compared with the child’s baselines. To prevent infective endocarditis, some children with unrepaired cardiac defects require antibiotic prophylaxis for certain dental and surgical procedures until the defect is repaired and for several months afterward. This includes children who have had infective endocarditis previously; therefore, the nurse needs to document this.
The child may become dyspneic during feeding, crying, and other activities that require exertion and may have difficulty keeping up with peers. Children with cyanosis may have frequent respiratory infections, may miss more school days, and may, as a result, academically lag behind their classmates, although they are often developmentally normal. If the child requires an IV line for hydration or another indication, it is imperative that the nurse assess IV line patency and inspect IV tubing for the presence of air in the line because even a small amount of air in the circulation increases the risk of systemic air emboli causing a stroke or heart attack. The use of an air filter is recommended.
Nursing Diagnosis and Planning
Nursing diagnoses and expected outcomes typical for the child with cyanosis and the child’s family include the following:
Expected Outcomes
The parents, and child if age appropriate, will explain the disease process, treatment, and interventions and will demonstrate the ability to perform home care treatments, including medication administration.
Expected Outcomes
The parents will express positive feelings for their child and for each other and will demonstrate the ability to meet the needs of the child, each other, and other family members.
Expected Outcomes
The child will demonstrate adequate growth according to an optimal growth curve for age and condition and will perform motor, social, and expressive skills typical of age-group within the scope of the child’s present capabilities. The parents will describe any developmental delay or deviation and make plans for intervention.
• Ineffective Tissue Perfusion related to hypercyanotic episodes.
Expected Outcomes
The child will remain free of decreased tissue perfusion, as evidenced by the absence of profound cyanosis and by the child’s activity level, affect, respiratory status, and oxygenation all being normal. The parents will list signs and symptoms that would signal the onset of hypercyanotic episodes.
Expected Outcomes
The child will remain free of endocardial infection. The parents will understand and carry out any ordered antibiotic prophylaxis; and the parents will list when to seek medical attention for fevers.
• Deficient Knowledge related to unfamiliarity with the systemic complications from increased risk of clotting.
Expected Outcome
The parents will describe the signs and symptoms to report immediately, including increasing cyanosis, vomiting, and fever (signs of clotting of the pulmonary shunt), and new-onset facial or extremity weakness, slurred speech, clumsiness, or breathing difficulty (signs of a CNS clot).
Interventions
Cyanotic heart disease is usually diagnosed in the newborn period. The initial nursing interventions are directed toward stabilizing and preparing the child for medical or surgical intervention. Pulmonary blood flow in cyanotic heart disease may depend on the persistence of the ductus arteriosus. Prostaglandin E1 (PGE1), a vasodilator, is often administered to maintain ductal patency and restore pulmonary or systemic blood flow. Continuous infusion of the drug may improve arterial oxygen saturation and tissue perfusion, allowing the infant to be stabilized in anticipation of further diagnostic and treatment interventions. PGE1 is rapidly metabolized through the pulmonary circulation and excreted through the renal system. It must be infused by continuous IV administration. The major side effect is apnea, and infants frequently require intubation. The nurse is responsible for monitoring PGE1 infusion flow and evaluating peripheral perfusion and respiratory status.
Parental teaching and support at the time of diagnosis are a priority. Parents receive complicated information and are often asked to make important decisions that may affect their child’s current therapy and, perhaps, future interventions. Parents need simple, yet thorough, explanations to help them make informed choices. The nurse may need to repeat the information several times. Help parents identify sources of emotional support and encourage communication within the family.
Parents of children with cardiovascular disease respond with a variety of reactions. Knowing this, the nurse has a responsibility to educate parents about their child’s disease and to stress the importance of the child interacting with the environment as normally as possible. For effective planning and provision of care, the child’s condition must be placed in the context of the family’s life.
In the child with cyanosis, careful monitoring of fluid status is necessary to prevent hemoconcentration. Intake and output are closely monitored, and daily weights may be recorded during hospitalization. Teach parents to recognize illnesses that place their child at risk for dehydration and to seek medical attention when their child has fluid losses.
Parents have concerns about worsening cyanosis and fear hypercyanotic episodes. Teach parents to recognize events that may trigger an episode and to respond calmly and place the infant in a knee-chest position. Review indications to seek medical care. Because most children with cyanosis limit their own physical activities, parents do not need to strictly limit the child’s activities.
Prevention of infective endocarditis may be necessary for select patients and is accomplished through antibiotic prophylaxis. Parents may be given a copy of the AHA prevention guidelines. Compared with previous guidelines, fewer patients are candidates to receive prophylaxis.
Children with cyanosis are prone to frequent respiratory infections. Respiratory infections may increase cardiac workload and lead to increased cyanosis and desaturation. Careful hand hygiene is necessary to reduce the risk of infection. Teach parents to avoid crowded areas and contact between their child and other people with respiratory infections.
The nurse’s role includes the astute and vigilant assessment, monitoring, and collaborative treatment of a child with known or potential cardiovascular alterations. Rapid changes in acuity and decompensation can occur in children with certain congenital and acquired heart diseases. Thus the skills required of the pediatric nurse must be focused and refined to identify clinically significant changes that may impact this often complex population.
Evaluation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree