CHAPTER 19
Superficial Thrombophlebitis and Deep Vein Thrombosis
Melody Heffline
M. Kate Schmidt
First Edition Authors: Kim Zurat and Karla Mees
OBJECTIVES
1. Describe the clinical significance of superficial and deep venous thrombosis.
2. Identify the risk factors for both superficial thrombophlebitis (STP) and deep vein thrombosis (DVT).
3. Identify diagnostic tests and procedures frequently used in the evaluation and treatment of venous thromboembolism.
4. Discuss the medial and surgical treatments for both STP and DVT.
5. Describe treatment modalities and essential elements of nursing care in management of thromboembolism.
Introduction
Venous thromboembolism is a major health problem. The Center for Disease Control (CDC) estimates the prevalence of venous disease to affect between 300,000 and 600,000 annually in the United States; of those 200,000 to 400,000 are diagnosed with DVT and 100,000 to 200,000 of those individuals will embolize to the pulmonary system. Around 30,000 to 60,000 of patients, who sustain pulmonary emboli (PE), will die (Office of the Surgeon General [US], 2008).
Venous thrombosis (clot formation) can occur in the superficial or deep venous systems. Superficial thrombus is clot formed in the superficial system producing pain, swelling, and erythematous streaking. Superficial venous thrombosis (SVT) are common affecting at least 125,000 people annually, with the incidence higher in women during pregnancy and in the postpartum period. The overall incidence has risen with the increased use of intravenous catheters and occurs more frequently when plastic catheters are inserted in the lower extremities (Ferri, 2011). STPs are generally treated symptomatically and rarely extend into the perforating veins and into the deep venous system.
DVT carries a higher risk of morbidity and mortality than STPs. One of the most serious complications is PE which occurs in more than one third of patients with DVT (Hirsh, Guyatt, Albers, Harrington, & Schunemann, 2008).
Venous disease also has a tremendous impact on the quality of life for thousands of Americans and presents a significant economic burden to the health care system. Increased awareness and early detection is important to reduce long-term sequelae and potential extension of the disease with the accompanied increased risk of death.
 I. Anatomy (see also Chapter 3, Anatomy and Physiology of the Vascular System)
I. Anatomy (see also Chapter 3, Anatomy and Physiology of the Vascular System)
A. Deep Venous System
1. Names correspond to the named arteries
2. Includes larger, deep veins
3. Lie below fascia and bound by muscle fascia
a. Abdomen
1) Inferior vena cava, mesenteric, renal, hepatic (in order of flow)
b. Lower extremity
1) Tibial, peroneal, popliteal, femoral, iliac
c. Upper extremity
1) Radial, ulnar, brachial, axillary, subclavian, jugular, innominate, superior vena cava
B. Superficial Venous System
1. Smaller veins, closer to the skin
2. Lie within subcutaneous tissue
a. Lower extremity
1) Greater saphenous
2) Lesser saphenous
b. Upper extremity
1) Cephalic
2) Basilic
C. Perforating Veins
1. Connect the deep and superficial venous systems by penetrating through the fascia (see Fig. 19-1)
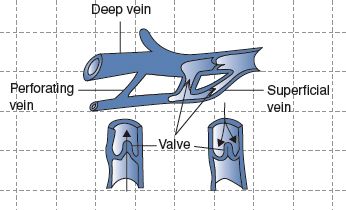
FIGURE 19.1 Anatomy of the venous system.
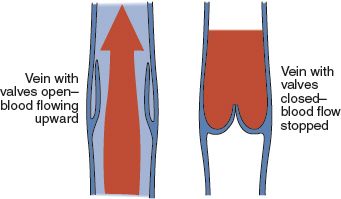
FIGURE 19.2 Venous valvular function.
D. Vein Structure
1. Tunica intima
a. Innermost layer
b. Endothelium, connective tissue, basement membrane
2. Tunica media
a. Smooth muscle and classic fibers
3. Tunica externa
a. Thin layer of connective tissue containing graduated and collagenous fibers
E. Valves (see Fig. 19-2)
1. One-way valves located within the veins
2. Open in diastole and closed in systole
3. Larger number of valves in distal veins (peroneal, tibial, popliteal); few in femoral and iliac; vena cava has no valves
4. Provide unidirectional blood flow back to heart (McCance & Huether, 2010)
 II. Pathophysiology
II. Pathophysiology
A. Inflammatory response triggered by the clotting cascade (tissue factor pathway)
B. Coagulation Cascade (tissue factor pathway)
1. Initiated by vascular injury
a. Exposes the connective tissue matrix leading to platelet adhesion and formation of hemostatic plug
b. Tissue factor collaborates with secreted platelet factor and activated platelets to activate the clotting system to form fibrin clots
c. The fibrin/platelet clot contracts to form a more permanent plug
d. Regulatory pathways are activated (fibrinolysis) to limit the size of the plug and begin the healing process
C. Virchow Triad
1. Three factors that result in DVT known also as Virchow triad
a. Local venous trauma
b. Stasis of venous blood flow
c. Systemic coagulation abnormalities (McCance & Huether, 2010)
2. Risk factors for STP and DVT
a. Trauma is common cause of STP—either a direct blow or iatrogenic injury (e.g., intravenous line or cannula with infusion of substances caustic to the vein wall)
1) Hypertonic solution: potassium chloride
2) Injurious compounds: antibiotics, barbiturates, benzodiazepines, chemotherapeutic agents
3) Needle insertion, which causes local inflammatory response
4) Incidence of catheter related is 0.1%, 100 out of 100,000 (Ferri, 2011)
5) Sclerotherapy of varicose vein is an example of beneficial effects of traumatic thrombophlebitis: a hypertonic agent is injected into the telangiectasia or varix to cause local inflammation and subsequent permanent scarring with closure of the vessel
b. Infection
D. Potential Outcomes of a VTE
1. Superficial VTE
a. Extension into deep vein system
1) Lower extremity: at the saphenofemoral junction thrombus moves from greater saphenous vein into common femoral vein; at lesser saphenous vein thrombus moves into popliteal vein
2) Upper extremity: thrombus moves from cephalic into axillary and subclavian vein
3) 20% of STP cases are associated with occult DVT (Ferri, 2011)
b. Signs and symptoms of superficial VTE
1) Subcutaneous vein is palpable, tender; cord present with erythema and edema of the overlying skin and subcutaneous tissue
2) Induration, redness, and tenderness are localized along the course of the vein. This linear appearance rather than circular appearance is useful to distinguish thrombophlebitis from other conditions (cellulitis, erythema nodosum)
3) No significant swelling of the limb
4) Low-grade fever may be present; high-grade fevers are suggestive of septic phlebitis
c. Suppurative thrombophlebitis: infectious process, usually at intravenous puncture site
1) Serious, potentially lethal; requires rapid treatment
2) Usual pathogens: Staphylococcus aureus was the most common pathogen, found in 65% to 78% of the cases of superficial suppurative thrombophlebitis before 1970; now the most cases are caused by Enterobacteriaceae, especially Klebsiella–Enterobacter spp. Usually acquired nosocomially and often resistant to multiple antibiotics (Ferri, 2011)
3) Signs and symptoms
a) Pus at intravenous line site (fever), elevated white blood cell count, intense pain at site (Hingorani & Ascher, 2009)
b) Superficial suppurative thrombophlebitis may be difficult to identify because local findings of inflammation may be absent. Fever is present in >70% of cases but rigors are rare
c) Local finding (warmth, erythema, tenderness, swelling, lymphangitis) are present in only one third of patients (Ferri, 2011)
d. Migratory thrombophlebitis
1) Multiple STPs at various sites most common in lower extremities
2) May be associated with carcinoma and may precede diagnosis of the carcinoma by several years
3) Workup for occult malignancy may be warranted (Hingorani & Ascher, 2009)
4) Other causes of migratory STP
a) Hypercoagulable states: protein C resistance, deficiencies of anti-thrombin III, protein C, and protein S
b) Estrogen therapy and birth control pills
c) Vasculitis: patients who have Buerger disease may develop “phlebitis migrans”; also seen in patients who have polyarteritis nodosa
d) Mondor disease: STP of the thoracoepigastric vein of the breast and chest wall. Thought to be associated with breast carcinoma or hypercoagulable state but some reported with no identifiable cause. Term also applied to SVT of dorsal vein of the penis. Treatment consists of conservative measures with warm compresses and NSAIDs (Hingorani & Ascher, 2009)
e) If multiple STPs occur in younger patient, suspect inflammatory vasculitis process; in an older patient, suspect malignancy
2. DVT
a. Pulmonary embolism, possibly fatal (refer to Chapter 20, Pulmonary Embolism)
b. Increased risk of long-term morbidity from post-thrombotic syndromes and chronic venous insufficiency (refer to Chapter 21, Chronic Venous Disease and Venous Ulcers)
1) Chronic changes in size, shape, and skin texture of the lower extremity caused by increased ambulatory venous pressure
2) Limb initially develops chronic swelling; over time, shape changes to that of an inverted champagne bottle because of distal leg skin sclerosis of subcutaneous fat layer (lipodermatosclerosis)
3) Nonhealing venous ulcers may form on medial and lateral malleolar surface because of effects of high venous pressure and absence of skin elasticity
4) 20% to 50% of patients with DVT develop post-thrombotic syndrome characterized by leg edema, pain, venous ectasia, skin induration, and ulcerations. Patients with extensive DVT and those with more severe post-thrombotic manifestations 1 month after DVT have poor long-term outcomes (Ferri, 2011)
c. High costs related to diagnosis, hospital readmissions, treatment of VTE, and long-term complications
d. Phlegmasia alba dolens
1) Advanced iliofemoral venous thrombosis commonly seen in pregnant women
2) Limb is swollen, pale, cool; nonpalpable pulses
3) Appearance of leg is caused by “severe perivenous inflammatory reaction extending to the peri-arterial sympathetic nerve fibers, producing arterial spasm or compression” (Walsh & Rice, 2004)
e. Phlegmasia cerulean dolens (also known as venous gangrene)
1) Near-total occlusion of venous outflow with increased venous pressure inhibiting arterial inflow
2) Seen in advanced carcinomas, especially of the pelvis or abdomen
3) Gangrene may result from combined arterial insufficiency with severe venous congestion
4) Patient complains of sudden deep pain, massive edema, and cyanosis of the limb (Walsh & Rice, 2004)
 III. Etiology/Precipitating Risk Factors
III. Etiology/Precipitating Risk Factors
A. Age
1. Most consistently associated with increased risk of DVT
2. Increases by factor of 200 between ages 20 and 80 with relative risk of 1.9 for each 10 year increase in age
3. Appears related to several age-associated factors
a. Decreased mobility
b. Increased number of major thrombotic risk factors
c. Age-related hypercoagulability
d. Changes in the venous system
B. Surgery
1. Multifactorial
a. Perioperative immobilization with resulting venous stasis
b. Activated coagulation
c. Transient depression of fibrinolysis
d. Increases in thrombin activation
e. Elevated levels of plasminogen and activator inhibitor
2. Degree of risk varies by
a. Age of the patient
b. Length and type of procedure
c. Presence of other thrombotic risk factors
3. Incidence of DVT based on type of surgery
a. 25% general surgery
b. 32% retropubic prostatectomy
c. 22% and 14% for gynecological procedures with and without malignancy
d. 22% for elective neurosurgical procedures
e. 45%, 51%, and 47% among those having surgery for hip fracture, hip arthroplasty, and knee arthroplasty, respectively (Meissner, 2009)
4. Postoperative patients at risk during entire period of immobility, especially after discharge when support for ambulation and activity may be lessened
C. Hormones
1. Oral contraceptives
a. Those that contain both estrogen and progestin increase the risk of a blood clot by 2 to 8 fold
b. The risk may be even greater with patches that contain transdermal contraceptives, since the amount of estrogen absorbed can be 60% higher
c. The absolute risk of women of fertile age using oral contraceptives is fairly low at 2 to 8 per 10,000 person-years, substantially lower that the risk faced by older men and women (US Dept of HR, 2008)
d. The highest risk of VTE occurs during the first year of use (Walsh & Rice, 2004)
e. Risk remains until the third month following discontinuation (McBane & Heit, 2009)
2. Hormone replacement therapy (HRT)
a. The relative risk of HRT is reported to be comparable to that of oral contraceptives despite a significantly lower amount of estrogen (Walsh & Rice, 2004)
3. Pregnancy
a. Risk of VTE increases 3 to 6 fold during pregnancy and is evenly distributed across all three trimesters
b. Rate of VTE after pregnancy is 172 to 199 per 100,000 deliveries
c. Risk is higher after cesarean section than vaginal delivery
d. Risks may be related to
1) High estrogen levels
2) Venous stasis
3) Pelvic trauma with delivery
4) Acquired hypercoagulability related to elevated procoagulants variables such as fibrinogen, von Willebrand factor, and factor VIII as well as decreased natural anticoagulants such as protein S
e. Risk factors associated with thrombosis during pregnancy include
1) Increasing age (>35 years)
2) Immobility
3) Obesity
4) Previous DVT
5) African Americans appear to have a greater risk than Caucasians (McBane & Heit, 2009)
D. Cancer
1. Incidence of VTE in patients with an active malignancy may be as high as 11% (McBane & Heit, 2009)
2. Tumors secrete clotting factor—tissue thromboplastin—like substance
3. High risk for development of thrombophlebitis
a. Risk of venous thrombosis after surgery for malignancy is 2 to 5 fold increased risk for postoperative VTE than surgery for nonmalignant conditions (Caprini & Arcelus, 2007)
b. Decreased fibrinolytic activity
c. Compression or infiltration of vein by tumor
d. Increased age
e. Greater likelihood of needing surgical procedure
f. Exposure to chemotherapeutic agents
g. Multiple invasive procedures and indwelling catheters
4. Patients with cancer have a >2 fold increased risk for VTE recurrence than those without cancer (Caprini & Arcelus, 2007)
5. Active cancers account for 20% of incidental VTE occurring in the community
6. Highest rates of venous thrombosis occur in carcinomas involving the kidney, stomach, pancreas, brain, and lymphoma
7. Lowest rates occurred in cancers of the head/neck, bladder, breast, esophagus, uterus, and cervix (Walsh & Rice, 2004)
8. Idiopathic venous thrombosis associated with 7.6% of malignancy; recurrent venous thrombosis associated with 17.1% incidence of malignancy (Mcbane & Heit, 2009)
E. Immobilization: bed rest of 72 hours or longer
1. Venous return is compromised due to lack of calf muscle pump activity
2. Blood pools in the extremities, increasing the risk of clot formation
3. Can be secondary to surgery, trauma, illness, or stroke resulting in paralysis
F. Heart Disease
1. Promotes stasis and emboli formation
2. Congestive heart failure; elevated central venous pressure and patients are often less mobile
3. Atrial fibrillation, cardiomyopathy, myocardial infarction, prosthetic heart valve, and valvular heart disease
G. Dehydration/Sepsis (relative hypercoagulable state)
H. Venipuncture/Numerous Central Catheters
I. Pulmonary Disease: severe chronic obstructive pulmonary disease with increased right heart pressure/failure reducing venous return
J. Previous History of DVT, PE, or Venous Disease
1. 30% develop recurrence within 10 years
2. 20% with optimal therapy will have extension or recurrence (Caprini & Arcelus, 2007)
K. Morbid Obesity
1. Increased incidence of DVT noted in patients weighing 20% above ideal weight for age, sex, and frame size, but obesity is not yet identified as an independent risk factor
2. Impaired fibrinolytic system
3. Increased immobility
L. Medications: chemotherapy and hormone therapy
M. Varicose Veins: the most frequent predisposing risk factor for SVT is presence of varicose veins, which occurs in 62% of patients (Hingorani & Ascher, 2009).
1. Varicosities adjacent to venous ulcers are more likely to become phlebitic.
2. Greater and lesser saphenous vein varicosities more often involved.
3. Spontaneous bleeding from grapelike clusters of telangiectasias near ankle can occur because of high venous pressure in these small superficial vessels. Small ulcers may then occur at the site of bleeding.
N. Inherited Factors
1. Antithrombin III deficiency
2. Protein C & S deficiency
3. Factor V Leiden mutation
4. Prothrombin 20210 mutation
5. Dysfibrinogenemia
O. Travel
1. Association between travel and VTE controversial
2. Association appears related to duration of travel
3. Most individuals with VTE associated with prolonged travel have additional risk factors (Mcbane & Heit, 2009)
P. Others
1. Myeloproliferative disorders
2. Polycythemia vera
 IV. Primary Prevention
IV. Primary Prevention
A. Ambulatory/Outpatient Setting
1. Assess for presence of risk factors
2. Promote education regarding risk factors
3. Promote healthy lifestyle
4. Special coagulations studies
a. May be obtained if there is a family history of thrombophlebitis, especially in family members under the age of 40 with no other risk factors for DVT
b. May be used to assess if patient is a high risk for development of thrombophlebitis
5. Nonpharmacologic methods for VTE prophylaxis
a. Exercise
1) Walking as much as possible
2) Dorsi/plantar flexion of feet with prolonged sitting or bed rest to promote venous return
6. Nonpharmacologic methods for VTE prophylaxis
a. Early mobilization
1) Routine part of postoperative care
2) Sole prophylaxis in lower-risk patients (<40 years of age without additional risk factors having minor surgery; less than 45 minutes) (Caprini & Arcelus, 2007)
b. Graduated compression stockings, 15 to 20 mm Hg pressure
1) Less effective than other methods
2) Recommended as an adjunct for most moderate or higher-risk patients
3) In the absence of palpable dorsalis pedis or posterior tibial pulses, an ABI is required
4) Contraindicated in patients with ABI <0.5
c. Intermittent pneumatic compression (IPC)
1) Recommended in combination with compression stocking after neurosurgery (Ferri, 2011)
2) Has been recommended in multiple trauma patients when anticoagulation is not feasible, especially in the setting of active bleeding (Mcbane & Heit, 2009)
3) Research suggests IPC can reduce risk in general surgical patients; strong evidence for use of IPC as sole therapy in moderate risk patients and as adjunctive therapy in high risk patients but more research is needed (Caprini & Arcelus, 2007)
d. Consider inferior vena cava filter placement, if other forms of prophylaxis are contraindicated
B. Pharmacologic Methods for VTE Prophylaxis
1. Low-dose unfractionated heparin (UFH)
a. Facilitates the binding of antithrombin III with the activated clotting factors thrombin, factor IXa and Xa
b. Subcutaneous administration, usually with twice-daily injections; typical dose is 2,500 to 5,000 units
c. Half-life is 0.5 to 2.5 hours
d. Use is decreasing because of the availability of low-molecular-weight heparin (LMWH)
2. LMWH
a. Lovenox (enoxaparin), Fragmin, (dalteparin), Innohep (tinzaparin)
b. Facilitates the binding of antithrombin III with activated clotting proteins, catalyzing inactivation of thrombin and other clotting factors
c. Can be administered subcutaneously as an outpatient or inpatient
3. Direct thrombin inhibitor
4. Factor Xa inhibitor
5. Oral vitamin K antagonist: warfarin may be recommended prophylactically for high-risk patients with a history of previous VTE
6. Oral direct thrombin inhibitor
C. Surgical Hospitalized Patients: example of an institution-specific protocol for DVT prevention (see Table 19-1)
 V. Assessment
V. Assessment
A. Risk Factors
1. Family history of venous thrombosis and or familial thrombophilia
2. Acquired thrombophilia
3. Assess for predisposing factors contributing to venous thrombosis as listed above
B. Patient History
1. Subjective
a. Focused history of patient, previous episodes of thrombophlebitis
b. Family history of venous thrombophlebitis or familial thrombophilia
c. Symptoms and duration of symptoms
d. Pain assessment, severity and location
2. Objective
a. Thrombophlebitis documented by diagnostic testing
TABLE 19-1 Prevention of Deep Vein Thrombosis (DVT) in Hospitalized Patients
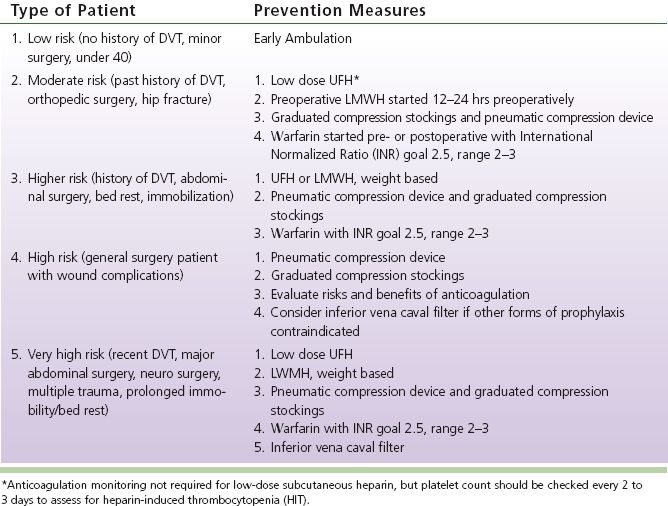
b. STP: unilateral limb swelling along an erythematous, tender, palpable cord. If limb is generally swollen or STP is in high, suspect coexistent DVT
C. Clinical Diagnosis of DVT is Unreliable
1. Many DVTs are clinically silent or asymptomatic, especially if obstruction is only partial, thrombus is small or collateral vessels have developed around complete obstruction (Walsh & Rice, 2004)
2. Up to 70% of patients presenting with complaints consistent with DVT will not have the disease and many patients with DVT will be asymptomatic (Lohr, Kin, & Krallman, 2009)
3. Homan sign (pain in the upper calf during forced dorsiflexion of the foot) is insensitive and nonspecific
4. Other nonspecific signs and symptoms: leg cramps, redness, cyanosis, varicose vein distention
D. Physical Examination
1. Inspection
a. Swelling, erythema, streaking, cord
1) Compare contralateral limb
2) Distended neck or chest veins in upper extremity and/or central thrombosis
3) Presence of dilated superficial veins
4) Presence of cyanosis or inflammation
b. Varicosities or signs of chronic venous disease (skin changes, ulceration)
c. Oral mucosa for evidence of petechiae or bleeding
2. Palpation
a. Assess pulses
b. Amount of edema: measure circumference of both limbs at similar levels and document
c. Skin: check for warmth, erythema
d. Palpable cord
e. Liver or spleen enlargement
3. Auscultation
a. Lung and cardiac examination for tachypnea, tachycardia, or murmurs
4. Vital signs
a. Temperature: presence of a fever
b. Blood pressure: hypo- or hypertension
c. Respiratory rate: tachypnea, congestion
d. Heart rate, rhythm and sounds for presence of arrhythmia, tachycardia, or murmurs
5. Height and weight
6. Homan sign: limited usefulness because of lack of sensitivity and specificity
E. Differential Diagnosis (Ferri, 2011)
1. SVT
a. Lymphangitis
b. Cellulitis
c. Erythema nodosum
d. Panniculitis
e. Kaposi sarcoma
2. DVT
a. Postphlebitic syndrome
b. STP
c. Ruptured Baker’s cyst
d. Cellulitis or lymphangitis, Achilles tendonitis
e. Hematoma
f. Muscle or soft tissue injury, stress fracture
g. Varicose veins, lymphedema
h. Arterial insufficiency
i. Abscess
j. Claudication
k. Venous stasis
F. Considerations Across the Life Span
1. Young adulthood
a. Participation in contact sports
b. Oral contraceptive
c. Alcohol, tobacco, or illicit drug use
d. Compliance
e. Presence of other disease
f. Diabetes
g. Cancer
h. Recent surgery or trauma, high-risk occupations such as long distance drivers or travelers, and sedentary office workers
i. Consider familial hypercoagulability if no other known risk factors present
j. Nutritional status and dietary habits, especially vegetarianism
k. Other medications and potential interactions with anticoagulants
2. Women of childbearing age
a. Oral contraceptive use
b. Conception planned
1) Warfarin crosses placenta and causes neural tube birth defects
2) Consider low-dose UFH or LMWH, which does not cross the placenta; if on warfarin (Coumadin) prior to conception for previous thromboembolic event, may need therapeutic dosing of LMWH throughout pregnancy rather than low dose
c. Alcohol, tobacco, or illicit drug use
d. Compliance
e. Nutritional status and dietary habits
f. Other medications and potential interactions with warfarin
3. Elderly
a. HRT
b. Safety issues
1) History of falls
2) Home environment
3) Presence of support system
4) Memory issues
5) Increased risk of bleeding
6) Compliance
c. General health and nutrition status: may require a lower dose of warfarin
d. Use of alcohol, tobacco, or illicit drugs
e. Other medications
1) Interaction with warfarin
2) Clues to treatment for medical comorbidities
 VI. Pertinent Diagnostic Testing
VI. Pertinent Diagnostic Testing
A. Noninvasive
1. Duplex ultrasound (also refer to Chapter 5, Vascular Diagnostic Studies)
a. First-line test; ≥95% sensitive and specific for proximal DVT
b. Real-time sonography allows visualization of veins and thrombi in transverse and longitudinal views, including “floating” thrombi that could embolize (Walsh & Rice, 2004)
c. Detects venous flow in both gray scale and color; can assess for antegrade and retrograde (reflux) flow direction
2. Computed tomography
a. Not usually ordered to diagnose thrombophlebitis, but thrombophlebitis may be visualized
b. Useful in pelvic or abdominal DVT diagnosis
B. Invasive
1. Ascending venography
a. Seldom performed because of high sensitivity of duplex scan, cost, and potential complications of invasive procedure
b. Involves placement of catheter in the dorsum of the foot, radiopaque contrast medium injection and use of x-ray
c. Provides accurate visualization of the veins and thrombi
d. Criteria for DVT diagnosis: intraluminal filling defect seen in multiple views
e. Useful for equivocal duplex scans
f. Complications: pain at injection site; hyperosmolar contrast may cause damage to endothelium of vessel and lead to STP or DVT; hypersensitivity reaction, renal failure, and potential for skin necrosis due to extravasation of contrast at the injection site
C. Other Less Commonly Used Diagnostic Tests
1. Air or impedance plethysmography (refer to Chapter 5, Vascular Diagnostic Studies); used when other testing modalities are not available
2. Nuclear scan with radioactive isotope, fibrinogen I-125 (Walsh & Rice, 2004)
a. Identifies fibrinogen attached to thrombi
b. Injection of radioactive fibrinogen is followed in 12 to 24 hours by nuclear scan
c. Highly accurate for DVT, including tibial and peroneal vessel thrombi
d. Disadvantages: costly, unable to detect thrombi that do not contain fibrinogen, time delay for diagnosis
D. Laboratory Tests
1. Baseline tests before initiation of treatment
a. Complete blood cell count with platelet count
b. Activated partial thromboplastin time (APTT)
c. Prothrombin time (PT)
d. INR
e. Fecal occult blood to detect gastrointestinal bleeding
f. Creatinine
g. Fibrinogen levels if plan tPa
2. Special coagulation testing when indicated
a. Familial thrombophilia
1) Factor V R 5060Q (Leiden) gene mutation
2) Prothrombin 20210
3) Homocysteine level
4) Protein S level (must draw before initiating warfarin)
5) Protein C level (must draw before initiating warfarin)
6) Antithrombin III level (must draw before initiating UFH/LMWH)
7) Activated protein C resistance
b. Acquired thrombophilia
1) Antiphospholipid antibodies
2) Antinuclear antibodies
3. D-dimer (refer to Chapter 20, Pulmonary Embolism)
4. Fibrin degradation: indicative of the presence of active clotting
 VII. Medical Management
VII. Medical Management
A. SVT
1. Prevention of SVT (Geerts et al., 2008)
a. Rotate intravenous sites every 3 days.
b. Dilute irritating intravenous infusions (e.g., potassium chloride).
c. Change intravenous tubing routinely under sterile conditions.
d. Avoid insertion of three-way stopcocks directly into hub of catheter.
2. Treatment of SVT (Hofmann, 2009)
a. Remove in-dwelling cannulae immediately.
b. Early use of extremity may speed recovery.
c. Treat pain with moist heat, elevation of limb, and oral or topical nonsteroidal anti-inflammatory medication for symptom relief unless contraindicated.
d. Consider appropriate prophylaxis for DVT as indicated by risk factors.
e. Frequent evaluation of site.
f. Symptoms usually subside in 2 to 3 weeks.
g. For spontaneous SVT may use LMWH, UFH, or vitamin K antagonist for 4 weeks. Not recommended to use oral anticoagulants and anti-inflammatory agents together.
3. Suppurative STP: inflammation and thrombosis in the setting of bacteremia (Mermel et al., 2009)
a. Monitor for signs of systemic illness
b. Culture catheter
c. Start empiric antibiotic therapy while awaiting culture results: vancomycin is drug of choice where MRSA is prevalent
d. Antibiotics per culture sensitivity until cellulitis resolved, or up to 6 weeks for bacteremia
e. Uncomplicated exit sites should be treated with topical antimicrobial agents on the basis of culture; mupirocin for S. aureus and ketoconazole or lotrimin for Candida
f. For trauma patients, prevention of suppurative STP is critical because of high rate of contamination of lines in first rescue efforts; all intravenous lines removed in first 24 hours
4. STP associated with varicose veins
a. Treatment directed at symptomatic relief: moist heat, rest, limb elevation, anti-inflammatory agents if not contraindicated
b. Activity encouraged
c. Compression support stocking if tolerated: may need to wait until initial pain and inflammation subside
d. Special coagulation studies if known recurrence
B. Deep Vein Thrombosis (Ageno et al., 2012; Hirsh et al., 2008)
1. Preliminary considerations
a. Baseline laboratory tests before initiation of treatment
1) APTT, PT (INR), complete blood cell count, creatinine, glomerular filtration rate (GFR)
2) Other coagulation studies may be ordered. Results of some studies may be affected by degree of clot burden and results may not be accurate in the acute phase of thrombosis. Should be drawn prior to starting any anticoagulation therapy if used
b. Assess for contraindication to anticoagulation therapy
1) History of HIT: will change choice of anticoagulants used
2) Recent gastrointestinal bleed or hemorrhagic cerebrovascular accident within past 3 months
3) Allergy
4) Recent major surgery
2. Intravenous UFH (Guyatt, Akl, Crowther, Gutterman & Shuünemann, 2012; Holbrook et al., 2012)
a. Mechanism of action
1) Heparin and antithrombin inactivate thrombin (factor IIa) and factors IXa, Xa, XIa, XIIa to stop clot propagation and decrease risk of embolization
2) Thrombolytic system begins to lyse thrombus naturally. UFH has no effect on existing thrombus
3) Half-life: 60 to 90 minutes
4) Does not cross placental barrier: safe in pregnancy
b. Dosing guidelines
1) Initial bolus 80 units/kg and infusion of 18 units/kg
2) Discontinue heparin infusion when INR at therapeutic range for 2 days with overlapping warfarin. Warfarin initiated on day 1 of treatment
3) Rate of recurrent thrombus found to be significantly lower with weight-based dosing
c. Monitoring UFH
1) APTT used to measure activity of thrombus activators and inhibitors. Endpoint is amount of time in seconds for the generation of a fibrin clot.
2) Usual target range 1.5 to 2.5 time control. Due to institutional monitoring variations, APTT range should be based on responsiveness of the reagent and coagulometer used (Ansell et al., 2008).
3) Obtain baseline APTT and platelet count prior to initiation and follow daily platelet count to assess for HIT.
d. Rapid achievement of therapeutic APTT critical for prevention of recurrent DVT
1) In patients without significant comorbid risk factors for bleeding (e.g., renal or liver failure), supratherapeutic APTT is not associated with increase in clinically significant bleeding
2) Goal is to achieve therapeutic APTT within first 24 hours using weight-based dosing
e. Contraindications: allergies to heparin, LMWH, previous history of HIT, recent history of bleeding/active bleeding, hemophilia, shock
f. Complications associated with UFH
1) Hemorrhagic: bleeding risk varies between 0% and 2% depending on underlying disease, other medications, and intensity/duration of therapy. Most common sites include GI tract, urinary tract, soft tissue, and oropharynx. Co-morbid conditions, particularly cancer, heart, renal or liver disease, and recent surgery or trauma increase risk of bleeding. Risk of bleeding increases with concomitant use of thrombolytic agents or GP IIb/IIIa inhibitors. Reversal of UFH is accomplished with protamine sulfate. Dose is 1 mg for every 100 units of heparin administered over the previous 4 hours. Administered IV slowly to prevent hypotension.
2) Nonhemorrhagic: heparin-induced thrombocytopenia (HIT) is a rare but serious complication occurring due to the development of heparin-specific antibodies binding to the platelet membrane. Results in drop in platelet count due to platelet aggregation. Mild thrombocytopenia may occur between days 2 and 6 of treatment. This usually resolves with discontinuation of UFH. More severe reductions in platelet count seen between days 4 and 15 may lead to significant decreases in platelet count of 30% to 40% from baseline with levels below 100,000. Skin necrosis from thrombosis may occur (Warkentin, Grienacher, Koster, & Lincoff, 2008).
3) Other: anaphylaxis, bronchoconstriction, urticaria, alopecia, osteoporosis (with long-term use such as during pregnancy)
3. LMWH (Holbrook et al., 2012)
a. Mechanism of action: produces major anticoagulant effect by activating antithrombin. Produce a more predictable anticoagulant response than heparin. Half-life of 3 to 6 hours after injection with peak levels in 3 to 5 hours. Half-life is dose dependent; cleared by kidneys.
b. Dosing and monitoring
1) No monitoring is recommended in routine patients. Patients treated during pregnancy should be monitored with anti-Xa levels and dose adjusted for weight gain. Levels must be drawn 3 to 5 hours after injection during the peak of action. Target anti-Xa levels are dependent on the agent used and the dosing regimen, for example, q12h dosing versus q24h dosing.
2) Dosing: weight based. Weight-based dosing recommended in obese rather than fixed dose. For severe renal disease (CrCl <30 mL/min) recommendation is for UFH or reduction in LMWH dose by 50%.
3) Reversal: protamine sulfate 1 mg per 100 anti-Xa units of LMWH for LMWH given within 8 hours. Administer second dose of 0.5 mg per 100 anti-Xa units if bleeding continues.
c. Contraindications: same as for heparin
d. Complications: bleeding, HIT less frequency than with UFH, osteoporosis less likely than with UFH
4. Fondaparinux (Garcia, Baglin, Weitz, & Samama, 2012; Holbrook et al., 2012)
a. Synthetic pentasaccharide, anti-Xa activity higher than LMWH, half-life about 17 hours; safe for patients with HIT
b. Administered SQ once daily in fixed doses, no laboratory monitoring
c. Prophylactic dose is 2.5 mg daily. Treatment of DVT or PE 7.5 mg for weight 50 to 100 kg. Decrease to 5 mg for <50 kg, increase to 10 mg for >100 kg.
d. Contraindicated in severe renal disease (CrCl <30 mL/min), dose reduction of 50% with CrCl <50 mL/min.
e. No reversal. May administer recombinant factor VIIa for uncontrolled bleeding.
f. Complications: no reports of HIT; may cause urticarial reactions, local skin necrosis.
5. Intravenous direct thrombin inhibitor (Garcia et al., 2012)
a. Agents: lepirudin, desirudin, bivalirudin, argatroban. Used in patients who have suspected or confirmed HIT
b. Dosing and monitoring
1) Reduced dose in renal disease with CrCl <60 mL/min but >30 mL/min for lepirudin or desirudin and monitor with APTT. Do not use lepirudin or desirudin with CrCl <30 mL/min.
2) Argatroban not used with hepatic dysfunction. Monitor with factor X levels due to variable effect on INR. Cannot stop infusion until INR reaches therapeutic level with oral vitamin K antagonist to decrease risk of thrombosis. Factor X levels <45% associated with INR value >2. Factor X levels safer than aiming for INR of 4 or greater before stopping infusion.
6. Oral direct thrombin inhibitors: dabigatran (Pradaxa) (Ageno et al., 2012; Prescriber’s Letter, 2013; Schulman et al., 2009)
a. Predictable anticoagulant effect with fixed dosing
b. No monitoring required, no reversal agent
c. Half-life 17 to 21 hours
d. Approved for stroke prevention in atrial fibrillation and prophylaxis for DVT in elective hip or knee arthroplasty
e. Avoid in patients with severe renal failure. Check renal function at baseline and yearly
f. US approval for prevention of stroke in nonvalvular afib and available only in 150 mg doses dosed twice daily. Off-label use for prevention of VTE posthip and knee replacement
g. Canada approved for stroke prevention and VTE prevention posthip or knee replacement. For stroke prevention dosed 150 mg twice daily under age 80. Over age 80, 110 mg twice daily. For VTE prevention posthip/knee 220 mg daily for 10 days postknee and 28 to 35 days posthip. Initial dose 110 mg if started 1 to 4 hours postoperatively. Consider 150 mg daily for patients over age 75 with initial dose 75 mg.
h. Dosing based on balance between efficacy and bleeding risk. Major risk is bleeding.
i. Must be used within 4 months of opening bottle. Store in original package only. Take at least 2 hours before antacids.
j. P-glycoprotein inhibitors may increase dabigatran levels, for example, dronedarone, ketoconazole, verapamil, quinidine, amiodarone, clarithromycin. P-glycoprotein inducers may decrease dabigatran efficacy. Avoid P-glycoprotein inducers, for example, rifampin, carbamazepine, St. John’s wort.
7. Vitamin K antagonists (Ageno et al., 2012)
a. Warfarin (Coumadin): inhibits synthesis of vitamin K-dependent proteins (factors II, VII, IX, X and proteins C and S) involved in the coagulation process; highly water soluble and rapidly absorbed from GI tract; circulates bound to plasma proteins; accumulates in the liver.
b. Produces anticoagulant and antithrombotic effects; anticoagulant effect occurs 12 to 24 hours after first dose; antithrombotic effect occurs 2 to 7 days after steady state has been achieved. Time to steady state related to long half-life of warfarin (36 to 42 hours) and long half-life of some factors (up to 90 hours).
c. Dosing
1) Initiate UFH/LMWH and start warfarin on same day
2) Adjust doses based on INR with testing beginning at baseline and after two or three doses or oral anticoagulation therapy
3) Overlap UFH/LMWH therapy for 5 days or until INR therapeutic for 2 days to allow for further reduction in factor II and X
4) Initial dosing may range from 5 to 10 mg; not recommended to use high initial dosing in elderly, debilitated, malnourished, CHF, liver disease, recent major surgery, or taking medications known to increase sensitivity to warfarin
d. Monitoring
1) INR used to monitor stability; consistent measure from one laboratory to another
2) Monitoring may be done through office point-of-care testing by advanced practice nurse, pharmacist, or nurse via protocol; self-testing may be done by qualified patients with scheduled reporting
3) Contraindications
a) Same as for other anticoagulants; use with caution in patients with drainage tubes, malabsorption disorders, dietary deficiencies, gastrointestinal ulcers, renal or liver dysfunction
b) Absolute: pregnancy—warfarin may cause teratogenic effect in the first trimester; crosses placental barrier and may cause fetal bleeding; for high-risk women with mechanical valves vitamin K antagonist may be used after 13th week with LMWH or UFH resumed close to delivery
e. Complications
1) Bleeding: proportional to elevation of INR and other risk factors; may be corrected with use of vitamin K, preferably administered orally; dose dependent on levels of INR and presence/absence of significant bleeding. Oral administration achieves reversal in approximately 24 hours. Low doses <5 mg are usually sufficient. Parenteral administration achieves quicker reversal but may lead to difficulty in achieving therapeutic levels after bleeding under control due to long half-life. For INR >5 but <9 and no significant bleeding, omit one or two doses and lower dose, monitor more frequently until stabilized. For serious bleeding, omit dose and supplement vitamin K reversal with FFP.
2) Food and drug interactions: associated with alteration in vitamin K availability/intake, reduced warfarin absorption, changes in warfarin protein binding, effects on drug metabolism. Vigilance and stability in dietary intake and concomitant medications is required. Any changes in medication warrant testing to monitor for interactions.
3) Skin necrosis: usually observed on third to eighth day; caused by extensive thrombosis of venules and capillaries in subcutaneous fat; may also cause limb gangrene due to outflow obstruction of venous circulation. Treated with discontinuation of warfarin, resumption of heparin, comfort measures, surgical debridement. May require skin grafting or amputation of limb if severe.
8. Direct Factor Xa inhibitor rivaroxaban (Xarelto) (Ageno et al., 2012; Guyatt et al., 2012; Prescriber’s Letter, 2013)
a. Dosing
1) Prevention of DVT 10 mg daily beginning 6 to 10 hours postoperatively for 2 weeks after total knee arthroplasty and 5 to 6 weeks after total hip replacement. Take with food.
2) Treatment of DVT/PE 15 mg twice daily for 3 weeks and then 20 mg daily. Take with food.
b. No monitoring necessary, no reversal agent
c. Not for use in patients with CrCl <15 mL/min, hepatic disease with coagulopathy. Monitor renal function periodically.
d. Do not use with strong CYP3A4 inducers or inhibitors, for example, ketoconazole, itraconazole, voriconazole, posaconazole, fluconazole protease inhibitor, clarithromycin, erythromycin.
9. Length of therapy
a. First episode: 3 to 6 months depending on location of thrombus, presence of reversible risk factor; long-term treatment recommended for unprovoked proximal DVT when no risk of bleeding and good anticoagulant monitoring is available
b. For recurrent DVT or thrombophilia, therapy may be lifelong
C. Supportive Measures
1. Graduated compression stockings to reduce swelling and promote venous return
2. Elevation of the affected extremity 10 to 20 degrees above heart level
3. Progressive ambulation as allowed; avoid or delay for significant limb edema
D. Thrombolysis of DVT (Gogainiceanu et al., 2009)
1. Dissolution of intraluminal thrombus via chemical lysis to restore vessel patency; recommended use in selected patients with extensive proximal DVT, such as ilio-femoral, with low risk of bleeding; directly infused into the venous thrombus via multi-side-hole catheter
2. Balloon angioplasty and stents recommended for correction of underlying venous lesions
3. Indications
a. Phlegmasia cerulea dolens resulting in compromised arterial circulation
b. Extensive ilio-femoral DVT
4. Benefits
a. Lysis of thrombus may prevent late destruction of venous valves thereby reducing the risk of chronic postphlebitic syndrome (chronic swelling, ulceration)
b. Performed percutaneously with direct catheter placement into thrombosed area under local anesthesia
c. Rapid acting and rapidly cleared from the body: half-life 5 to 8 minutes, administered by IV infusion
5. Disadvantages
a. High risk of systemic bleeding complications; risk of bleeding differs with respect to underlying disorders and co-morbid conditions; appears to be associated with 1.5 to 2-fold increase in risk of major bleeding
b. Definitions of major bleeding differ among studies: most ominous bleed is intracerebral
c. Frequent angiograms needed to check extent of thrombolysis requiring increased contrast load and risk of subsequent contrast-induced nephropathy
d. Intensive care monitoring required
6. FDA-approved thrombolytic agents
a. Streptokinase
1) First agent approved
2) Disadvantages: not as rapid in clot lysis as other agents, frequent hypersensitivity reactions; seldom used
b. Urokinase
1) More effective than streptokinase in clot lysis but less effective in restoring venous flow than recombinant tissue plasminogen activator (tPa)
c. Recombinant tissue plasminogen activator (tPa)
1) More rapid and complete thrombus dissolution than urokinase
2) Shortest treatment time
7. Contraindications to thrombolytic therapy
a. Absolute: active bleeding, cerebrovascular disease/event or procedure within past 2 months
b. Relative: recent major surgery or trauma (within 10 days), postpartum, cardiopulmonary resuscitation with rib fractures, thoracentesis, paracentesis, lumbar puncture, any condition requiring maintenance of normal clotting or healing
c. Potentially serious bleeding: uncontrolled coagulation defects, severe hypertension, pregnancy, any condition with potential bleeding risk (refer Chapter 20, Pulmonary Embolism, for patient-care guidelines)
 VIII. Interventional Management (Vedantham, 2009; Walsh & Rice, 2004)
VIII. Interventional Management (Vedantham, 2009; Walsh & Rice, 2004)
A. DVT Thrombectomy
1. General/spinal anesthesia
2. May be done percutaneously or through incision; thrombus removed by fragmentation and aspiration of clot
3. Requires anticoagulation with heparin and warfarin once hemostasis is achieved
4. Usually performed in conjunction with thrombolytic therapy
5. Indications: same as for thrombolysis, DVT not responsive to medical intervention
6. Best outcome if performed within 2 to 3 days of thrombus formation
B. Vena Cava Filter Placement
1. Indications
a. Unable to use anticoagulant therapy due to contraindications
b. Prevention of pulmonary embolization of thrombus (originating in the lower extremity or pelvic veins) in high-risk patient
c. Recurrent thromboembolism despite adequate anticoagulation
d. Patient undergoing pulmonary embolectomy
e. Chronic recurring pulmonary embolism
f. Elderly patients at risk of falling with severe consequences if receiving anticoagulation therapy
g. Free-floating proximal thrombus
h. Mentally unstable patient
i. Low cardiac reserve or pulmonary hypertension
j. Massive pulmonary embolism
2. See Chapter 20, Pulmonary Embolism for devices, insertion technique, and complications
C. Saphenofemoral Junction Ligation for SVT
1. Indication: prevention of DVT when superficial venous thrombophlebitis extends into the saphenofemoral junction
2. Surgical procedure with a small groin incision under local or general anesthesia, ligation or disassociation of the superficial saphenous vein from the deep femoral vein
3. Outpatient procedure or 1-day hospital stay depending on patient circumstances
D. Suppurative STP (Mermel et al., 2009)
1. Surgical resection limited to patients with purulent superficial veins or when infection extends beyond vessel wall or failure of conservative therapy
2. Wound left open for secondary healing
3. Skin graft may be needed once infection resolved and patient stable
E. Varicose Vein STP: phlebectomy for recurrent episodes; refer to Chapter 21, Chronic Venous Disease and Venous Ulcers for various techniques
 IX. Upper Extremity DVT (Munoz et al., 2008; Persson, Arnhjort, Larfars, & Rosfors, 2006)
IX. Upper Extremity DVT (Munoz et al., 2008; Persson, Arnhjort, Larfars, & Rosfors, 2006)
A. DVT of Axillary, Subclavian, Brachiocephalic
1. Precipitating factors similar to lower extremity, but may also include
a. Increased use of subclavian vein for central line
b. Use of irritating intravenous solutions or drug abuse
c. Muscular effort of arms, especially in athletes such as swimmers, weightlifters, rowers, painters, wrestlers (Paget–Schroetter syndrome)
d. Thoracic outlet syndrome
e. Pacemaker wires
f. Tumor
g. Congenital venous malformations
2. Signs and symptoms
a. May be asymptomatic
b. Pain and local tenderness
c. Warmth
d. Edema
e. Supraclavicular fullness
f. Palpable cord
g. Increased venous pattern on chest wall
h. Upper limb cyanosis
3. Complications
a. Chronic venous insufficiency
b. Upper limb ulceration
c. Phlebitis
d. Thoracic duct obstruction
e. Superior vena cava syndrome
f. Brachial plexopathy
g. Pulmonary embolism
4. Diagnosis established by
a. Clinical history and physical examination
b. Venous duplex ultrasound
c. Venography
d. Magnetic resonance venography: valuable for central veins
e. CT venography: useful in detecting external compression of the venous system
5. Medical management: elevation, moist heat, anticoagulation, and/or thrombolytic agent
6. Interventional/surgical management
a. Superior vena cava filter
b. Venous thrombectomy (early re-thrombosis common)
c. Transaxillary first rib resection: most useful for thoracic outlet syndrome or effort thrombosis after initial thrombolytic or anticoagulation therapy
B. Superior Vena Cava Syndrome (Sajid, Ahmed, Desai, Baker, & Hamilton, 2007)
1. Most common causes
a. Bronchogenic cancer
b. Lymphoma
c. Metastatic tumor from breast or testicles
d. Radiation therapy
e. Central line placement, especially Swan–Ganz, Hickman, or transthoracic pacemakers
f. Other causes: aortic aneurysm, polyarteritis nodosa, pericardial hematoma, congenital anomaly
2. Pathophysiology
a. Extrinsic compression from slowly progressive tumor that eventually obstructs superior vena cava
b. Direct invasion of vessel or acute thrombosis causes sudden, dramatic symptoms
3. Signs and symptoms
a. Upper extremity and facial edema, including conjunctival edema
b. Dilation of superficial veins on chest, neck, or arms
c. Cough, hoarseness, shortness of breath with stridor due to laryngeal edema
d. Dysphagia due to esophageal edema
e. Dizziness, headache, visual complaints, somnolence, change in mental status (including coma)
f. Symptoms worse with lying, stooping, bending
4. Diagnosis
a. Venous duplex may be done initially but venography confirms diagnosis
b. Chest radiography and CT scan identify underlying problem
c. Biopsy through a percutaneous approach for tumor/mass
5. Treatment
a. Bed rest, diuretics, head elevation
b. Steroids for laryngeal and esophageal edema
c. Anticoagulation and/or thrombolysis if thrombosis of superior vena cava has occurred
d. Surgical reconstruction may be attempted in some cases
 X. Nursing Management/Diagnoses
X. Nursing Management/Diagnoses
A. Alteration in tissue perfusion related to venous thrombosis as evidenced by swelling
1. Expected patient outcomes
a. Patient will have decreased swelling
b. Patient will have continued arterial flow
2. Interventions
a. Leg/arm elevation above the level of the heart
b. Graduated compression stockings
c. Anticoagulation as indicated
d. Avoid prolonged sitting or standing when allowed out of bed
3. Evaluation
a. Measure circumference of affected limb
b. CMS checks (circulation, motion, and sensation)
B. Alteration in tissue perfusion related to venous thrombosis as evidenced by pain
1. Expected patient outcomes
a. Patient will be pain free
b. Patient will be able to perform activities of daily living
2. Interventions
a. Analgesia as needed
b. Graduated compression stockings
c. Bed rest/elevation of affected limb
d. Position for comfort (do not gatch bed at knees and avoid extreme flexion of hips)
3. Evaluation
a. Assess pain using a scale of 1 to 10 every 4 to 8 hours
b. Note usage of analgesia and response
c. Observe activity level the patient is able to tolerate
d. Obtain physical and occupational therapy consults as needed to help patient return to normal activities of daily living
C. Potential for injury/bleeding related to anticoagulation
1. Expected patient outcomes
a. Patient will show no signs of bleeding
2. Interventions
a. Avoid intramuscular injections
b. Recommend use of electric razors and soft toothbrushes
c. Apply pressure to all venipuncture sites for 3 to 5 minutes
d. Check IV site with each physical assessment
e. Monitor PTT/PT/INR/platelet count
3. Evaluation
a. Assess for signs of epistaxis, bleeding gums, melena, hematuria, bruising, and petechiae with each physical assessment
b. Monitor for internal bleeding as noted by a change in vital signs
1) Increased heart rate
2) Decreased blood pressure
3) Pallor
 XI. Patient/Family Education
XI. Patient/Family Education
A. Disease Prevention/Health Promotion and Secondary Prevention
1. Discuss etiology/pathophysiology of venous thrombosis
2. Identify/discuss and evaluate patient/family understanding of risk factors
3. Explain treatment goals
B. Prevention of Subsequent Thrombosis/Embolism/Post-Thrombotic Syndrome
1. Wearing compression garment
2. Maintenance of baseline activities of daily living
3. Elevate as necessary
C. Medication Education
1. Tablet strength, color, how many, and how often to take
2. INR goal: define, give expected range (2 to 3), and explain that adjustments may be necessary periodically
3. Side effects
a. Bleeding/easily bruised
b. New rash
c. Seek medical attention if any side effects occur
4. Interactions with other medications
a. No aspirin/anti-inflammatory drug unless prescribed by health care provider
b. Multiple drug interactions with warfarin
5. Subcutaneous injection technique teaching if self-administering LMWH
6. Discuss estrogen therapy risks with medical provider
D. Symptom Management
1. Infection: call health care provider
2. Increased pain, swelling, warmth, erythema: go to emergency department
3. Pulmonary embolism
a. Chest pain, rapid pulse, diaphoresis, anxiety, fainting, hemoptysis
b. Call 911
4. Bleeding
a. Major: call 911 or go to emergency department
b. Minor: alert health care provider
5. Unilateral paralysis, numbness, facial droop, vision/speech change, loss of coordination, or change in mental status: seek emergency care, call 911
E. Home Care Considerations
1. Diet: issues involving vitamin K
a. Diet should be consistent and moderate
b. Provide list of foods moderate to high in vitamin K
1) Green leafy vegetables are high in vitamin K
2) Green tea and tobacco are very high in vitamin K and should be avoided
c. Avoid alcohol use
d. Encourage patient to check with health care provider before using any herbal remedies
2. Activity
a. Walk regularly, may also jog, swim, cycle, or engage in low-impact sports after acute phase of thrombosis
b. Avoid prolonged sitting/standing
c. Elevate legs whenever nonambulatory
d. Avoid crossing legs and constrictive clothing
3. Compression garment should be measured and fitted by an experienced professional
4. Safety precautions
a. Protect the feet
b. Exercise caution when using sharp items (e.g., scissors, broken glass)
c. Wear a helmet when riding a bicycle
d. Wear or carry identification that indicates anticoagulation therapy
e. Seek immediate care for serious injury or head injury
f. Avoid contact sports
5. Home environment
a. Ability to come in for routine INR checks
b. Ability to administer own medications accurately
c. Assess for risk of falls
d. Self-testing for select patients based on diagnosis, dexterity, and compliance
6. Notify health care provider
a. Changes in medication, diet, missed dose
b. Questions or symptoms
CASE STUDY
A 22-year-old female, Ms. K., with no significant past medical history, sustains severe trauma to her right knee in a skiing accident. She undergoes knee reconstruction. A tourniquet is placed above the affected knee during the surgical procedure to minimize operative bleeding. Twenty-four hours after the procedure, the patient reports increased pain and swelling of her right leg.
Physical examination includes the following:
• Integument: warm, red right leg above the level of her dressing with increased swelling compared with previous postoperative examination. Incision is intact and without erythema or evidence of wound infection. All other skin intact and normal
• Cardiovascular: apical pulse 84, regular rate and rhythm, no murmurs, rubs, or gallops, blood pressure 138/74, palpable pulses throughout with moderate edema from right foot to mid-thigh level
• Respiratory: clear bilaterally, even respirations, rate 20
• Gastrointestinal: hypoactive bowel sounds, no masses or tenderness
• Neurologic: alert and oriented × 3, appears in mild distress, tearful; sensation and motion of extremities normal, negative Homan sign
• Additional vital signs: temperature: 37.8°C
• Pain scale rating: 9 on scale of 1 to 10
Social history: independent with activities of daily living; single, lives with two roommates in a one-story apartment and attends local college; nonsmoker, drinks socially on the weekends; has taken birth control pills for the last 4 years.
Family history: mother, father, one brother alive and well; mother had two miscarriages, reasons unknown.
Laboratory values reveal the following:
Hematocrit 36 (normal)
Hemoglobin 14 (normal)
Creatinine 0.7 (normal)
Oxygen level 99% pulse oximetry (normal)
Platelets 176,000 (normal)
Initial diagnosis: suspicious for right leg postoperative DVT
Review Questions
1. What signs and symptoms are suspicious for venous thrombosis?
a. Pain
b. Swelling
c. Homan sign
d. a and b
2. What diagnostic test would the provider order next?
a. Antithrombin III
b. Venous duplex ultrasound
c. CT scan
d. Venography
3. What was the most important precipitating factor that resulted in her DVT?
a. Immobilization
b. Systemic coagulation abnormalities
c. Birth control pills
d. Venous stasis
e. Venous trauma
f. d and e.
4. What immediate care measures need to be instituted to aid in preventing a pulmonary embolism?
a. Elevation of the affected extremity
b. Anticoagulation with warfarin
c. Anticoagulation with heparin or low-molecular-weight heparin
d. Compression therapy
5. What teaching and instructions need to be included in her discharge plan?
a. Continued avoidance of all tobacco products
b. Discontinuation of birth control pills
c. Compression therapy
d. Medication teaching
e. All of the above
Answers/Rationales
1. d. a and b. Pain, swelling, and tenderness are typically seen with venous thrombosis; however, the signs and symptoms can vary depending on the extent and location of the clot. Physical findings may be completely absent (silent DVT) when there is minimal obstruction or adequate collateral flow. Homan sign is an unreliable indicator for presence of DVT.
2. b. Venous duplex ultrasound. Acute leg pain and swelling may have many causes, especially in this postoperative patient with total knee reconstruction. Diagnosing DVT is difficult on clinical grounds alone. Noninvasive studies have become the most useful tools in diagnosing a DVT; duplex ultrasound is ordered most frequently because of its high sensitivity and specificity of >95% for proximal vessels. A CT scan can show a DVT, but it is not the first-line choice. Venography is invasive and therefore is used less than other diagnostic tests, although it is still the most reliable. Venography may be indicated in patients suspected of having DVT if there is an inconclusive test in a patient who has a strong clinical suspicion for DVT. Because of its risk, discomfort, and expense, venography should only be used when it is imperative to establish a definitive diagnosis. Checking the antithrombin III level will not help diagnose the DVT. It will only tell if the patient has a clotting abnormality. Ms. K. was sent for a duplex ultrasound, which confirmed a right popliteal to femoral DVT.
3. f. Venous stasis and venous trauma. The tight tourniquet placed around the patient’s leg during surgery, combined with severe trauma, probably injured the leg veins and also caused blood stasis. Although the patient is young and probably has a low propensity for thrombosis, the vessel injury/stasis in this setting was of sufficient magnitude to precipitate a DVT. Oral contraceptives and immobilization are predisposing risk factors to the development of DVT. However, Virchow triad (stasis of venous blood, local venous trauma, and coagulation abnormalities) is still believed to be of primary importance in the formation of thrombus in the systemic veins.
4. c. Anticoagulation with heparin or low-molecular-weight heparin. This patient needs immediate anticoagulation to prevent propagation of the thrombus or embolism. Treatment consists of therapeutic-dose UFH or LMWH for at least 5 days, overlapped with and followed by treatment with Coumadin for 3 to 6 months. Lethal pulmonary embolism most often originates in the femoral or iliac veins, and thrombosis in this area absolutely requires anticoagulation. Isolated calf vein or tibial vein thromboses rarely lead to clinically detectable pulmonary embolus; thus this type of thrombus may not require full anticoagulation therapy. Elevation is recommended to help prevent swelling and will help with comfort measures; the more the leg swells, the more it hurts but it will not prevent pulmonary embolism. Compression therapy is used after the acute phase of thrombosis to help with the swelling and to prevent long-term postphlebitic complications. Ms. K’s INR is 2.6 on postoperative day 4, and she is being discharged.
5. e. All of the above. Patient and family education and understanding of the treatment, prevention, and risk factors associated with DVT is of primary importance. Anticoagulation therapy instruction should focus on its purpose, side effects, drug interactions, dietary and physical activity restrictions, and the need for continued follow-up and frequent blood tests. Inform the patient of the risks of smoking and taking oral contraceptives in someone who has had a DVT, as well as the risk of recurrence in certain situations (e.g., air travel). The risks associated with alcohol consumption and warfarin therapy should be reviewed, especially because this patient admits to social drinking. Inform the patient that the graduated compression stocking needs to be worn to promote venous blood return, decrease leg swelling, and help prevent the long-term complications of postphlebitic syndrome. Education is critical to the success of a patient’s overall treatment plan. Taking the time to instill an understanding of why interventions and life-style modifications are necessary will help the patient comply for better short-term and long-term health.
REFERENCES
Ageno, W., Gallus, A., Wittkowshy, A., Crowther, M., Hylek, E., & Palareti, G. (2012). Oral anticoagulant therapy; Antithrombotic therapy and prevention of thrombosis 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest, 141(2 Suppl.), 44S–88S.
Ansell, J., Hirsh, J., Hylek, E., Jacobson, A., Crowther, M., & Palareti, G. (2008). Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines. Chest, 133, 160S–198S.
Caprini, J., & Arcelus, J. (2007). Venous thromboembolism prophylaxis in the general surgical patient. In J. Bergan (Ed.), The vein book (pp. 369–380). Burlington, MA: Elsevier.
Ferri, F. J. (2011). Ferri’s clinical advisor 2011: Instant diagnosis and treatment (pp. 310–312; 1084). St. Louis, MO: Elsevier, Mosby.
Garcia, D., Baglin, T., Weitz, J., & Samama, M. (2012). Parenteral anticoagulants antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest, 141(2 Suppl.), 24S–43S.
Geerts, W., Bergqvist, D., Pineo, G., Heit, J., Samama, C., Lasses, M., & Colwell, C. (2008). Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th ed.). Chest, 133, 381S–453S
Gogainiceanu, P., Johnston, C. J., Kahlid, U., Holt, P., Hincliffe, R., Loftus, I., & Thompson, M., (2009). Indications for thrombolysis in deep venous thrombosis. European Journal of Vascular and Endovascular Surgery, 38, 192–198.
Guyatt, G., Akl, E., Crowther, M., Gutterman, D., & Shuünemann, H. (2012). Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physician evidence-based clinical practice guidelines. Chest, 141(2 Suppl.), 7S–46S.
Hingorani, A., & Ascher, R. (2009). Superficial venous thrombophlebitis. In Handbook of venous disorders: Guideline of the American venous forum (pp. 314–319). London, UK: Hodder Arnold.
Hirsh, J., Bauer, K., Donati, M., Gould, M., Samama, M., & Weitz, J. (2008). Parenteral anticoagulants: American college of chest physician evidence-based guidelines (8th ed.). Chest, 133, 141S–159S.
Hirsh, J., Guyatt, G., Albers, G., Harrington, R., & Schunemann, H. (2008). Antithrombotic and thrombolytic therapy: American college of chest physicians, evidence-based clinical practice guidelines (8th ed.). Chest, 133, 110S–112S.
Hofmann, L. (2009). Therapies for acute and chronic deep venous thrombosis. Clinical Advances in Hematology & Oncology, 7, 301–303.
Holbrook, A., Schulman, S., Witt, D. M., Vandvik, P. O., Fish, J., Kovacs, M., … Guyatt, G. H. (2012). Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physician evidence-based clinical practice guidelines. Chest, 141(2 Suppl.), 152S–177S.
Lohr, J., Kin, D., & Krallman, K. (2009). Diagnostic algorithms for acute deep vein thrombosis and pulmonary embolism. Handbook of venous disorders: Guideline of the American venous forum (pp. 208–220). London, UK: Hodder Arnold.
Mcbane, R., & Heit, J. (2009). Current recommendations for prevention of deep vein thrombosis. In Handbook of venous disorders: Guideline of the American venous forum, (pp.277–291). London, UK: Hodder Arnold.
McCance, K., & Huether, S. (2010). Pathophysiology: The biologic basis for disease in adults and children (6th ed., pp. 952–988 & 1091–1141). Maryland Heights, MO: Mosby.
Meissner, M. (2009). The epidemiology of and risk factors for acute deep venous thrombosis. In Handbook of venous disorders: Guideline of the American venous forum, (pp.94–104). London, UK: Hodder Arnold.
Mermel, L., Allon, M., Bouza, E., Craven, D., Flynn, P., O’Grady, N., … Warren, D. (2009). Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious disease society of America. Clinical Infectious Diseases, 49, 1–45.
Munoz, F., Mismetti, P., Poggio, R., Valle, R., Barron, M., Guil, M., & Monreal, M. (2008). Clinical outcome of patients with upper-extremity deep vein thrombosis. Chest, 133, 143–148.
Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General (US); 2008. Available from http://www.ncbi.nlm.nih.gov/book/NBK44189
Persson, L., Arnhjort, T., Larfars, G., & Rosfors, S. (2006). Hemodynamic and morphologic evaluation of sequelae of primary upper extremity deep venous thrombosis treated with anticoagulation. Journal of Vascular Surgery, 43, 1230–1235.
Prescriber’s Letter. (2013). Comparison of oral antithrombotics, Prescriber’s Letter, Detail document #290201, Retrieved from www.PrescribersLetter.com, accessed June 28, 2013.
Sajid, M., Ahmed, N., Desai, M., Baker, D., & Hamilton, G. (2007). Upper limb deep vein thrombosis: A literature review to streamline the protocol for management. Acta Haematologica, 118, 10–18.
Schulman, S., Kearon, C., Kakkar, A. A., Mismetti, P., Schellong, S., Eriksson, H., … Goldhaber, S. (2009). Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New England Journal of Medicine, 361, 2342–2352.
Vedantham, S. (2009). Deep venous thrombosis: The opportunity at hand. American Journal of Radiology, 193, 922–927.
Walsh, M. E., & Rice, K. L. (2004). Venous thromboembolic disease. In V. Fahey (Ed.), Vascular nursing (4th ed., pp. 365–398) St. Louis, MO: Saunders.
Warkentin, R., Greinacher, A., Koster, A., & Lincoff, A. (2008). Treatment of heparin-induced thrombocytopenia: American college of chest physicians evidence-based clinical practice guidelines (8th ed.). Chest, 133, 340S–380S.
SUGGESTED READINGS
Bregenzer, T., Conen, D., Sakmann, P., & Widmer, A. F. (1998). Is routine replacement of peripheral intravenous catheters necessary? Archives of Internal Medicine, 158(2), 151–156.
Geerts, W., Pineo, G., Heit, J., Berquist, D., Lassen, M., Colwell, C., & Ray, J. (2004). Prevention of venous thromboembolism: The seventh ACCP conference on antithrombotic therapy. Chest, 126, 338–400.
Heit, J. (2009). The epidemiology of venous thromboembolism in the community: Implications for prevention and management. In J. Bergan (Ed.), The vein book (pp. 323–330) Burlington, MA: Elsevier.
Ligush, J., & Johnson, G. (1996). Superficial thrombophlebitis. In P. Gloloviczki & J. S. T. Yao (Eds.), Handbook of venous disorders: Guidelines of the American venous forum (pp. 235–243). London, UK: Chapman & Hall Medical.
Schulman, S., Beyth, R., Kearon, C., & Levine, M. (2008). Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guideline (8th ed.). Chest, 133, 257S–298S.
< div class='tao-gold-member'>



